
Metformin prolonged the survival of diffuse large B-cell lymphoma and grade 3b follicular lymphoma patients responding to first-line treatment with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone: a prospective phase II clinical trial
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin’s lymphoma, accounting for 35–40% of the patients. Follicular lymphoma grade 3b (FL3b) shares similar biology characteristics of DLBCL and is recommended to be treated as DLBCL (1). Rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone, R-CHOP) has become the standard first-line treatment for DLBCL, with 75–80% of the response rate. However, 30–40% of the responded patients inevitably relapse (2,3). Although autologous stem-cell transplantation (ASCT) decreased the relapse rate in high-risk DLBCL patients (4), the toxicity of ASCT limits its application in elder and fragile patients. New therapeutic strategies need to be explored in terms of maintenance therapy in DLBCL.
Metformin belongs to the biguanide class of oral hypoglycemic agents. Experimental data support that metformin can inhibit tumor cell growth in breast, prostate, colon and pancreatic cancer (5-7). Clinical studies also confirm the effect of metformin to improve the prognosis of breast, lung, and bladder cancer patients (8-10). As for hematological malignancies, metformin improves clinical outcomes and reduces mortality in patients with multiple myeloma and acute lymphoblastic leukemia (11,12). Recent mechanism study revealed the activation of adenosine monophosphate (AMP)-activated protein kinase (AMPK) in metformin-treated cells and xenograft models (13). Moreover, combination of oral metformin with doxorubicin may induce cell autophagy and increase chemosensitivity (14).
In the present study, we designed a single-arm prospective study to assess clinical efficacy and safety of metformin in patients with DLBCL and FL3b.
Methods
Patients
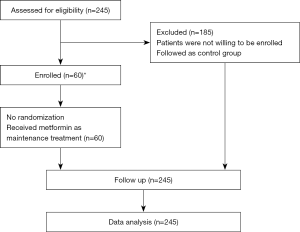
We conducted a prospective, single-arm phase II clinical trial, evaluating the therapeutic effect of metformin as maintenance therapy in patients with DLBCL or FL3b achieved complete remission. From 2013 January to 2017 July, 245 DLBCL or FL3b patients achieved complete response (CR) after 6 cycles of R-CHOP-21 were enrolled. Among them, 60 patients were willing to take part in this prospective clinical trial with metformin maintenance, while the rest 185 patients were referred as the control group (Figure 1). All the patients signed their informed consent. The study was performed in accordance with the Declaration of Helsinki and the protocol was approved by the Ethics Committee of Shanghai Rui Jin Hospital (2012-26). The trial was registered on http://www.chictr.org.cn (ChiCTR-OIN-17012130).
Diagnosis and staging systems
Pathological diagnosis was established according to World Health Organization (WHO) classification (15). The staging work-up included history and physical examination, blood cell counts and serum chemistry, bone marrow aspiration or biopsy, endoscopy of gastrointestinal tracts, chest and abdominal tomography scan or positron emission tomography and computed tomography (PET-CT).
Diabetes diagnosis was based on the 1999 WHO diagnostic criteria (16) for diabetes: (I) typical symptoms of diabetes (including polydipsia, polyuria and unexplained weight loss), random blood glucose ≥11.1 mmol/L (random blood glucose refers to any time of blood glucose); (II) or fasting blood glucose ≥7.0 mmol/L; (III) or 2 hours blood glucose ≥11.1 mmol/L in 75 g glucose load test. Peoples with no symptoms of diabetes need to repeat the blood glucose another day to determine the diagnosis. Impaired glucose tolerance (IGT) definition: fasting blood glucose <7.0 mmol/L, postprandial 2 h blood glucose 7.8–11.1 mmol/L. Fasting blood glucose damage (IFG) definition: fasting blood glucose 6.1–7.0 mmol/L, 2 h postprandial blood glucose <7.8 mmol/L.
Treatment and response
All of the 245 patients received chemotherapy (6 standard dose of CHOP regimens) combined with Rituximab (375 mg/m2). All of the 245 patients achieved CR, according to the WHO response criteria (17). Briefly, CR was defined as no evidence of residual disease, partial response (PR) with at least a 50% reduction in tumor burden from the onset of treatment, progressive disease (PD) or relapse was defined as the presence of a newly developed lesion or more than 25% increase in the product of 2 diameters of at least one tumor. Stable disease (SD) was defined as the state of neither PR nor PD. Assessment of treatment response was evaluated by follow-up clinical, radiological, or laboratory studies, as determined by the clinician. The patients had metformin orally at a dose of 1.0 g, twice a day for 2-year maintenance. If adverse events, such as diarrhea, occurred, metformin was reduced to 1.0 g qd. If the events resolved, the dose of metformin was restored to 1.0 g bid.
Immunohistochemistry assay
Immunohistochemistry was performed on 5 µm-paraffin sections with an indirect method (EnVison) using the primary antibody against MYC (1:100), CD10 (1:100), Bcl-6 (1:100), MUM-1 (1:100), Ki67 (1:100), (Abcam, Cambridge, USA), and anti-rabbit/rat-IgG antibody from Dako (Carpinteria, CA, USA) as the second antibody. Bcl-2, Bcl-6 and MYC positive were determined as previously reported (18-20).
Statistical analysis
The progression-free survival (PFS) time is defined as the time of diagnosis to the progression of disease/relapse or death. The overall survival (OS) time is defined as the time of diagnosis to death or the last follow-up time. Survival functions were estimated using the Kaplan-Meier method and compared by the log-rank test. Chi-square was used in comparison the clinical data of patients with different treatment. Multivariate survival analysis was performed using a Cox regression model. Significant variables in the univariate analysis were selected as variables in the multivariate analysis for survival. P<0.05 was considered statistically significant. All statistical analyses were evaluated using Statistical Package for the Social Sciences (SPSS) 23.0 software (SPSS Inc., Chicago, Illinois, USA).
Results
Patient characteristics
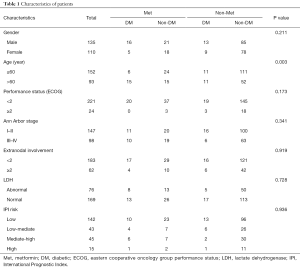
The characteristics of the 245 patients at diagnosis were summarized in Table 1. The median follow-up was 24.3 months (range, 5.8–46.5 months), the median age was 57 years (range, 19–79 years), and the male-to-female ratio was 1.23:1. Most patients had good performance status [Eastern cooperative oncology group (ECOG) performance status 0–1, 90.2%] and the International Prognostic Index (IPI) indicating low-risk was 75.9%.
Full table
Taking into account that some of the patients in the group had diabetes, we divided the patients into four groups: diabetic patients with metformin maintenance (DM + Met, n=21), diabetic patients without metformin maintenance (DM + non-Met, n=22), non-diabetic patients with metformin maintenance (non-DM + Met, n=39), and non-diabetic patients without metformin maintenance (non-DM + non-Met, n=163), there were no statistical difference of gender, Ann Arbor stage, ECOG score, IPI and serum lactate dehydrogenase (LDH) among the 4 groups (Table 1). Higher percentage of elderly patients was present in the group of diabetic patients with metformin maintenance (Table 1).
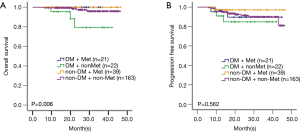
Patients with metformin maintenance had longer survival time when compared with those without metformin maintenance
The 3-year OS and PFS of 245 patients was 95.2% and 90.2%, respectively. The OS of the four groups had significant difference (P=0.006): the DM + Met group and non-DM + Met had the best prognosis with 3-year OS both as 100%, followed by non-DM + non-Met and DM + non-Met group. The PFS of these four groups remained similar (P=0.562, Figure 2).
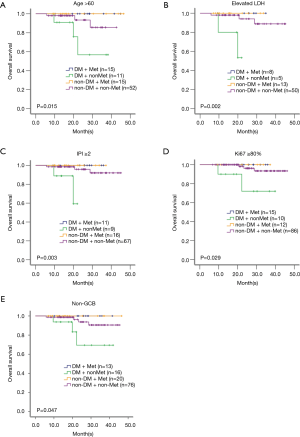
Metformin improved the survival time of patients with age >60, elevated LDH, and IPI ≥2
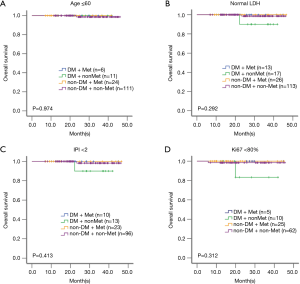
To further define the benefit of the patients from metformin maintenance, different unfavorable factors were analyzed. The survival data showed that among the DLBCL group with age >60 (P=0.015), elevated serum LDH (P=0.002), or IPI ≥2 (P=0.003), the DM patients without metformin maintenance had worse survival than those of non-DM patients (Figure 3A,B,C). However, among the patients with age ≤60, or normal LDH, or IPI <2, there was no statistical difference among the four groups (Figures S1A,B,C).
Metformin improved the survival time of patients with tumor cells Ki67 (≥80%) and non-germinal center B-cell-like (non-GCB) phenotype
Among all, 123 patients presented Ki67 ≥80%, and 125 were non-GCB subtype according to the Hans algorithm. In the group with tumor cells Ki67 ≥80% or with non-GCB subtype, distinct differences were observed among the four groups (P=0.029 and P=0.047, Figure 3D,E). However, among the tumor samples with Ki67 <80%, there was no statistical difference (Figure S1D). Since all the patients with GCB subtype, no matter in Met group or in non-Met group, are still alive, no difference was found among the four groups (data not shown).
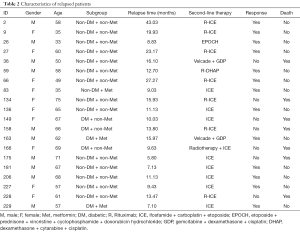
Metformin reduced the relapse rate and mortality
During metformin treatment as a maintenance agent, the relapse rate was much lower than the patients without metformin (5.0% vs. 9.2%). The median relapse time of the patients with or without metformin was 9.0 and 12.7 months, respectively, without significant difference between the two groups (P=0.422). There were only three patients relapsed with metformin maintenance, all of which presented with IPI score ≥2. During metformin maintenance, the mortality of the patients was 0, while that without metformin was 3.8%. There were 7 patients passed away during the follow-up, all of them were related with lymphoma relapse and progression (Table 2).
Full table
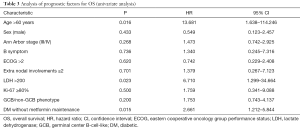
Age >60, elevated serum LDH and diabetics patients without metformin maintenance were the three independent unfavorable factors in DLBCL
In univariate analysis, age >60, elevated serum LDH and diabetics patients without metformin maintenance were unfavorable factors for OS (Table 3). These three parameters were further included in the multivariate analysis, age >60 (P=0.045, HR 9.368, 95% CI: 1.051–83.546), elevated serum LDH (P=0.024, HR 7.293, 95% CI: 1.302–40.864) and diabetics patients without metformin maintenance (P=0.044, HR 2.625, 95% CI: 1.027–6.713) were independent prognostic factors for the inferior OS.
Full table
Metformin was tolerated well in the patients
There were about 10% of patients had diarrhea within the first month of metformin administration. Metformin was thus reduced into 1.0 g qd, until the diarrhea resolved, the dose of metformin was restored to 1.0 g bid. More than 50% of these diarrhea patients could tolerate 1.0 g bid of metformin afterwards. In addition, almost all the patients experienced a slight weight loss after metformin administration, which could be tolerated. The fasting glucose was regularly monitored in the patients in this trial every 3 months. The fasting glucose was normal during the follow-up in the non-diabetic patients. However, the median fasting glucoses was 5.96 mmol/L (range, 4.44–9.92) and 7.05 mmol/L (range, 5.19–13.37), in the DM patients with and without metformin maintenance, respectively.
Discussion
To our knowledge, this is the first prospective study of metformin as maintenance therapy in patients with DLBCL or FL3b achieved complete remission. Metformin is a widely used and economic oral agent for type 2 diabetes with mild adverse effects. The anti-tumor mechanism of metformin is multiple (21). As previously reported by our group, metformin activates AMPK through LKB1-dependent mechanism, resulting in inhibition of mTOR, induction of autophagic death, and increased sensitivity of chemotherapy (14). Anti-cancer activity of metformin was also due to inhibition of PP2A-dependent phosphatase activity through disruption of PP2A complex (22). Iglesias et al. (23) studied xenograft mouse models of endometrial cancer and found that metformin displaces active K-Ras from cell membrane via a PKC-dependent pathway and inhibits Ras signaling.
Previous retrospective studies showed that metformin can increase the remission rate and survival time of the patients with lung, breast, bladder and multiple myeloma (8-12), but rarely reported in non-Hodgkin’s lymphoma. Diabetic patients have a higher prevalence and mortality of tumors than non-diabetic patients. An analysis of 196 centers, including 178,547 patients in Asian showed diabetes was closely associated with a high risk of tumor mortality (HR 1.26, 95% CI: 1.21–1.31), including lymphoma (HR 1.39, 95% CI: 1.04–1.86) (24). Consistent with these results, our prospective study showed that metformin maintenance can significantly prolong the survival time of the DLBCL patients, especially in diabetic subgroup. Koo et al. (25) retrospectively analyzed the influence of concomitant drugs on the prognosis of DLBCL patients receiving rituximab-based regiments. Among the 213 DLBCL patients, 47 had statins, 182 had metformin and 186 had aspirin. Survival analysis was made according to these concomitant medicines, and results showed that patients receiving metformin during the chemotherapy had no significant advantage in survival when compared with those without metformin. Another retrospective study was conducted to compare the efficacy of metformin usage on prognosis in 24 DLBCL diabetics patients, and showed that metformin usage could improve objective response rate, CR rate and PFS, as compared with other diabetic agents (26). The opposite results of the two studies, probably, were due to the different population of patients; the former was conducted in the whole DLBCL cohort and the latter in the diabetic subgroup.
The survival time of the patients with DLBCL and FL3b had been greatly improved by the R-CHOP regimen, but the relapse remains the obstacle for CR patients to obtain long-term survival. Accumulating data revealed that elder, elevated serum LDH, high Ki67, non-GCB subtype are the risk factors correlated with lymphoma relapse (24,27-29). Our study showed that metformin maintenance can effectively extend the survival time in these high-risk patients.
There was a significant improvement on OS rather than PFS in the patients with metformin maintenance, perhaps due to the relapsed patients in the metformin maintenance group, are still alive after second-line chemotherapy. The relapsed patients, on the contrary, in non-metformin maintenance group died after second-line treatment. The difference might be related to the growth-inhibitory and drug-sensitizing effect of metformin on lymphoma cells, targeting mTOR pathway through AMPK activation (14).
Finally, both univariate and multivariate analysis showed that age >60, elevated serum LDH and diabetic patients without metformin maintenance were the three independent unfavorable factors for patients with DLBCL and FL3b.
In conclusion, this study provides novel rationale of a new strategy for elderly and high-risk patients unsuitable for ASCT.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.07.20). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was performed in accordance with the Declaration of Helsinki and the protocol was approved by the Ethics Committee of Shanghai Ruijin Hospital (2012-26). The trial was registered on http://www.chictr.org.cn (ChiCTR-OIN-17012130). All the patients signed their informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
Guidelines NCCN - Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood 2010;116:2040-5. [Crossref] [PubMed]
- Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol 2013;14:525-33. [Crossref] [PubMed]
- Stiff PJ, Dahlberg S, Forman SJ, et al. Autologous bone marrow transplantation for patients with relapsed or refractory diffuse aggressive non-Hodgkin’s lymphoma: value of augmented preparative regimens--a Southwest Oncology Group trial. J Clin Oncol 1998;16:48-55. [Crossref] [PubMed]
- Dowling RJ, Zakikhani M, Fantus IG, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res 2007;67:10804-12. [Crossref] [PubMed]
- Zakikhani M, Dowling RJ, Sonenberg N, et al. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila) 2008;1:369-75. [Crossref] [PubMed]
- Wang LW, Li ZS, Zou DW, et al. Metformin induces apoptosis of pancreatic cancer cells. World J Gastroenterol 2008;14:7192-8. [Crossref] [PubMed]
- Mazzone PJ, Rai H, Beukemann M, et al. The effect of metformin and thiazolidinedione use on lung cancer in diabetics. BMC Cancer 2012;12:410. [Crossref] [PubMed]
- Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 2009;27:3297-302. [Crossref] [PubMed]
- Peng M, Su Q, Zeng Q, et al. High efficacy of intravesical treatment of metformin on bladder cancer in preclinical model. Oncotarget 2016;7:9102-17. [PubMed]
- Vu K, Busaidy N, Cabanillas ME, et al. A randomized controlled trial of an intensive insulin regimen in patients with hyperglycemic acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk 2012;12:355-62. [Crossref] [PubMed]
- Wu W, Merriman K, Nabaah A, et al. The association of diabetes and anti-diabetic medications with clinical outcomes in multiple myeloma. Br J Cancer 2014;111:628-36. [Crossref] [PubMed]
- Green AS, Chapuis N, Maciel TT, et al. The LKB1/AMPK signaling pathway has tumor suppressor activity in acute myeloid leukemia through the repression of mTOR-dependent oncogenic mRNA translation. Blood 2010;116:4262-73. [Crossref] [PubMed]
- Shi WY, Xiao D, Wang L, et al. Therapeutic metformin/AMPK activation blocked lymphoma cell growth via inhibition of mTOR pathway and induction of autophagy. Cell Death Dis 2012;3:e275 [Crossref] [PubMed]
- Swerdlow SH, Campo E, Harris NL, et al. editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th Edition. Lyon: IARC, 2008.
- World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO Consultation. Part 1. Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization, 1999.
- Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999;17:1244. [Crossref] [PubMed]
- Chen YW, Hu XT, Liang AC, et al. High BCL6 expression predicts better prognosis, independent of BCL6 translocation status, translocation partner, or BCL6-deregulating mutations, in gastric lymphoma. Blood 2006;108:2373-83. [Crossref] [PubMed]
- Barrans S, Crouch S, Smith A, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 2010;28:3360-5. [Crossref] [PubMed]
- Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275-82. [Crossref] [PubMed]
- Safe S, Naira V, Karki K. Metformin-induced anticancer activities: recent insights. Biol Chem 2018;399:321-35. [Crossref] [PubMed]
- Kickstein E, Krauss S, Thornhill P, et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci U S A 2010;107:21830-5. [Crossref] [PubMed]
- Iglesias DA, Yates MS, van der Hoeven D, et al. Another surprise from Metformin: novel mechanism of action via K-Ras influences endometrial cancer response to therapy. Mol Cancer Ther 2013;12:2847-56. [Crossref] [PubMed]
- Chen Y, Wu F, Saito E, et al. Association between type 2 diabetes and risk of cancer mortality: a pooled analysis of over 771,000 individuals in the Asia Cohort Consortium. Diabetologia 2017;60:1022-32. [Crossref] [PubMed]
- Koo YX, Tan DS, Tan IB, et al. Effect of concomitant statin, metformin, or aspirin on rituximab treatment for diffuse large B-cell lymphoma. Leuk Lymphoma 2011;52:1509-16. [Crossref] [PubMed]
- Alkhatib Y, Abdel Rahman Z, Kuriakose P. Clinical impact of metformin in diabetic diffuse large B-cell lymphoma patients: a case-control study. Leuk Lymphoma 2017;58:1130-4. [Crossref] [PubMed]
- Park JH, Yoon DH, Kim DY, et al. The highest prognostic impact of LDH among International Prognostic Indices (IPIs): an explorative study of five IPI factors among patients with DLBCL in the era of rituximab. Ann Hematol 2014;93:1755-64. [Crossref] [PubMed]
- Song MK, Chung JS, Lee JJ, et al. High Ki-67 expression in involved bone marrow predicts worse clinical outcome in diffuse large B cell lymphoma patients treated with R-CHOP therapy. Int J Hematol 2015;101:140-7. [Crossref] [PubMed]
- Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937-47. [Crossref] [PubMed]


