
This article has an erratum available at: http://dx.doi.org/10.21037/tcr-2023-08 the article has been update on 2024-01-08 at here.
The targeting of endoglin on vascular endothelial cells affects the infiltration of M2 macrophages into the breast cancer microenvironment by modulating the interleukin-6 (IL-6) level
Introduction
Breast cancer is the most common malignancy in women and the second leading cause of death in the world. The American Cancer Society estimates that 167,114 cases of breast cancer were newly diagnosed and 521,907 patients died of breast cancer in the US in 2012 (1). Most patients diagnosed early with breast cancer can achieve long-term survival, and some patients can achieve disease-free survival. However, advanced breast cancer is very difficult to treat. Chemotherapy plus anti-angiogenic therapy is an effective option for advanced breast cancer that improves the disease-free survival but not the long-term survival of patients (2-4). Many studies have discovered angiogenesis-related signalling pathways in tumours and have revealed that the blockade of a certain signalling pathway might activate other signalling pathways, and thereby enhance angiogenesis in a tumour, leading to failure of anti-angiogenic therapies (5,6). Recent studies have shown that anti-angiogenic therapies modulate the tumour microenvironment.
The tumour microenvironment refers to the internal environment for tumour growth, which consists of the tumour parenchyma and interstitium. The interstitium includes tumour-associated fibroblasts, the extracellular matrix, immune cells, tissue fluid, and infiltrating biological molecules (7). The immune cells in the microenvironment play an important role in tumour immunity, which affects the outcome of tumour treatment. Tumour associated macrophages (TAMs) are macrophages that infiltrate tumour tissue and are the most abundant immune cells in the tumour microenvironment (approximately 50%) (8). Macrophages are highly plastic and include both M1 macrophages, which are activated via conventional pathways, and M2 macrophages, which are activated via alternative signalling pathways according to the activation state and function (9). M1 macrophages kill tumour cells and inhibit lymphangiogenesis and neovascularization. In contrast, M2 macrophages are primary tumour-associated macrophages that synthesize and release various cytokines, such as vascular endothelial growth factor (VEGF) and tumour growth factor β (TGF-β), to suppress the inflammatory response and promote tumour growth and metastasis (10). Previous studies have shown that anti-angiogenic agents targeting the VEGF pathway enhance tumour immunity. Sunitinib and endostatin reduce the amount of regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs) and B cells (11), promotes the maturation of dendritic cells, and restores the function of dendritic cells (12). A Low-dose of DC101 reduces the M2/M1 ratio (13). However, other studies have shown that anti-angiogenic therapy may inhibit the immunity of the tumour microenvironment and thus promote tumour progression. For example, some studies have shown that sorafenib, an anti-angiogenic agent, increases the amount of M2 macrophages and Tregs (14). The number of Tregs is also increased during anti-angiogenic therapies of renal cancer, and this increase has adverse effects on overall survival and progression-free survival (15). As anti-angiogenic agents both promote and inhibit tumour immunity, there was a controversy over the effects of anti-angiogenic agents on tumour immunity.
Endoglin is a membrane glycoprotein on the surface of the TGF-β receptor complex and a membrane antigen on the surface of endothelial cells that is related to proliferation (16). In addition, endoglin is closely associated with tumour development and progression, is highly expressed only in endothelial cells of new vessels, and is a marker of tumour angiogenesis. During tumour progression, endoglin promotes the proliferation and migration of vascular endothelial cells, which ultimately leads to neovascularization (17). The targeted blockage of endoglin reduces angiogenesis and inhibits tumour growth (18). Matsuno et al. showed that grafted tumours are completely eliminated in nude mice after treatment with SN6j and SN6k, which are anti-endoglin monoclonal antibodies (19). Mizutani et al. showed that an anti-endoglin vaccine inhibits tumour growth by effectively suppressing angiogenesis (20). Phase Ib/II clinical trials have shown that chemotherapy plus TRC105, an anti-angiogenic agent targeting endoglin on vascular endothelial cells, is superior to chemotherapy alone in extending the median progression-free survival but not overall survival of patients with advanced tumours (3,4,20). However, it is unknown whether anti-angiogenic therapy targeting endoglin on vascular endothelial cells affects the treatment outcome by altering the tumour microenvironment.
In this study, we used a conditional inducible endoglin knockout mouse model of orthotopic breast cancer to investigate the effect of endoglin knockout on tumour growth, macrophage infiltration, phenotype and related mechanisms with the aim of improving the efficacy of anti-angiogenic therapy for cancer patients.
Methods
Cell culture
The mouse breast cancer cell line EO771 was derived from a spontaneous mammary tumour in C57BL/6 mouse and widely used in the study of breast cancer research. This cell line was generously provided by Professor Helen Arthur (Institute of Genetic Medicine, Newcastle University, UK). EO771 cell line is reported to be negative expression of estrogen receptor alpha (ERα), progesterone (PR) and without human epidermal growth factor receptor 2 (HER2) amplification (21). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Corning, New Jersey, USA) containing 10% fetal bovine serum and 1% penicillin-streptomycin solution at 37 °C in humidified atmosphere containing 5% CO2.
Mice and tumour establishment
Female Eng-iKOe (Engfl/fl VE-Cre) mice and control (Engfl/fl) mice, aged eight to twelve weeks, were obtained from Newcastle University (Newcastle upon Tyne, UK). The mice was injected intraperitoneally every other day with 2 mg of tamoxifen for a total of 5 times (T6448-1G, Sigma), and a conditional endoglin knockout (Eng-iKOe) group and a control (Engfl/fl) group (n=10 for each group) were established (22).
EO771 cells (5×105) were injected orthotopically into the fourth right mammary fat pad of Eng-iKOe and control mice to establish EO771 tumours. Tumour growth was measured using a calliper every day, and the tumour volume was calculated using the following formula: volume = length×width2/2.
Immunofluorescence staining and evaluation
The tumour tissues were harvested on the 7th and 14th days after inoculation, fixed in 4% PFA at 4 °C for 12 hours, maintained overnight in 30% sucrose at 4 °C, and embedded in optimal cutting temperature compound (OCT) (4583, Sakura) at −80 °C for 2 hours. Then, 8-µm sections were cut from each specimen for immunofluorescence staining. Prior to immunofluorescence staining, frozen tumours tissues sections were removed from the freezer, and defrosted to room temperature, rinsed with PBS and blocked with 10% bovine serum albumin (A1933, Sigma) for 1 hour at room temperature. The sections were then incubated with primary antibodies against CD31 (550274, BD Pharmingen,1:50), CD105 (550546, BD Pharmingen, 1:100), F4/80 (123140, Biolegend, 1:200), CD206 (Sc-48758, Santa Cruz Biotechnology, 1:200) overnight at 4 °C, and then with Alexa Fluor 488-conjugated secondary antibodies (R37116, Alexa Fluor, 1:200) at room temperature for 1 hour. The sections were subsequently stained with DAPI (P-36931, Invitrogen) under dark condition, and visualized under a fluorescence microscope (Axio Imager M2, Carl Zeiss, Jena, Germany).
To identify “hot spots” of CD31-positive microvessels or F4/80-positive macrophages, the entire stained sections were observed under a fluorescence microscope. The expression of CD31 and CD105 at the same location in two serial sections were evaluated at high magnification (×200), and the numbers of F4/80- and CD206-positive macrophages were then counted, respectively (23,24).
Immunohistochemistry staining and quantitation
Tumour tissues, which were collected using a protocol as described in immunofluorescence staining, were fixed in 4%paraformaldehyde for 12 hours, fixed in 40%, 70%, 90%, and 100% ethanol for dehydration and embedded in paraffin wax at 65 °C. For immunohistochemistry staining, 3-µm sections were cut from each specimen. The specimens were blocked with 1% bovine serum albumin (A1933, Sigma) and 5% normal goat serum (S-1000; Vector Laboratories, Inc.) in PBS supplemented with 0.04% Tween 20 at room temperature for 1 hour. The tumour sections were then incubated overnight at 4 °C with primary antibodies specific for interleukin-6 (IL-6) (WL02138, Wanlei Bio, 1:100) and IL-10 (WL01124, Wanlei Bio, 1:200), and then treated with a 2-step plus Poly-HRP anti-mouse/rabbit IgG detection system (PV-9000, ZSGB-BIO). Antibody binding was detected with 3,3'-diaminobenzidine tetrahydrochloride (DAB kit) (ZLI-9019, ZSGB-BIO), according to the manufacturer’s recommended protocols. After a final wash with distilled water, the sections were counterstained with Mayer’s haematoxylin (MHS16, Sigma-Aldrich), dehydrated, cleared and mounted with neutral balsam (G8590, Solarbio).After staining, the cells were observed under a light microscope (qualitative evaluation), and the IL-6 and IL-10 expression levels were evaluated under high magnification (×200). ImageJ Pro software (version ImageJ 1.51k, National Institutes of Health, Bethesda, MD, USA) was used to determine the cumulative absorbance of positive staining in each of five, non-overlapping field, and the mean value was then calculated.
Western blotting
Tumour tissues were harvested on the 7th and 14th days after inoculation, lysed with ice-cold RIPA Lysis Buffer (P0013B, Beyotime) containing 1% PMSF for 30 minutes on ice and homogenized with a tissue homogenizer. The lysates were centrifuged at 12,000 rpm and at 4 °C for 20 minutes. The protein concentration of the supernatant was determined using the BCA Protein Assay (P0009, Beyotime). Equivalent samples were subjected to SDS-PAGE on a 12% gel, and the proteins were then transferred to polyvinylidene difluoride (PVDF) membranes (IPVH000 10, Merck Millipore). The membranes were incubated overnight at 4 °C with primary antibodies against IL-6 (WL02138, Wanlei Bio, 1:1,000), JAK2 (WL02188,Wanlei Bio, 1:500), p-JAK2 (WL02997, Wanlei Bio, 1:500), STAT3 (WL01836,Wanlei Bio, 1:300), p-STAT3 (WLP2412, Wanlei Bio, 1:1,000) and β-actin (WL0962a,Wanlei Bio, 1:1,500) and then with horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody (31431, Invitrogen, 1:3,000) for 1 hour at room temperature. The proteins that reacted with the primary antibodies were detected using an Electrochemi-Luminescence (ECL) Plus kit (32132, Thermo).
Statistical analysis
The analyses are performed using SPSS version 16.0 (IBM SPSS, Inc.). Normally distributed quantitative data was presented as the mean ± standard deviation (SD). The statistical significance of the differences between the Eng-IKOe group and the control group in tumour size, macrophage numbers, IL-6 expression, and IL-10 expression were determined using Student’s t-test on days 7 and 14. Pearson’s correlation test was used to evaluate the correlations among IL-6, IL-10 and M2 macrophages on days 7 and 14, respectively. Statistical significance was set at P<0.05.
Results
Endoglin knockout inhibits tumour angiogenesis and tumour growth in mouse breast cancer animal models (Figure 1)
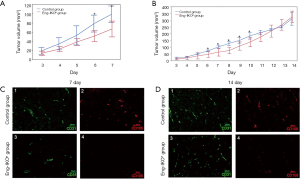
In order to evaluate the effect of endoglin on angiogenesis and tumour growth in breast cancer, EO771 breast cancer cells were injected into mouse mammary glands of Eng-iKOe and control mice. After 2 days, tumour volume was measured daily. On day 7 after engraftment, the tumour volume was significantly smaller in the Eng-iKOe group than in the control group (P<0.05; Figure 1A,B).
To confirm anti-angiogenic effects of endoglin knockout on breast cancer, 5 mice were sacrificed in each group. Breast tumours were obtained. CD31 and CD105 immunofluorescence staining was performed to confirm the knockout of endoglin. The results showed that CD31 and CD105 were expressed in vascular endothelial cells in breast cancer tissue from the control group, whereas CD31, but not CD105, was expressed in the Eng-IKOe group, confirming that endoglin was knocked out and that the mouse model was successfully established (Figure 1C,D). Moreover, CD31 expression was lower in the Eng-iKOe group compared with the control group on day 7 (Figure 1C), which showed that tumour angiogenesis was inhibited by targeting endoglin.
Further observation was conducted on the left 10 mice (n=5 in each group) on tumour growth, however, the initial difference in tumour vascularization and volume between the two group gradually disappeared. On day 14 after engraftment, no significant between-group difference was observed (P>0.05; Figure 1B). Subsequently, CD31 and CD105 expression were also examined, positive CD31, but not CD105 was observed in the Eng-iKOe group (Figure 1D). Taken together, the results showed that targeted endoglin therapy inhibited breast tumour growth by reducing angiogenesis, however, the short-term effect was neutralized, and more pronounced tumour angiogenesis was detected.
TAM and M2 macrophages infiltration and the expression of IL-6 and IL-10 in breast cancer tissue (Figures 2,3)
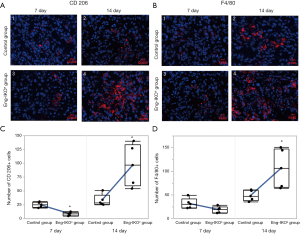
Tumour immunity is an important factor for the outcome of cancer treatment, whereas infiltrating TAMs are essential for tumour immunity. Moreover, M2 macrophages have been shown to promote tumour angiogenesis (25). Therefore, in this study, infiltration of macrophages into the tumour microenvironment was investigated after endoglin knockout. TAMs and M2 macrophages, which are characterized by positive expression of F4/80 and CD206, respectively (26-28), were examined and evaluated in tumour tissue on days 7 and 14 after engraftment. On day 7, no difference in the F4/80-positive cells was found between the Eng-IKOe group and the control group (P>0.05; Figure 2B), however, less CD206 positive cells were observed in the Eng-IKOe group than in the control group (P<0.05; Figure 2A). On day 14, more F4/80- and CD206-positive cells were observed in the Eng-IKOe group than in the control group (P<0.05; Figure 2A,B). These results indicate that CD206-positive macrophages might be the primary cause leading to failure of anti-endoglin therapy.
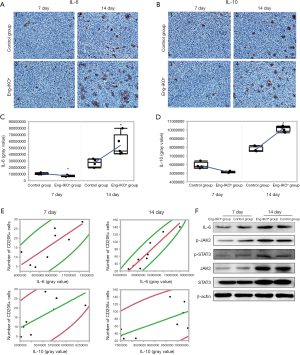
IL-6 and IL-10 are closely associated with the polarization of macrophages in the tumour microenvironment during tumour development (29-31). The analysis of IL-6 and IL-10 expression revealed that IL-6 and IL-10 expression in tumour tissue was significantly lower on day 7 (P<0.05) and significantly higher on day 14 in the Eng-IKOe group compared with the control group (P<0.05; Figure 3A,B,C,D). On day 7, tumour growth was inhibited, at which time, the expression of IL-6 and IL-10 in the Eng-IKOe group was downregulated. On day 14, inhibited tumour growth was neutralized and the expression of IL-6 and IL-10 in the Eng-IKOe group was upregulated. The results indicate efficacy of anti-endoglin therapy might be affected by IL-6 and IL-10.
Correlation between IL-6 and IL-10 levels and number of M2 macrophages
In tumour tissues with more infiltration of M2 macrophages, increased expressions of IL-6 and IL-10 were found. Furthermore, to evaluate the role of IL-6 and IL-10 on the infiltration of M2 macrophages, the correlation between IL-6 or IL-10 expression and M2 macrophages were statistically analyzed. The results showed significant positive correlations between the IL-6 level and the number of M2 macrophages in tumour tissue on days 7 (P=0.017, r=0.80) and 14 (P<0.01, r=0.94) (Figure 3E). Positive correlation between the IL-10 level and the number of M2 macrophages wasn’t observed on day 7 (P=0.055), but found on day 14 (P=0.043, r=0.682) (Figure 3E). These results suggest that IL-6 might be the main factor that affects the immune status related to the polarization of tumour-associated macrophages, which is associated with increased number of M2 macrophages after endoglin-targeted therapies.
Level of IL-6/JAK2/STAT3 proteins in tumour tissue
Previous clinical studies revealed that IL-6 was upregulated after endoglin-targeted therapy. The activation of JAK2/STAT3 signalling pathway was reported to promote the polarization of macrophages into M2 macrophages, which could be induced by IL-6 (30). To investigate the status of IL-6/JAK2/STAT3 signaling pathway in the tumour tissue after endoglin-targeted therapy, the levels of IL-6, JAK2, STAT3, and phosphorylation of JAK2 (p-JAK2) and STAT3 (p-STAT3) proteins were evaluated. No difference was observed in the level of JAK2 or STAT3 between-group on day 7 and day 14 (Figure 3F). But, the levels of IL-6, p-JAK2, and p-STAT3 were lower on day 7 and higher on day 14 in the Eng-IKOe group compared with the control group. The results indicate that the activation of JAK2/STAT3 pathway, which is characterized by increased levels of p-JAK2 and p-STAT3, might be directly associated with the expression of IL-6 after endoglin-targeted therapy.
Discussion
Recent studies have shown that the effects of anti-angiogenic agents in patients with advanced or progressive tumours show initial good responses followed by tumour relapses (4,32). In Eng-IKOe mice, the phenomenon of initial inhibition followed by rebound of tumour growth was observed. The present study indicated that increased infiltration of CD206-positive M2 macrophages and that this increased infiltration might lead to failure of anti-angiogenic therapy (33). Moreover, we found that the increased number of infiltrated M2 macrophages might be due to activation of the IL-6/JAK2/STAT3 signalling pathway in the tumour microenvironment.
It was widely discussed that, after a short-time efficacy of anti-angiogenic therapies, the rebound of tumour growth was observed. In the context of VEGF inhibitions, this accelerated growth may result from an increase in pro-angiogenic factors, which can subsequently lead to rapidly out-of-control neovascularization (34). In tumour microenvironment, immune cells were an important population that closely associated with treatment effects and angiogenesis. Moreover, anti-angiogenic therapies reportedly affect tumour immunity, which might in turn have an influence on the treatment effects. Previous studies have shown that sunitinib and bevacizumab appear to improve the function of antigen presenting cells (APCs) and T cells by reducing the number of immunosuppressive cells, such as Tregs and MDSCs (2,11). However, cessation of sunitinib treatment resulted in an immunosuppressive rebound effect (34). Endoglin, an important regulator of sprouting in angiogenesis, was also found to interact with tumour immunity in our study. In the tumour-engraftment mice model, we observed a short-term effect of endoglin-targeted therapy on tumor angiogenesis and growth, and in the treated tumours, exhibited fewer number of infiltrated M2 macrophages. However, the efficacy of the therapy in the endoglin knockout mice gradually disappeared, and unfettered tumour growth with increased M2 macrophage infiltration was observed. Previous studies have shown that the number of M2 macrophages is positively correlated with the density of tumour vessels in breast, lung, and endometrial cancer (25,26). Moreover, macrophages are demonstrated to promote tumour angiogenesis by releasing a variety of cytokines, such as VEGF, nitric oxide (NO) and IL-10 (27). In addition, increased number of M2 macrophages is reportedly the cause of a weakened anti-angiogenic effect. Taken together, these findings indicate that increased infiltration of M2 macrophages might partially explain the inefficiency of endoglin-targeted therapy.
Furthermore, when the tumour growth is uncontrolled, infiltration of TAMs in the Eng-iKOe group was significantly increased compared with the control group. Previous studies reported that monocyte-derived tissue-resident macrophages were recruited to tumour microenvironment as a pro-inflammatory factor (M1 macrophages) or tumour promoter (M2 macrophages). Thus, the increased number of M2 macrophages observed in our study likely resulted from the polarization of an increased number of macrophages. A clinical study revealed that circulating biomarkers, such as Ang-2, C-reactive protein, intercellular adhesion molecule-1 (ICAM-1), IGFBF-1, IL-6, TSP-2, and vascular cell adhesion molecule-1 (VCAM-1), were upregulated following TRC105 treatment, and these effects might contribute to the polarization of macrophages towards M2 (35). Apart from IL-4, IL-13 and IL-10, IL-6 also plays a role in promoting the polarization of M2 macrophages via the JAK2/STAT3 pathway (28). Additionally, in the tumour microenvironment, IL-6 can sustain a pro-tumour milieu by supporting angiogenesis and tumour evasion of immune surveillance to promote tumour progression (36). Consistently, in the present study, efficient or inefficient treatment was accordingly accompanied by decreased or increased IL-6 expression during endoglin-targeted therapy. Our findings also show a strong positive correlation between the expression of IL-6 and the number of M2 macrophages on both day 7 and day 14. In addition, M2 macrophages can release IL-10, which activates M2 macrophages via positive feedback (37). However, even though IL-10 expression was positively associated with increased M2 macrophages on day 14, no correlation was observed on day 7. Altogether, on day 7, at which time the tumour growth was inhibited by endoglin-targeted therapy, lower expression of IL-6 and IL-10 accompanied by less infiltrated M2 macrophages was detected. However, on day 14, at which time the inhibited effect on tumour growth was neutralized, both higher expression of IL-6 and IL-10, as well as more infiltration of M2 macrophages were found. As IL-10 could be released into the tumour microenvironment by M2 macrophages and in turn promoted M2 macrophages polarization, the results indicate that the expression of IL-6 might the direct cause for changes of the number of infiltrated M2 macrophages and thereby an inefficient effect of endoglin-targeted therapy on anti-angiogenesis.
STAT3 is considered a primary signaling molecule in the process of macrophage polarization towards the M2 phenotype (29). IL-6 is a common activator of the JAK2/STAT3 signaling pathway (31), which is an important pathway in tumour progression. An inhibitor of the JAK2/STAT3 pathway could block M2 polarization. Consistent with the positive correlations between the expression of IL-6 and the number of M2 macrophages, the expression of proteins involved in the activated JAK2/STAT3 signalling pathway was changed by changes in the number of M2 macrophages and the expression of IL-6 (31). However, in our study, we didn’t evaluate changes of anti-angiogenic efficacies, M2 macrophages by blocking IL-6, and definitely, our further studies will focus on it. Taken together, the result suggest that anti-angiogenic therapies targeting endoglin on vascular endothelial cells affects the infiltration of M2 macrophages in breast cancer tissue by modulating the presentation of IL-6 and activating the JAK2/STAT3 signalling pathway.
In summary, this finding provides non-mature conclusive but accumulating evidence that suggests that the efficacy of anti-angiogenic therapies targeting endoglin on vascular endothelial cells might be blunted by increased infiltration of M2 macrophages associated with higher IL-6 expression and activation of the IL-6/JAK2/STAT3 signalling pathway. The results of the present study provide evidence indicating that IL-6 could serve as an indicator for monitoring therapeutic efficacy and a potential target for improving the efficacy of antiangiogenic therapy. Nevertheless, additional studies are required to further investigate the interactions between anti-angiogenic therapy and M2 macrophage infiltration, as well as the involvement of the IL-6/JAK2/STAT3 pathway.
Acknowledgments
The authors do appreciate Professor Helen Arthur from the Institute of Genetic Medicine, Newcastle University, UK, generously provided the cell line and genetic mouse models. It can’t be a publishable paper without her and her team’s warm-hearted attributions.
Funding: This work was supported by the First Hospital Affiliated to Jinzhou Medical University and the National Natural Science Foundation of China (grant No. 81472460).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.06.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Jinzhou Medical University Laboratory Animal Welfare and Animal Experimental Ethical Committee, Jinzhou, China [No. SCXK(Liao)2014-0004].
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac J Cancer Prev 2016;17:43-6. [Crossref] [PubMed]
- Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357:2666-76. [Crossref] [PubMed]
- Rosen LS, Hurwitz HI, Wong MK, et al. A phase I first-in-human study of TRC105 (Anti-Endoglin Antibody) in patients with advanced cancer. Clin Cancer Res 2012;18:4820-9. [Crossref] [PubMed]
- Apolo AB, Karzai FH, Trepel JB, et al. A Phase II Clinical Trial of TRC105 (Anti-Endoglin Antibody) in Adults With Advanced/Metastatic Urothelial Carcinoma. Clin Genitourin Cancer 2017;15:77-85. [Crossref] [PubMed]
- Crawford Y, Kasman I, Yu L, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 2009;15:21-34. [Crossref] [PubMed]
- Patel PH, Chaganti RS, Motzer RJ. Targeted therapy for metastatic renal cell carcinoma. Br J Cancer 2006;94:614-9. [Crossref] [PubMed]
- Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet 2009;25:30-8. [Crossref] [PubMed]
- Solinas G, Germano G, Mantovani A, et al. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 2009;86:1065-73. [Crossref] [PubMed]
- Komohara Y, Jinushi M, Takeya M. Clinical significance of macrophage heterogeneity in human malignant tumors. Cancer Sci 2014;105:1-8. [Crossref] [PubMed]
- Costa NL, Valadares MC, Souza PP, et al. Tumor-associated macrophages and the profile of inflammatory cytokines in oral squamous cell carcinoma. Oral Oncol 2013;49:216-23. [Crossref] [PubMed]
- Abe F, Younos I, Westphal S, et al. Therapeutic activity of sunitinib for Her2/neu induced mammary cancer in FVB mice. Int Immunopharmacol 2010;10:140-5. [Crossref] [PubMed]
- Ozao-Choy J, Ma G, Kao J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res 2009;69:2514-22. [Crossref] [PubMed]
- Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A 2012;109:17561-6. [Crossref] [PubMed]
- Chen Y, Ramjiawan RR, Reiberger T, et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 2015;61:1591-602. [Crossref] [PubMed]
- Tamaskar I, Dhillon J, Pili R. Resistance to angiogenesis inhibitors in renal cell carcinoma. Clin Adv Hematol Oncol 2011;9:101-10. [PubMed]
- Martinez LM, Labovsky V, Calcagno ML, et al. CD105 expression on CD34-negative spindle-shaped stromal cells of primary tumor is an unfavorable prognostic marker in early breast cancer patients. PLoS One 2015;10:e0121421 [Crossref] [PubMed]
- Duff SE, Li C, Garland JM, et al. CD105 is important for angiogenesis: evidence and potential applications. FASEB J 2003;17:984-92. [Crossref] [PubMed]
- Duwel A, Eleno N, Jerkic M, et al. Reduced tumor growth and angiogenesis in endoglin-haploinsufficient mice. Tumour Biol 2007;28:1-8. [Crossref] [PubMed]
- Matsuno F, Haruta Y, Kondo M, et al. Induction of lasting complete regression of preformed distinct solid tumors by targeting the tumor vasculature using two new anti-endoglin monoclonal antibodies. Clin Cancer Res 1999;5:371-82. [PubMed]
- Mizutani N, Luo Y, Mizutani M, et al. DNA vaccines suppress angiogenesis and protect against growth of breast cancer metastases. Breast Dis 2004;20:81-91. [Crossref] [PubMed]
- Johnstone CN, Smith YE, Cao Y, et al. Functional and molecular characterisation of EO771.LMB tumours, a new C57BL/6-mouse-derived model of spontaneously metastatic mammary cancer. Dis Model Mech 2015;8:237-51. [Crossref] [PubMed]
- Allinson KR, Carvalho RL, van den Brink S, et al. Generation of a floxed allele of the mouse Endoglin gene. Genesis 2007;45:391-5. [Crossref] [PubMed]
- Gwak JM, Jang MH, Kim DI, et al. Prognostic value of tumor-associated macrophages according to histologic locations and hormone receptor status in breast cancer. PLoS One 2015;10:e0125728 [Crossref] [PubMed]
- Di Paolo V, Russo I, Boldrini R, et al. Evaluation of Endoglin (CD105) expression in pediatric rhabdomyosarcoma. BMC Cancer 2018;18:31. [Crossref] [PubMed]
- Wu H, Xu JB, He YL, et al. Tumor-associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol 2012;106:462-8. [Crossref] [PubMed]
- Du Q, Tsuboi N, Shi Y, et al. Transfusion of CD206+ M2 Macrophages Ameliorates Antibody-Mediated Glomerulonephritis in Mice. Am J Pathol 2016;186:3176-88. [Crossref] [PubMed]
- Almatroodi SA, McDonald CF, Darby IA, et al. Characterization of M1/M2 Tumour-Associated Macrophages (TAMs) and Th1/Th2 Cytokine Profiles in Patients with NSCLC. Cancer Microenviron 2016;9:1-11. [Crossref] [PubMed]
- Draijer C, Boorsma CE, Robbe P, et al. Human asthma is characterized by more IRF5+ M1 and CD206+ M2 macrophages and less IL-10+ M2-like macrophages around airways compared with healthy airways. J Allergy Clin Immunol 2017;140:280-3.e3. [Crossref] [PubMed]
- Fu XL, Duan W, Su CY, et al. Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol Immunother 2017;66:1597-608. [Crossref] [PubMed]
- Lopes RL, Borges TJ, Zanin RF, et al. IL-10 is required for polarization of macrophages to M2-like phenotype by mycobacterial DnaK (heat shock protein 70). Cytokine 2016;85:123-9. [Crossref] [PubMed]
- Yuan F, Fu X, Shi H, et al. Induction of murine macrophage M2 polarization by cigarette smoke extract via the JAK2/STAT3 pathway. PLoS One 2014;9:e107063 [Crossref] [PubMed]
- de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225-33. [Crossref] [PubMed]
- Liu Y, Tu L, Wang L, et al. The accumulation of macrophages attenuates the effect of recombinant human endostatin on lung cancer. Onco Targets Ther 2016;9:6581-95. [Crossref] [PubMed]
- Griffioen AW, Mans LA, de Graaf AMA, et al. Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin Cancer Res 2012;18:3961-71. [Crossref] [PubMed]
- Liu Y, Starr MD, Brady JC, et al. Modulation of circulating protein biomarkers following TRC105 (anti-endoglin antibody) treatment in patients with advanced cancer. Cancer Med 2014;3:580-91. [Crossref] [PubMed]
- Fisher DT, Appenheimer MM, Evans SS. The two faces of IL-6 in the tumor microenvironment. Semin Immunol 2014;26:38-47. [Crossref] [PubMed]
- Qi L, Yu H, Zhang Y, et al. IL-10 secreted by M2 macrophage promoted tumorigenesis through interaction with JAK2 in glioma. Oncotarget 2016;7:71673-85. [Crossref] [PubMed]


