
Technical note: simultaneous 90Y and 99mTc-MAA injection for two-stage selective internal radiation therapy (SIRT) of liver metastases
Introduction
Hepatocellular carcinoma (HCC) represents the sixth most common cancer worldwide (1). The liver is a common site of metastasis from malignancies such as colorectal carcinoma (CRC), neuroendocrine tumors (NETS), pancreatic carcinoma, and breast cancer (1). It creates a need for multimodal strategies in dealing with both primary liver cancer, as well as liver dominant or liver only metastatic disease. One technique is 90-Yttrium Transarterial Radioembolization (90Y TARE), commonly known as selective internal radiation therapy (SIRT) of liver malignancies. The therapy, consisting of a multistep, minimally invasive procedure with the aim to deliver a high dose of selective radiation using an intra-arterial infusion of microspheres loaded with 90Y is designed to be an outpatient based single cycle therapy. This catheter-based, tumor selective modality can be used for patients with primary or metastatic liver cancer, having a lower toxicity profile compared with traditional therapies (2,3). The appropriate selection of patients, meticulous planning and targeted delivery result in an acceptable incidence of complications (4). The most commonly reported complications arise from the excess deposition in extrahaptic sites or excess dose delivered to the liver (non-targeted embolization), which may include gastritis/duodenitis, gastrointestinal ulceration/bleeding, cholecytitis, pancreatitis, and radiation pneumonitis. Excessive exposure of radiation to non tumorous liver parenchyma may lead to radioembolization induced liver disease (REILD), histopathologically similar to veno-occlusive disease and may be associated with underlying cirrhosis (1,5-12). Furthermore, candidates with diffuse hepatic disease involvement often have concomitant impaired hepatic function. Sequential treatments are safer and therefore preferable to single-session whole-liver therapy. In practice, the therapies are administered at 30-45 days’ intervals (13,14).
Prior to radioembolization, hepatic arteriography and hepatic arterial perfusion (Tc-macroaggregated albumin, Tc-MAA) studies are performed to confirm particle localization and rule out extrahepatic shunting to the gastrointestinal tract and lungs (15). If the hepatic arterial anatomy precludes safe delivery or if 99mTc-MAA perfusion imaging demonstrates significant extrahepatic activity, selective permanent embolization of the relevant arteries can be performed. Generally, prophylactic embolizations of all extrahepatic vessels (gastroduedenal, right gastric) are performed before 99mTc-MAA scanning to avoid extrahepatic deposition (16). If extrahepatic accumulation of radiopharmaceutical is detected, repeat angiogram and coil embolization of aberrant arteries should be repeated until no extrahepatic accumulation is detected (15).
Generally, planar imaging and single photon computed tomography (SPECT) of the upper abdomen are performed to assess 99mTc-MAA distribution. Studies have shown that 99mTc-MAA SPECT/CT provides additional information when compared to planar and SPECT images alone and is therefore the modality of choice. 99mTc-MAA SPECT/CT can also be utilized to accurately evaluate the exact distribution of activity in the liver (15,17).
Recently, Ahmadzadehfar et al. have shown the feasibility of combined post-therapy Bremsstrahlung (BS) and 99mTc-MAA perfusion scan in a small case series (13). Here we present two cases of patients with diffuse NET hepatic metastases undergoing staged therapy for each lobe of the liver. We investigated the clinical role and potential applications of simultaneous TARE and injection of 99mTc-MAA.
Patient A
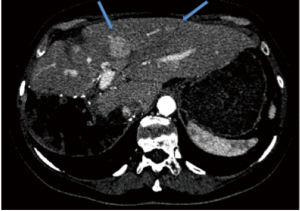
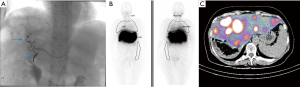
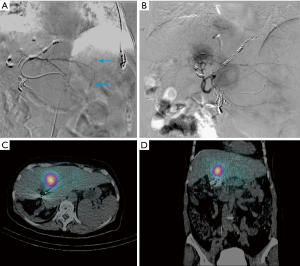
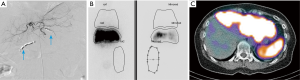
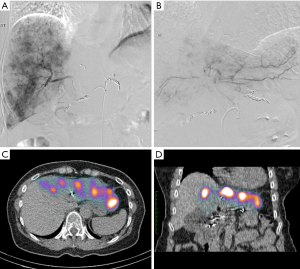
Patient A presented with multiple NET metastases to left lobe of the liver with no known primary site identified. Prior right hepatectomy had been performed (Figure 1). Interventional radiologists in the Department of Radiology performed the planning angiograms. The right gastric and gastroduodenal arteries were embolized to isolate and optimize hepatic perfusion. The microcatheter was retracted back to the proximal CHA and 185 MBq of 99mTc-MAA was administered (Figure 2A). The patient was transferred to the Department of nuclear medicine for 99mTc-MAA perfusion scan and pulmonary shunt analysis. Whole-body, planar and SPECT/CT images of the abdomen were obtained in the Nuclear Medicine Department approximately one hour after intra-arterial injection of 99mTc-MAA. A dual-detector gamma camera with a mounted single-row CT scanner (Symbia T, Siemens Healthcare) was utilized. Attenuation and scatter correction were performed on the SPECT images. The SPECT images were reconstructed into axial, sagittal and coronal planes. The co-registered CT and SPECT images were fused on OSIRIXMD software (version 1.4). Pulmonary shunt fraction was 3% and no gastrointestinal accumulation of activity was identified (Figure 2A-C). Two weeks later, the patient returned for first stage therapy. Super selective catheterization of left hepatic artery supplying Couinaud’s segments II and III was performed. Single administration of 1.4 GBq of 90Y impregnated glass microspheres utilizing an ‘EX’ protocol was applied, (TheraSphere, MDS Nordion Inc., Ottawa, Canada) for a total estimated absorbed dose of 80 Gy (Figure 3A). Selective catheterization of Couinaud’s segment IV of the liver was then performed. Tc-MAA (185 MBq) was injected intra-arterially into segment IV for subsequent therapy planning (Figure 3B) primarily to confirm shunt fraction of segment IV due to possible concerns of anatomical variation that was missed on the preprocedural CT and angiograms. Within two hours of the procedure, the patient was transferred to nuclear medicine for simultaneous 90Y BS and 99mTc-MAA SPECT/CT imaging (Figure 3C,D). The images did not show any gastrointestinal deposition of activity and the pulmonary shunt fraction was 4%.
Patient B
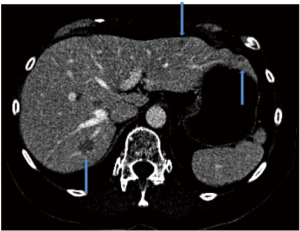
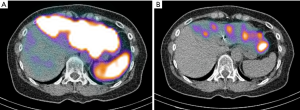
Patient B presented with metastases to both lobes of the liver and carcinoid syndrome, again no primary site was identified (Figure 4). Mesenteric angiogram with coil-embolization of the GDA was performed. We took note of prominent communicating collaterals between the right and left gastric arteries; thus we decided to embolize the branch of the left gastric artery. 99mTc-MAA was injected into the CHA and the patient underwent planar and SPECT/CT imaging. Heterogeneous activity was noted predominantly within the left lobe and spleen. The pulmonary shunt fraction was 2% (Figure 5). Two weeks later, the patient returned for first stage right lobe treatment. After selective right hepatic artery catheterization, 1.1 Gbq of 90Y glass microspheres (TheraSpheres) utilizing ‘EX’ protocol was administered for a total dose of 80 Gy (Figure 6A). The left hepatic artery was then catheterized and Tc-MAA was injected for planning of subsequent left lobe therapy (second stage) (Figure 6B). The patient underwent simultaneous 90Y BS and Tc-MAA SPECT/CT as per protocol detailed above. No significant pulmonary shunting was detected (<2%). The SPECT/CT images demonstrated accumulation of activity in multiple arterialized left lobar tumors with no gastrointestinal/splenic activity (Figure 6C,D).
Discussion
Whole-liver TARE is not desirable in candidates that present with an increased likelihood of REILD due to compromised hepatic reserve (1). In cases with diffuse hepatic involvement, superselective segmental TARE (radiation segmentectomy) has been shown to selectively deliver high dose to the tumor with minimal normal liver exposure (18). After optimization of hepatic vasculature, intraarterial 99mTc-MAA is injected in the target bed in order to confirm uptake of the tumor, determine pulmonary shunt fraction and exclude the possibility of non-targeted embolization of radioembolic. Commonly in order to establish total shunt perfusion fraction, 99mTc-MAA is injected in the proper hepatic artery with an assumption of uniform homogeneous distribution (and shunt) throughout the entire liver with the assumption that 99mTc-MAA must be injected into the hepatic artery similar to the application of microspheres. Neovascularity of tumors can lead to the formation of arteriovenous anastamoses or shunts, which can allow direct entry of microspheres into the venous system (19). 99mTc-MAA SPECT/CT is superior to SPECT or planar imaging alone for demonstration of gastrointestinal Tc-MAA deposition (17). 99mTc-MAA SPECT/CT is also valuable in accurate depiction of intrahepatic distribution of Tc-MAA (15,17). SPECT/CT can be particularly valuable in cases with heterogeneous intrahepatic Tc-MAA and when spare segments (segments without activity) are visualized. Subsequent test angiogram and Tc-MAA perfusion imaging of spare segments prior to treatment is necessary to exclude aberrant vessel to other organs (Figure 7) (15).
Inherent in this method are fundamentally incorrect assumptions based on a single compartment model. As regional target areas will demonstrate specific shunt fractions that may effect the ability to deposit high amounts of activity in disease active regions (radiation segmentectomy, radiation lobectomy). Therefore, in situations where geographical areas require interrogation with intended multisession therapy (e.g., radiation segementectomy on the background of planned whole liver therapy) a dual acquisition technique such as herein described would prove valuable in the determination of actual compartment shunt fraction as opposed to estimation based on whole liver 99mTc-MAA injection, also allowing for more advanced two compartment and dose kernel modelling of tumor uptake (20).
Furthermore, it has been documented that embolized vessels/organs can revascularize quickly (6). Thus planning angiogram and SIRT should be performed in a narrow time frame. Confirming vascular optimization of the non-treated segment(s) can be advantageous in terms of savings in- time, dose, resources and limiting risks of extra-procedures.
Other applications may be in determining optimal treatment strategy for large volume tumors with several vascular supplies, for large volume tumors in various segments can have differing hypervascularity. Simultaneous administration of 90Y and the injection of Tc-MAA into another lobe or segment for subsequent treatment planning may provide more accurate assessment of geographic uptake and shunt. In incidents where radiation doses to tumors are inferred from the partition model, this technique may provide a more accurate prediction of response rate and survival (21). Differential tumor burden and sequential treatment is not accounted for within the partition model for radiation dose to the lungs. RE with concurrent Tc-MAA injection could play a role in the confirmation of non-dose limiting exposure to the lungs in such cases. SPECT/CT images of Tc-MAA can also provide accurate 3D maps of MAA biodistribution at a lobar/segmental level. These quantitative 99mTc-MAA images can be used for 3D dosimetry calculations resulting in 3D maps of dose distribution arising from heterogeneous distribution of activity in the liver (22,23). Recently, several studies investigating feasibility of 90Y PET imaging reported very promising results in visualizing 90Y microsphere distribution (24-27), even attempting activity quantification (28) and dosimetry (29). Quantitative segmental/lobar 99mTc-MAA SPECT/CT can be used as a validation tool for 90Y PET.
The feasibility of TARE of a lobe/segments of a liver with simultaneous test angiogram/Tc-MAA of another has been demonstrated (15). The Tc-MAA SPECT/CT images in our two patients demonstrated pure Tc-MAA distribution. Exclusion of lower intensity BS radiation during SPECT/CT reconstruction of higher Tc-MAA gamma radiation resulted in elimination of scatter artifacts (15). We did not evaluate the distribution of 90Y in our candidates. However, Ahmadzadehfar et al. have shown that accurate BS scan can be performed at 48 hours when “washout” of technetium has occurred (15). We believe that any robust future sequential segmental/lobar therapy protocol should include simultaneous Tc-MAA and Y-90 BS SPECT/CT.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.01.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethical committee. Written informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodological considerations. J Vasc Interv Radiol 2006;17:1251-78. [PubMed]
- Goin JE, Salem R, Carr BI, et al. Treatment of unresectable hepatocellular carcinoma with intrahepatic yttrium 90 microspheres: factors associated with liver toxicities. J Vasc Interv Radiol 2005;16:205-13. [PubMed]
- Salem R, Lewandowski RJ, Atassi B, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol 2005;16:1627-39. [PubMed]
- Murthy R, Nunez R, Szklaruk J, et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics 2005;25:S41-55. [PubMed]
- Ahmadzadehfar H, Biersack HJ, Ezziddin S. Radioembolization of liver tumors with yttrium-90 microspheres. Semin Nucl Med 2010;40:105-21. [PubMed]
- Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68:13-23. [PubMed]
- Leung TW, Lau WY, Ho SK, et al. Radiation pneumonitis after selective internal radiation treatment with intra-arterial 90yttrium-microspheres for inoperable hepatic tumors. Int J Radiat Oncol Biol Phys 1995;33:919-24. [PubMed]
- Murthy R, Brown DB, Salem R, et al. Gastrointestinal complications associated with hepatic arterial yttrium-90 microsphere therapy. J Vasc Interv Radiol 2007;18:553-61; quiz 562. [PubMed]
- Carretero C, Munoz-Navas M, Betes M, et al. Gastroduodenal injury after radioembolization of hepatic tumors. Am J Gastroenterol 2007;102:1216-20. [PubMed]
- Salem R, Parikh P, Atassi B, et al. Incidence of radiation pneumonitis after hepatic intra-arterial radiotherapy with yttrium-90 microspheres assuming uniform lung distribution. Am J Clin Oncol 2008;31:431-8. [PubMed]
- Yip D, Allen R, Ashton C, et al. Radiation-induced ulceration of the stomach secondary to hepatic embolization with radioactive yttrium microspheres in the treatment of metastatic colon cancer. J Gastroenterol Hepatol 2004;19:347-9. [PubMed]
- Atassi B, Bangash AK, Lewandowski RJ, et al. Biliary sequelae following radioembolization with yttrium-90 microspheres. J Vasc Interv Radiol 2008;19:691-7. [PubMed]
- Ahmadzadehfar H, Sabet A, Muckle M, et al. 99mTc-MAA/90Y-Bremsstrahlung SPECT/CT after simultaneous Tc-MAA/90Y-microsphere injection for immediate treatment monitoring and further therapy planning for radioembolization Eur J Nucl Med Mol Imaging 2011;38:1281-8. [PubMed]
- Lewandowski RJ, Thurston KG, Goin JE, et al. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135–150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging. J Vasc Interv Radiol 2005;16:1641-51. [PubMed]
- Ahmadzadehfar H, Sabet A, Biermann K, et al. The significance of 99mTc-MAA SPECT/liver perfusion imaging in treatment planning for 90Ymicrosphere selective internal radiation treatment. J Nucl Med 2010;51:1206-12. [PubMed]
- Liu DM, Salem R, Bui JT, et al. Angiographic considerations in patients undergoing liver-directed therapy. J Vasc Interv Radiol 2005;16:911-35. [PubMed]
- Hamami ME, Poeppel TD, Müller S, et al. SPECT/CT with 99mTc-MAA in radioembolization with 90Y microspheres in patients with hepatocellular cancer. J Nucl Med 2009;50:688-92. [PubMed]
- Riaz A, Gates VL, Atassi B, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys 2011;79:163-71. [PubMed]
- Ahmadzadehfar H, Biersack H, Ezziddin S. Radioembolization of liver tumors with Yttrium-90 microspheres. Semin Nucl Med 2010;40:105-21. [PubMed]
- Kennedy A, Dezarn W, Weiss A. Patient specific 3D image-based radiation dose estimates for 90Y microsphere hepatic radioembolization in metastatic tumors. J Nucl Med Radiat Ther 2011;2:111.
- Ho S, Lau WY, Leung TW, et al. Partition model for estimating radiation doses from yttrium-90 microspheres in treating hepatic tumours. Eur J Nucl Med 1996;23:947-52. [PubMed]
- Bolch WE, Bouchet LG, Robertson JS, et al. MIRD pamphlet No. 17: the dosimetry of nonuniform activity distributions--radionuclide S values at the voxel level. Medical Internal Radiation Dose Committee. J Nucl Med 1999;40:11S-36S. [PubMed]
- Dieudonné A, Garin E, Laffont S, et al. Clinical feasibility of fast 3- dimensional dosimetry of the liver for treatment planning of hepatocellular carcinoma with 90Y microspheres. J Nucl Med 2011;52:1930-7. [PubMed]
- Nickles RJ, Roberts AD, Nye JA, et al. Assaying and PET imaging of yttrium-90: l> > 34 ppm > 0. IEEE Nucl Sci Symp Rec 2004;6:3412-4.
- Bagni O, D’Arienzo M, Chiaramida P, et al. 90Y-PET for the assessment of microsphere biodistribution after selective internal radiotherapy. Nucl Med Commun 2012;33:198-204. [PubMed]
- Gates VL, Esmail AA, Marshall K, et al. Internal pair production of 90Y permits hepatic localization of microspheres using routine PET: proof of concept. J Nucl Med 2011;52:72-6. [PubMed]
- Lhommel R, Goffette P, Van den Eynde M, et al. Yttrium-90 TOF PET scan demonstrates high-resolution biodistribution after liver SIRT. Eur J Nucl Med Mol Imaging 2009;36:1696. [PubMed]
- Werner MK, Brechtel K, Beyer T, et al. PET/CT for the assessment and quantification of 90Y biodistribution after selective internal radiotherapy (SIRT) of liver metastases. Eur J Nucl Med Mol Imaging 2010;37:407-8. [PubMed]
- Lhommel R, van Elmbt L, Goffette P, et al. Feasibility of 90Y TOF PET-based dosimetry in liver metastasis therapy using SIR-Spheres. Eur J Nucl Med Mol Imaging 2010;37:1654-62. [PubMed]


