
SLC5A12 is a prognostic marker in head and neck squamous cell carcinoma
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the seventh most common cancer worldwide, accounting for approximately 600,000 new cases and 300,000 deaths each year (1,2). HNSCC is classified by tumor location: in the mouth (oral cavity), in the middle part of the throat near the mouth (oropharynx), in the space behind the nose (nasal cavity and paranasal sinuses), in the upper part of the throat near the nasal cavity (nasopharynx), in the voice box (larynx), or in the lower part of the throat near the larynx (hypopharynx).
The strongest risk factors for HNSCC are tobacco use (both smoking and chewing tobacco) and heavy alcohol consumption. HNSCC is more common in men than in women, and most prevalent in men in their 50s or 60s, although the incidence among the younger population is increasing because of infection by the human papillomavirus (HPV) (3). The occurrence of HNSCC varies greatly in different regions of the world, which reflects the prevalence of these risk factors. For example, oropharyngeal cancer is more common in young populations because of HPV infection (4,5), nasopharyngeal cancer is more common in southern regions of China because of Epstein-Barr virus infection (6), while mouth and tongue cancers are more common in India where tobacco chewing is popular (7,8). Over 50% of HNSCC cases are diagnosed as regional advanced or metastatic disease with poor survival. However, the HNSCC prognosis varies according to disease location and associated risk factors, with women having a better prognosis than men (9,10), patients with larynx SCC having a better survival than those with hypopharynx SCC, and HPV-associated HNSCCs being associated with response to chemotherapy and improved survival (11,12). Despite our improved understanding of HNSCC risk factors, novel prognostic markers and therapeutic targets are urgently needed.
Tumor cells are characterized by increased aerobic glycolysis which rapidly generates ATP and converts glucose into precursors for biomolecular synthesis, thus supporting their high proliferation needs (13). This leads to an accumulation of intracellular lactate, which is subsequently transported across the plasma membrane to maintain an intracellular physiological pH. Although lactate is generated as a byproduct of altered tumor metabolism, it plays a critical role in tumor development, progression, and metastasis (14). Indeed, tumor lactate levels have been shown to correlate with tumor recurrence, increased metastasis, resistance to therapy, and poor outcome (15). Exported lactate can induce metabolic reprogramming in adjacent cancer, stroma, and vascular endothelial cells, as well as promoting tumor inflammation, inactivating immune surveillance, and stimulating tumor angiogenesis. It also acidifies the tumor microenvironment, which results in the development of resistance to chemotherapy (16).
Besides being transported across the plasma membrane by proton-coupled monocarboxylate transporters (MCT1–4) (16), lactate can also be transported by sodium-coupled monocarboxylate transporters (SMCTs). Thus far, two SMCTs have been identified: the high affinity SLC5A8 (SMCT1) and low affinity SLC5A12 (SMCT2) (17). Several studies have indicated that SLC5A8 functions as a tumor suppressor in different types of cancer, including colon and thyroid cancers (18,19). However, little is known about the role of SLC5A12 in tumor development and progression.
In the current study, we determined both mRNA and protein expression levels of SLC5A12 in HNSCC tissue samples by quantitative reverse transcription PCR (qRT-PCR), tissue microarray immunohistochemistry analysis (TMA-IHC), and western blotting, and correlated these with patient clinical characteristics.
Methods
Human tissue specimens and patient clinical information
A total of 336 HNSCC patients were included in this study as previously described (20). Twenty-eight HNSCC patients consented to inclusion and were enrolled before surgery, and 56 fresh tissue samples (HNSCC tissue and matched adjacent normal tissue from each patient) were collected and frozen at the time of surgery. Six pairs of fresh tumor and matched adjacent tissue were also obtained from patients undergoing surgery for HNSCC at the Affiliated Hospital of Nantong University (Jiangsu, China). Additionally, 336 HNSCC patients provided 479 archived formalin-fixed paraffin-embedded tissue blocks. These included 121 tongue squamous cell carcinoma (TSCC) tissues and 54 matched adjacent normal tissues, 90 buccal squamous cell carcinoma (BSCC) tissues and 38 matched adjacent normal tissues, and 125 laryngeal squamous cell carcinoma (LSCC) tissues and 51 matched adjacent normal tissues. Clinical characteristics were obtained from patients’ medical records. The study protocol was approved by the Human Research Ethics Committee of the Affiliated Hospital of Nantong University.
Bioinformatic analysis
The database UALCAN (http://ualcan.path.uab.edu/index.html) performs to in-depth analyses of TCGA gene expression data including 31 cancer types, to evaluate the expression pattern of SLC5A12 in massive tissue from GC patients.
One-step qRT-PCR
SLC5A12 mRNA levels were determined by qRT-PCR and normalized to the housekeeping gene GAPDH (21). Primer sequences were as follows: SLC5A12 forward primer (5'-TCCACAATGTATTAGAGCAAT-3') and SLC5A12 reverse primer (5'-ATAGATTCCGAGCCAAGTA-3'), GAPDH forward primer (5'-TGCACCACCAACTGCTTAGC-3') and GAPDH primer (5'-GGCATGGACTGTGGTCATGAG-3'). Amplification conditions were: 42 °C for 30 min for reverse transcription and 94 °C for 2 min for Taq activation followed by 40 cycles of 94 °C for 20 s, 55 °C for 20 s, and elongation at 72 °C for 30 s.
Western blotting
SLC5A12 protein expression was quantitatively detected by western blotting as described previously (21). Membranes wee incubated overnight at 4 °C with primary rabbit polyclonal anti-SLC5A12 (1:20 dilution; Atlas Antibodies, USA) and rabbit anti-GAPDH antibodies (1:2,000 dilution; Goodhere, Hangzhou, China). They were then incubated with a horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:1,500 dilution; Abcam, Cambridge, UK), and scanned by ECL (Beyotime Institute of Biotechnology, Shanghai, China). Data were analyzed by densitometry.
TMA-IHC and scoring
SLC5A12 protein expression in tissue blocks was determined using TMA-IHC (20) with a primary rabbit polyclonal anti-human SLC5A12 antibody (dilution 1:1,000; Atlas Antibodies) and a secondary goat anti-rabbit IgG antibody (dilution 1:1,000; Abbkine Inc., Redlands, CA, USA). The product of the percentages and intensity scores was used as the final staining score, and ranged from 0 (no staining) to 300 (+++ staining intensity). The cutoff point was determined as a score of 100, such that a score of 0–100 represented low SLC5A12 expression and a score of 100–300 represented high SLC5A12 expression.
Statistical analysis
SLC5A12 IHC data were scored using the semi-quantitative H-score method and analyzed using the X-tile software program (22-24). Statistical analysis was performed as described previously (25). The relationships between the expression of SLC5A12 and clinical parameters were calculated by using χ2 tests. Additionally, we used univariate analysis and multivariate analysis to evaluate the prognostic value for patients with HNSCC. SPSS 20.0 software (IBM Corporation, Armonk, NY, USA) and STATA 12.0 (Stata Corporation, College Station, TX, USA) were applied for data analysis. P<0.05 was considered to be statistically significant.
Results
SLC5A12 mRNA expression was significantly higher in HNSCC tissues than in adjacent normal tissues
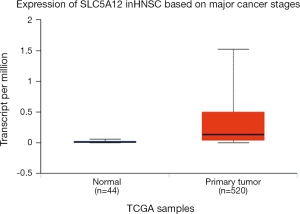
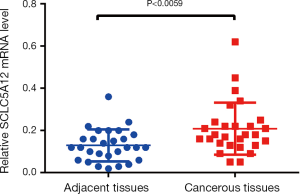
According to the database, SLC5A12 is more expressed in cancers compared with para-cancerous tissues (Figure 1). Moreover, we performed qRT-PCR and discovered that SLC5A12 mRNA expression was significantly higher in cancerous tissues (0.2082±0.02332) than in adjacent normal tissues (0.1296±0.01438) (P<0.0059) (Figure 2).
SLC5A12 protein expression was significantly higher in HNSCC tissues than adjacent normal tissues
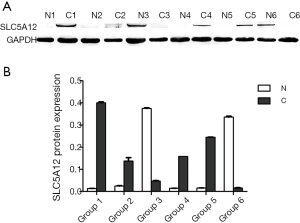
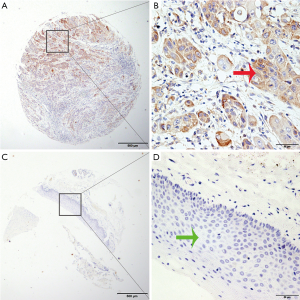
Western blotting revealed significantly higher SLC5A12 protein expression in four of six pairs of cancerous tissues compared with normal tissues (Figure 3). Of the 479 archived HNSCC tissue blocks, high SLC5A12 expression was detected in 51.2% of TSCC tissues, which is significantly higher than the high SLC5A12 expression detected in 27.8% of adjacent normal tissues (χ2=8.341, P=0.004); high SLC5A12 expression was also detected in 54.4% of BSCC tissues compared with 26.3% of adjacent normal tissues (χ2=5.508, P=0.004), and in 56.0% of LSCC tissues compared with 29.4% of adjacent normal tissues (χ2=10.254, P=0.001) (Table 1, Figure 4).

Full table
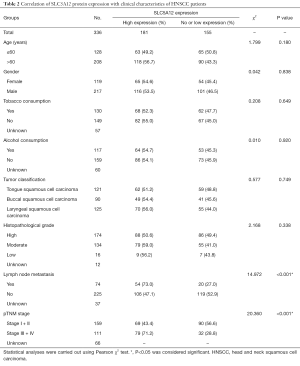
Association of SLC5A12 expression with HNSCC clinical characteristics
We next correlated SLC5A12 protein expression with HNSCC patient clinical characteristics, including tobacco and alcohol consumption. High SLC5A12 protein expression was significantly associated with the presence of lymph node metastasis (P<0.001) and higher staging [clinical tumor-node-metastasis (cTNM) stage III–IV, P<0.001] (Table 2).
Full table
High SLC5A12 expression predicts poor overall survival in HNSCC patients
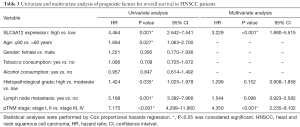
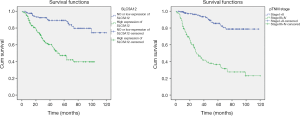
Finally, we analyzed prognostic factors in HNSCC patients using both univariate and multivariate analysis. Univariate analysis showed that high SLC5A12 expression [hazard ratio (HR), 4.464, 95% confidence interval (CI): 2.642–7.541, P=0.001], older age at diagnosis (HR, 1.694, 95% CI: 1.063–2.700, P=0.027), histopathological grade (HR, 1.424, 95% CI: 1.025–1.978, P=0.035), lymph node metastasis (HR, 5.198, 95% CI: 3.392–7.966, P=0.001), and cTNM stage (HR, 7.170, 95% CI: 4.299–11.960, P<0.001) were all significantly associated with overall survival. Multivariate analysis showed that high SLC5A12 expression (HR, 3.229, 95% CI: 1.890–5.515, P<0.001), and cTNM stage (HR, 4.350, 95% CI: 2.235–8.102, P<0.001) remained significantly associated with poor overall survival (Table 3, Figure 5).
Full table
Discussion
In the current study, we found that SLC5A12 mRNA and protein expression was significantly higher in HNSCC tissues than in adjacent normal tissues. High SLC5A12 protein expression was also associated with lymph node metastasis and high cTNM stages, and was shown to be an independent prognostic marker for poor overall survival in HNSCC patients.
Cancer cell metabolism is characterized by the increased utilization of aerobic glycolysis, a phenomenon known as the Warburg effect (16). This is directly regulated by signaling pathways linked to the activation of oncogenes or loss of tumor suppressors (14). The altered metabolism is not only essential for malignant transformation, but also facilitates tumor development and progression. Because glycolytic ATP production is associated with tumor malignancy (26), the targeting of cancer cell metabolism represents a promising novel strategy for early cancer diagnosis and treatment.
Lactate is one of the byproducts associated with tumor-specific aerobic glycolysis, and MCT1–4 are the main lactate transporters responsible for its removal from the cell. A recent meta-analysis study showed that expression of MCT4 and its chaperone CD147 protein correlates with decreased disease-free and overall survival in many cancer types including oral SCC (27,28). Moreover, the overexpression of MCT1, MCT4, and CD147 was associated with tumor progression in clear cell renal cell carcinoma (29), while high MCT1 and MCT4 expression levels were independent prognostic factors for poor overall survival and poor recurrence-free survival in urothelial carcinoma of the bladder (30).
In HNSCC, MCT1 expression correlates with cell proliferation, while MCT4 expression is associated with high oxidative stress and correlates with poor clinical outcome (31). Inhibiting both MCT1 and MCT4 therefore represents a new therapeutic strategy for HNSCC. Indeed, several in vitro studies suggest that MCT1 and MCT4 inhibition has anti-tumor activities. For example, MCT4 expression is higher in castration-resistant prostate cancer and is associated with early relapse, while MCT4 knockdown can inhibit tumor growth (32). In colon cancer cell lines, MCT1/4 inhibition reduces lactate export and tumor growth (33,34), suggesting that MCT inhibition is a potential cancer therapy. In non-small cell lung cancer cell lines, targeting lactate export can inhibit glycolysis and sensitize tumor cells to the mitochondrial inhibitor phenformin (35).
Lactate can also be transported out of the cell by sodium-coupled lactate transporters, such as SLC5A8 and SLC5A12. SCL5A8 was initially identified as a tumor suppressor in the human colon (36), while SLC5A12 was isolated as a SLC5A8 homologous gene from the mouse kidney cDNA library (37,38). Both can transport lactate, pyruvate, butyrate, and short-chain fatty acids with different affinities (17). The expression of SLC5A8 is epigenetically silenced in several types of cancer, including that of the colon, thyroid, stomach, brain, breast, pancreas, and kidney (18,19). It acts as a tumor suppressor by inducing apoptosis through the butyrate/pyruvate-dependent inhibition of histone deacetylases. Moreover, the downregulation of SLC5A8 has also been associated with drug resistance (39).
Very little is known about the exact function of SLC5A12. Because of its low affinity, it has been hypothesized that SLC5A12 is responsible for the bulk absorption of lactate in the kidney. SLC5A12 is also expressed in the small intestine but not in the colon, astrocytes, or glia (17). A recent study suggested that SLC5A12 is selectively expressed on CD4+ T cells and is responsible for the entrapment of CD4+ T cells in the chronically inflamed synovial tissue of rheumatoid arthritis patients (40), which suggests that SLC5A12 promotes tumorigenesis through inducing chronic inflammation.
Our study has several limitations. First, it was retrospective so was subject to sample selection bias; therefore, our conclusions cannot be directly extended to other populations without further validation. Second, only patients with oral cavity SCC (63%) and laryngeal SCC (37%) were included in our study. Because HNSCC is a heterogeneous disease, and prognosis varies by tumor location, we do not know whether our conclusions can be extended to HNSCC at other locations. Third, we did not determine the HPV status of HNSCC cases in the current study, and cannot speculate on the interaction between HPV oncogenes and SLC5A12. Fourth, we did not determine the expression of MCT1–4 in relation to SCL5A12, so cannot speculate on whether SCL5A12 is a better prognostic marker for HNSCC compared with MCT1–4. Finally, we have not suggested a mechanism for the role of SLC5A12 in tumor development. It remains unknown whether SLC5A12 expression is associated with cell proliferation or oxidative stress. Future in vitro studies are therefore needed to elucidate underlying molecular mechanisms.
In conclusion, our study demonstrates that SLC5A12 may act as an oncogene in HNSCC, and shows that SLC5A12 overexpression is an independent prognostic marker for HNSCC in the Chinese population. The function of SLC5A12 is tightly linked to tumor metabolic reprogramming, lactate transportation, and chronic inflammation, so SLC5A12 inhibition represents a novel cancer therapy for targeting the Warburg effect and tumor inflammation.
Acknowledgments
We thank Sarah Williams, PhD, from Liwen Bianji, Edanz Group China (
Funding: This work was supported by grants from the Jiangsu Provincial Health and Family Planning Commission (Grant No. H201523) and the key scientific research project of Nantong (No. MS22015111).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.07.19). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Human Research Ethics Committee of the Affiliated Hospital of Nantong University (No. 2018-K020). Informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Wyss A, Hashibe M, Chuang SC, et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am J Epidemiol 2013;178:679-90. [Crossref] [PubMed]
- Sankaranarayanan R, Masuyer E, Swaminathan R, et al. Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res 1998;18:4779-86. [PubMed]
- Gillison ML, Chaturvedi AK, Anderson WF, et al. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J Clin Oncol 2015;33:3235-42. [Crossref] [PubMed]
- Rettig EM, D'Souza G. Epidemiology of head and neck cancer. Surg Oncol Clin N Am 2015;24:379-96. [Crossref] [PubMed]
- Raghupathy R, Hui EP, Chan AT. Epstein-Barr virus as a paradigm in nasopharyngeal cancer: from lab to clinic. Am Soc Clin Oncol Educ Book 2014;149-53. [Crossref] [PubMed]
- Bray F, Ren JS, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2013;132:1133-45. [Crossref] [PubMed]
- Lambert R, Sauvaget C, de Camargo Cancela M, et al. Epidemiology of cancer from the oral cavity and oropharynx. Eur J Gastroenterol Hepatol 2011;23:633-41. [Crossref] [PubMed]
- Gatta G, Botta L, Sanchez MJ, et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur J Cancer 2015;51:2130-43. [Crossref] [PubMed]
- Curado MP, Hashibe M. Recent changes in the epidemiology of head and neck cancer. Curr Opin Oncol 2009;21:194-200. [Crossref] [PubMed]
- Mirghani H, Amen F, Tao Y, et al. Increased radiosensitivity of HPV-positive head and neck cancers: Molecular basis and therapeutic perspectives. Cancer Treat Rev 2015;41:844-52. [Crossref] [PubMed]
- Lewis A, Kang R, Levine A, et al. The New Face of Head and Neck Cancer: The HPV Epidemic. Oncology (Williston Park) 2015;29:616-26. [PubMed]
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 2012;21:297-308. [Crossref] [PubMed]
- Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest 2013;123:3685-92. [Crossref] [PubMed]
- Kennedy KM, Dewhirst MW. Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Future Oncol 2010;6:127-48. [Crossref] [PubMed]
- Miranda-Goncalves V, Reis RM, Baltazar F. Lactate transporters and pH regulation: potential therapeutic targets in glioblastomas. Curr Cancer Drug Targets 2016;16:388-99. [Crossref] [PubMed]
- Ganapathy V, Thangaraju M, Gopal E, et al. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J 2008;10:193-9. [Crossref] [PubMed]
- Ganapathy V, Gopal E, Miyauchi S, et al. Biological functions of SLC5A8, a candidate tumour suppressor. Biochem Soc Trans 2005;33:237-40. [Crossref] [PubMed]
- Gupta N, Martin PM, Prasad PD, et al. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci 2006;78:2419-25. [Crossref] [PubMed]
- Han L, Tang MM, Xu X, et al. LTBP2 is a prognostic marker in head and neck squamous cell carcinoma. Oncotarget 2016;7:45052-9. [PubMed]
- Huang J, Mei H, Tang Z, et al. Triple-amiRNA VEGFRs inhibition in pancreatic cancer improves the efficacy of chemotherapy through EMT regulation. J Control Release 2017;245:1-14. [Crossref] [PubMed]
- Detre S, Saclani Jotti G, Dowsett M A. "quickscore" method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol 1995;48:876-8. [Crossref] [PubMed]
- Huang J, Fan X, Wang X, et al. High ROR2 expression in tumor cells and stroma is correlated with poor prognosis in pancreatic ductal adenocarcinoma. Sci Rep 2015;5:12991. [Crossref] [PubMed]
- Lu C, Wang X, Zhu H, et al. Over-expression of ROR2 and Wnt5a cooperatively correlates with unfavorable prognosis in patients with non-small cell lung cancer. Oncotarget 2015;6:24912-21. [Crossref] [PubMed]
- Xu Y, Wang C, Zhang Y, et al. Overexpression of MAGE-A9 Is Predictive of Poor Prognosis in Epithelial Ovarian Cancer. Sci Rep 2015;5:12104. [Crossref] [PubMed]
- Li F, Xu Y, Chen C, et al. Pro-apoptotic and anti-proliferative effects of 3,3'-diindolylmethane in nasopharyngeal carcinoma cells via downregulation of telomerase activity. Mol Med Rep 2015;12:3815-20. [Crossref] [PubMed]
- Bovenzi CD, Hamilton J, Tassone P, et al. Prognostic Indications of Elevated MCT4 and CD147 across Cancer Types: A Meta-Analysis. Biomed Res Int 2015;2015:242437 [Crossref] [PubMed]
- Zhu J, Wu YN, Zhang W, et al. Monocarboxylate transporter 4 facilitates cell proliferation and migration and is associated with poor prognosis in oral squamous cell carcinoma patients. PLoS One 2014;9:e87904 [Crossref] [PubMed]
- Kim Y, Choi JW, Lee JH, et al. Expression of lactate/H(+) symporters MCT1 and MCT4 and their chaperone CD147 predicts tumor progression in clear cell renal cell carcinoma: immunohistochemical and The Cancer Genome Atlas data analyses. Hum Pathol 2015;46:104-12. [Crossref] [PubMed]
- Choi JW, Kim Y, Lee JH, et al. Prognostic significance of lactate/proton symporters MCT1, MCT4, and their chaperone CD147 expressions in urothelial carcinoma of the bladder. Urology 2014;84:245 e9-15.
- Curry JM, Tuluc M, Whitaker-Menezes D, et al. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle 2013;12:1371-84. [Crossref] [PubMed]
- Choi SY, Xue H, Wu R, et al. The MCT4 Gene: a Novel, Potential Target for Therapy of Advanced Prostate Cancer. Clin Cancer Res 2016.
- Le Floch R, Chiche J, Marchiq I, et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci U S A 2011;108:16663-8. [Crossref] [PubMed]
- Marchiq I, Le Floch R, Roux D, et al. Genetic disruption of lactate/H+ symporters (MCTs) and their subunit CD147/BASIGIN sensitizes glycolytic tumor cells to phenformin. Cancer Res 2015;75:171-80. [Crossref] [PubMed]
- Granja S, Marchiq I, Le Floch R, et al. Disruption of BASIGIN decreases lactic acid export and sensitizes non-small cell lung cancer to biguanides independently of the LKB1 status. Oncotarget 2015;6:6708-21. [Crossref] [PubMed]
- Li H, Myeroff L, Smiraglia D, et al. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci U S A 2003;100:8412-7. [Crossref] [PubMed]
- Gopal E, Umapathy NS, Martin PM, et al. Cloning and functional characterization of human SMCT2 (SLC5A12) and expression pattern of the transporter in kidney. Biochim Biophys Acta 2007;1768:2690-7. [Crossref] [PubMed]
- Srinivas SR, Gopal E, Zhuang L, et al. Cloning and functional identification of slc5a12 as a sodium-coupled low-affinity transporter for monocarboxylates (SMCT2). Biochem J 2005;392:655-64. [Crossref] [PubMed]
- Babu E, Ramachandran S. Role of SLC5A8, a plasma membrane transporter and a tumor suppressor, in the antitumor activity of dichloroacetate. Oncogene 2011;30:4026-37. [Crossref] [PubMed]
- Haas R, Smith J, Rocher-Ros V, et al. Lactate Regulates Metabolic and Pro-inflammatory Circuits in Control of T Cell Migration and Effector Functions. PLoS Biol 2015;13:e1002202 [Crossref] [PubMed]


