
KRAS and BRAF mutation frequencies in a series of Turkish colorectal cancer patients
Introduction
Colorectal cancer (CRC) is one of the most frequent cancers worldwide. Each year approximately one million new patients are diagnosed with CRC, and metastatic disease develops in 50% of these patients (1). Surgery is a curative option for most patients with early stage disease. New therapeutic strategies along with advances in surgery, chemotherapy and adjuvant therapy have increased the overall survival rate and prolonged the progression time of advanced CRC patients. Clinical trials conducted with metastatic CRC patients have demonstrated that the addition of monoclonal antibodies targeting the epidermal growth factor receptor (EGFR) pathway (such as cetuximab and panitumumab) to oxaliplatin/5-fluorouracil/leucovorin (FOLFOX) regimens improves the overall survival rate. This improvement is attributed to inhibition of EGFR signaling, tumor growth and proliferation (2). However, numerous clinical trials have shown that a set of CRC patients benefit from anti-EGFR therapies. In addition, negative predictors of response to EGFR-targeted therapies have been reported; anti-EGFR monoclonal antibodies are only effective in metastatic CRC patients harboring a wild type KRAS (Ki-ras2 Kirsten rat sarcoma viral oncogene homolog) gene (3).
The KRAS gene is an oncogene that encodes for the KRAS protein, which is a small membrane-bound G protein. KRAS protein is activated by receptor tyrosine kinases, and it plays a crucial role in the regulation of cell division by transferring external proliferation signals to the nucleus. KRAS gene mutations, especially in codon 12 and 13, increase its tyrosine kinase activity, thereby promoting cell transformation, aggressive tumors and resistance to chemotherapy and anti-EGFR-targeted biological therapies. Activating KRAS gene mutations have been detected in approximately 35-45% of CRCs, and these mutations are associated with poor therapeutic responses (4,5). Although a wild type KRAS gene is a negative predictor of EGFR-targeted therapeutic response, recent studies have indicated a wild type BRAF genotype is also required for anti-EGFR based therapeutic responses (6,7).
The BRAF gene encodes a protein kinase that is involved in intracellular signaling and cell growth. The BRAF protein is downstream of the KRAS protein in the RAS/RAF/MAPK cellular signaling pathway and serves a promising target in several cancers. Activating BRAF gene mutations promote enhanced protein activation. This over-activation triggers the MAPK pathway signaling cascade, uncontrolled cellular proliferation and resistance to apoptosis. Approximately 5% to 10% of CRC patients harbor BRAF gene mutations. These mutations are associated with poor treatment response and outcomes. Similar to the KRAS mutation status, BRAF gene mutations are an important factor for beneficial anti-EGFR therapy. The National Comprehensive Cancer Network recommends BRAF mutation testing in wild type KRAS metastatic CRC patients selected for anti-EGFR therapies (8).
Advances in personalized cancer managements, such as the development of target-specific cancer therapeutics or novel biomarkers as well as the characterization of the mutational status of specific targets or biomarkers, are needed. These improvements are outlined in cancer management guidelines. The frequency of oncogenic KRAS and BRAF mutations varies in distinct populations. Moreover, the frequency of KRAS and BRAF gene mutations in CRC patients is unknown in certain populations. The aim of our study is to identify the KRAS and BRAF gene mutation frequency in a series of Turkish CRC patients and to associate these mutations with demographic features in the Turkish population.
Methods
Patient selection and tissue samples
Two hundred twenty colorectal adenocarcinoma patients with sufficient archival formalin-fixed paraffin-embedded (FFPE) tumor tissues for molecular analysis were chosen for this study. A pathologist confirmed the colorectal adenocarcinoma histology and the presence of >75% tumor cells in hematoxylin & eosin-stained slides. We obtained 10-µm thick sections from FFPE tumor tissue blocks. Patient demographic data were obtained from hospital information systems. This data includes the patient’s age at diagnosis, gender, primary tumor site, tumor differentiation, tumor stage and metastasis.
DNA isolation
Genomic DNA was extracted from 10-µm thick tumor tissue sections using the QIAamp DNA FFPE Tissue Kit (Qiagen, Cat no: 56404, Hilden, Germany) according to the manufacturer’s instructions. Tumor tissues were deparaffinized in xylene, washed with absolute ethanol and air-dried. The lysis process was performed with proteinase K at 56 °C overnight. The extracted DNA concentration and quality were determined by spectrometric measurement.
KRAS-BRAF mutation analysis by chip array hybridization
Mutation assays were performed with isolated DNA using automated chip array hybridization-based genotyping technology. Chip array hybridization was performed in an automated INFINITI Analyzer (Autogenomics Inc., INFINITI Biofilm Chip Microarray, Vista, CA, USA) with the INFINITI KRAS-BRAF Assay (Autogenomics Inc., KRAS-BRAF Assay) according to the manufacturer’s instructions. The genomic DNA samples were assessed for mutations in codons 12 (G12A/C/D/F/R/S/V), 13 (G13A/C/D/R/S/V), and 61 (Q61E/H/K/L/P/R) for KRAS as well as codon 600 (V600A/D/E/KRM) for BRAF. The genomic regions were amplified using a multiplex polymerase chain reaction (PCR) in a thermal cycler (Applied Biosystems, 2720 Thermal Cycler, Singapore). An enzymatic cleanup with shrimp alkaline phosphatase and exonuclease I was performed after the multiplex PCR. Subsequently, the INFINITI Analyzer was used for allele-specific primer extension with fluorescently labeled nucleotides, capture via hybridization to the microarray, array scans and signal measurements.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) (Version 19.0; SPSS, Inc., Chicago, IL, USA) program was used for the statistical analyses. The Chi-square test was used to assess the association between mutation status and other variables. P values <0.05 were considered significant.
Ethics statement
The study was approved by the Non-invasive Research Ethics Committee of Dokuz Eylul University School of Medicine (Nos: 2010/04-25 and 2011/40-21).
Results
Patient demographics
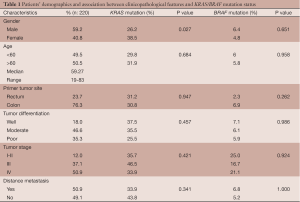
Two hundred twenty patients diagnosed with colorectal adenocarcinoma were included in this study. Data on patient characteristics are presented in Table 1. In total, 40.8% of patients were female, and 59.2% of patients were male. The median age at diagnosis was 59.27 years (range, 19-83 years). The primary tumor was localized in the colon and rectum in 76.3% and 23.7% of patients, respectively. In 18% of patients, the tumors were well differentiated. The tumors were moderately differentiated and poorly differentiated in 46.6% and 35.3% of patients. The majority of tumors were histopathological grade IV (50.9%). In addition, 37.1% of tumors were grade III, and 12.0% of tumors were grades I & II. Based on clinical inspection, 50.9% of patients had distant metastasis, whereas the remaining patients displayed no metastasis (49.1%).
Full table
Molecular results
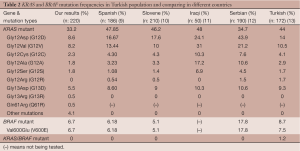
We analyzed the KRAS and BRAF mutation status in 220 Turkish CRC patients. The KRAS and BRAF mutation distributions are presented in Table 2. KRAS mutation analysis indicated that 66.8% of patients possess a wild type KRAS genotype, whereas 33.2% of cases carry a KRAS mutation at one of three codons examined (codons 12, 13 and 61). The following mutation frequencies were observed: Gly12Asp (8.6%), Gly12Val (8.2%), Gly12Cys (2.3%), Gly12Ala (1.8%), Gly12Ser (1.8%), Gly13Asp (5.5%), Gly13Arg (0.5%), Gln61Arg (0.5%) and other mutations (4.1%). The most common mutations were Gly12Asp (8.6% of all analyzed mutations) and Gly12Val (8.2% of all analyzed mutations). We did not observe any patient with more than one KRAS gene mutation at codons 12, 13 and 61.
Full table
BRAF mutation analysis indicates that 6.7% of cases harbored mutations. The V600E mutation was identified in all BRAF mutant patients. All patients carrying the V600E BRAF mutation possessed wild type KRAS genotypes.
A significant difference was observed for the KRAS mutation frequency with respect to gender (P value =0.027). Female patients displayed a higher KRAS mutation frequency than male patients. No significant difference was observed between the BRAF mutation frequency and gender (P value =0.44). No significant differences in KRAS and BRAF mutation frequencies with respect to patient age, primary tumor site, tumor differentiation, tumor stage or metastasis were observed (Table 1).
Discussion
CRC is one of the most frequent cancers worldwide and is a leading cause of cancer mortality. In CRC, KRAS and BRAF mutations are crucial for carcinogenesis and the success of anti-EGFR treatments. Mutant KRAS and BRAF alleles impair the therapeutic efficacy of anti-EGFR targeted agents, such as cetuximab or panitumumab. KRAS and BRAF gene alterations promote uncontrolled cell proliferation and survival independent of the EGFR pathway. Large clinical trials have demonstrated that wild type KRAS and BRAF are required for the response to anti-EGFR therapies. Genotype analyses could be used to select patients eligible for the treatment. In this study, we detected KRAS and BRAF gene mutations in 220 Turkish CRC patients. The KRAS gene was genotyped for mutations at codons 12, 13, and 61, and the BRAF gene was analyzed for mutations in codon 600 in this study.
KRAS mutations were identified in 33.2% of the current CRC samples. The most frequent mutations were at codon 12: Gly12Asp (8.6%) and Gly12Val (8.2%). The KRAS mutation frequency varies in different populations. Table 2 presents a comparison of KRAS-BRAF mutational frequencies in various countries. The KRAS mutation frequency was 48% in a Spanish population, 46.2% in a Slovene population, 48% in an Iraqi population, 35% in a Serbian population, 32% in a Saudi Arabian population, 29.3% in a Greek population, and 23.91% in a Moroccan population. The frequencies were different from each paper. Our results are similar to the Slovene, Greek and Saudi Arabian population results. This result was expected given that Turkey’s geographical region is in close proximity to Serbia, Greece and Saudi Arabia. However, the ethnicity categories of Turkey are different compared with Europe and Asia, and information regarding KRAS mutation differences in different ethnicities is limited (9-12,14-16).
The KRAS mutation profiles can be different in the same population. Our KRAS mutation frequencies are different from other studies conducted with limited patient groups in Turkey. The following are KRAS mutation frequencies reported in four studies: 11% (total n: 53), 40% (total n: 35), 44% (total n: 172) and 49.05% (total n: 53) (13,17-19). The case numbers of the other Turkish study groups were reduced compared with our group (total n: 220), thereby resulting in this frequency difference. Large study groups should be used for genotyping studies to obtain the most accurate data. Cancer mutation profiles are influenced by cultural life style and ethnicity. The KRAS mutation frequency varies according to a patient’s ethnicity (more frequent in Caucasians than Asians) (20,21). Turkey is a country that is located among Europe, the Middle East and the Caucasus region. Thus, Turkey is comprised of many ethnic groups with European, Middle Eastern, Caucasian or Asian origins. This difference can be primarily attributed to ethnicity. However, information about ethnicity-based KRAS genotype differences in Turkey is limited.
The most common mutations were Gly12Asp, Gly12Val and Gly13Asp. Most population studies indicate that these are the most commonly reported KRAS mutations in CRC. When prescribing anti-EGFR-based therapies, oncologists should know whether the patient carries a wild type KRAS gene. However, some papers demonstrate that KRAS gene mutation type may alter a tumor’s biological behavior. Various tumor behavior functions have been observed for Gly12Asp and Gly12Val; the Gly12Val mutation was classified as more aggressive than Gly12Asp with regard to resistance to apoptosis and uncontrolled cellular proliferation. The most common mutation types (Gly12Val, Gly12Asp and Gly13Asp) are similar in the majority of population-based studies. However, the frequencies of other mutations were different. Thus, additional mutations and their effect on tumor biological behavior should be studied in large population-based studies (22).
A significant difference was observed in the KRAS mutation frequency with respect to gender (P value =0.027). Female patients displayed increased KRAS mutation frequencies compared with male patients. Thus, females are potentially resistant to anti-EGFR therapies. Increased KRAS mutations in females are potentially indicative of a relationship between KRAS mutations and hormones. We observed this relationship in our previous analysis, which was conducted in a limited CRC patient group (23).
BRAF mutations were identified in 6.7% of current CRC samples. The V600E mutation was identified in all BRAF mutant patients. All patients with the V600E BRAF mutation possessed a wild type KRAS genotype. Based on our results, we hypothesize that BRAF mutations are exclusively present in wild type KRAS patients.
The BRAF mutation frequency is similar in various populations. The BRAF mutation frequency was reported as 5.1% in a Slovene population, 5.1% in a Chinese population, and 5.43% in a Moroccan population. The BRAF mutation frequency reported in this study was increased compared with other studies involving other ethnic groups (10,16,24). No significant differences in BRAF mutation frequencies were observed with respect to patient age, gender, primary tumor site, tumor differentiation, tumor stage and metastasis.
In addition to ethnicity, environmental factors and lifestyle may alter the epigenetic regulation of oncogenes and/or tumor suppressor genes in various ethnic groups. Low dietary folate, Western-style diets, cigarette smoking and alcohol consumption are associated with colorectal carcinogenesis-related oncogenes/tumor suppressor genes. KRAS oncogene mutations in CRC are associated with low dietary folate. For example, in various Middle Eastern populations, the extracts of several wild plants that possess anti-inflammatory activity are consumed to cure gastrointestinal system disorders via the inhibition of cyclooxygenase-2, which is an important factor in CRC. CRC prevention can be developed with informative genotypic data about CRC pathogenesis. Given their diverse lifestyle patterns and environmental conditions, developing countries offer various opportunities to understand the heterogeneity of CRC. A comprehensive understanding of the international differences in the molecular pathogenesis of CRC may provide insight for novel preventive and therapeutic strategies directed at lifestyle and environmental factors (25-27).
In addition to ethnicity, lifestyle and environmental factors, the detection of gene mutation frequencies can be influenced by assay methodology and the percentage of neoplastic cells. A variety of mutation detection methods are used for KRAS-BRAF mutations. Differences in method sensitivities can affect the mutation frequencies. More sensitive assays can detect mutations less than 1%. In our clinical practice, we use the validated INFINITI Analyzer for molecular testing. Given that molecular testing is becoming critical component of clinical laboratories, validated molecular testing platforms should be used to provide desired quality, reliability and robustness. The percentage of neoplastic cells used for mutation analysis and the clinical response to anti-EGFR therapies may vary. Tumor heterogeneity varies within different regions of the tumor. Sampling errors cause false-negative results. Sample selection should be informed by immunohistochemistry and performed by a pathologist (28).
Genotypic analyses are important in CRC management. Recently, important molecular discoveries in CRC genotypes have altered the clinical management of metastatic CRC. KRAS and BRAF gene alterations may determine the therapeutic response to anti-EGFR treatments (cetuximab or panitumumab). Therefore, patients should be classified into genotypic subgroups for the selection of appropriate therapeutic agents. In Turkish oncology practices, oncologists administer personalized drug therapies based on a patient’s tumor genotype, especially in the management of metastatic CRC. FOLFOX (oxaliplatin/5-fluorouracil/leucovorin) or FOLFIRI (irinotecan/5-FU/leucovorin) in combination with cetuximab or panitumumab are used as the first line therapy for metastatic CRC patients with wild type KRAS and BRAF genes as KRAS and BRAF mutations may worsen prognosis in this group.
Ethnic groups, environmental conditions and lifestyle influence the genotypic classification results. KRAS mutation frequencies vary among the majority of population studies, whereas BRAF mutation frequencies are generally similar. Given this information, we can understand CRC carcinogenesis within various populations. This information can also aid in more efficient targeting of cancer cells based on KRAS-BRAF markers that play a crucial role in the management of CRC (29). By correlating genotyping studies with clinical findings, we clarified the clinical utility of these markers. Our data suggest that KRAS mutations might be present more frequently in females than males. Further research involving larger study groups is necessary to confirm this finding. Advances in population-based genotyping studies may aid in the diagnostic and therapeutic decision processes.
Acknowledgments
Funding: This study was supported by Dokuz Eylul University Research Foundation (DEU-BAP 2012.KB.SAG.63).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.04.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Non-invasive Research Ethics Committee of Dokuz Eylul University School of Medicine (No. 2010/04-25 and 2011/40-21). Written informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tougeron D, Cortes U, Ferru A, et al. Epidermal growth factor receptor (EGFR) and KRAS mutations during chemotherapy plus anti-EGFR monoclonal antibody treatment in metastatic colorectal cancer. Cancer Chemother Pharmacol 2013;72:397-403. [PubMed]
- Lamparella NE, Saroya BS, Yang Z, et al. Contradictory KRAS mutation test results in a patient with metastatic colon cancer: a clinical dilemma in the era of personalized medicine. Cancer Biol Ther 2013;14:699-702. [PubMed]
- Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009;27:2091-6. [PubMed]
- Tan C, Du X. KRAS mutation testing in metastatic colorectal cancer. World J Gastroenterol 2012;18:5171-80. [PubMed]
- Jancík S, Drábek J, Radzioch D, et al. Clinical relevance of KRAS in human cancers. J Biomed Biotechnol 2010;2010:150960.
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757-65. [PubMed]
- Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705-12. [PubMed]
- Kalady MF, Dejulius KL, Sanchez JA, et al. BRAF mutations in colorectal cancer are associated with distinct clinical characteristics and worse prognosis. Dis Colon Rectum 2012;55:128-33. [PubMed]
- Herreros-Villanueva M, Rodrigo M, Claver M, et al. KRAS, BRAF, EGFR and HER2 gene status in a Spanish population of colorectal cancer. Mol Biol Rep 2011;38:1315-20. [PubMed]
- Ličar A, Cerkovnik P, Novaković S. Distribution of some activating KRAS and BRAF mutations in Slovene patients with colorectal cancer. Med Oncol 2011;28:1048-53. [PubMed]
- Al-Allawi NA, Ismaeel AT, Ahmed NY, et al. The frequency and spectrum of K-ras mutations among Iraqi patients with sporadic colorectal carcinoma. Indian J Cancer 2012;49:163-8. [PubMed]
- Jakovljevic K, Malisic E, Cavic M, et al. KRAS and BRAF mutations in Serbian patients with colorectal cancer. J BUON 2012;17:575-80. [PubMed]
- Selcukbiricik F, Erdamar S, Ozkurt CU, et al. The role of K-RAS and B-RAF mutations as biomarkers in metastatic colorectal cancer. J BUON 2013;18:116-23. [PubMed]
- Zekri J, Rizvi A, Al-Maghrabi J, et al. K-ras in Colorectal Cancer Tumors From Saudi Patients: Frequency, Clinco-pathological Association and Clinical Outcome. The Open Colorectal Cancer Journal 2012;5:22-7.
- Symvoulakis EK, Zaravinos A, Panutsopulos D, et al. Highly conserved sequence of exon 15 BRAF gene and KRAS codon 12 mutation among Greek patients with colorectal cancer. Int J Biol Markers 2007;22:12-8. [PubMed]
- Marchoudi N, Amrani Hassani Joutei H, Jouali F, et al. Distribution of KRAS and BRAF mutations in Moroccan patients with advanced colorectal cancer. Pathol Biol (Paris) 2013;61:273-6. [PubMed]
- Akkiprik M, Celikel CA, Düşünceli F, et al. Relationship between overexpression of ras p21 oncoprotein and K-ras codon 12 and 13 mutations in Turkish colorectal cancer patients. Turk J Gastroenterol 2008;19:22-7. [PubMed]
- Demiralay E, Saglam Y, Altaca G, et al. The Frequency of K-ras Mutation in Colorectal Adenocarcinomas with Absence of Distant Metastasis at Diagnosis. Surgical Science 2012;3:111-5.
- Ozen F, Ozdemir S, Zemheri E, et al. The proto-oncogene KRAS and BRAF profiles and some clinical characteristics in colorectal cancer in the Turkish population. Genet Test Mol Biomarkers 2013;17:135-9. [PubMed]
- Riely GJ, Kris MG, Rosenbaum D, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res 2008;14:5731-4. [PubMed]
- Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc 2009;6:201-5. [PubMed]
- Monticone M, Biollo E, Maffei M, et al. Gene expression deregulation by KRAS G12D and G12V in a BRAF V600E context. Mol Cancer 2008;7:92. [PubMed]
- Baskin Y, Calibasi G, Canda E, et al. Unusual high frequency of KRAS mutations in female colorectal cancer patients. In Vivo 2011;25:519.
- Gao J, Wang TT, Yu JW, et al. Wild-Type KRAS and BRAF Could Predict Response to Cetuximab in Chinese Colorectal Cancer Patients. Chin J Cancer Res 2011;23:271-5. [PubMed]
- Chan AO, Soliman AS, Zhang Q, et al. Differing DNA methylation patterns and gene mutation frequencies in colorectal carcinomas from Middle Eastern countries. Clin Cancer Res 2005;11:8281-7. [PubMed]
- Slattery ML, Anderson K, Curtin K, et al. Lifestyle factors and Ki-ras mutations in colon cancer tumors. Mutat Res 2001;483:73-81. [PubMed]
- Slattery ML, Curtin K, Anderson K, et al. Associations between dietary intake and Ki-ras mutations in colon tumors: a population-based study. Cancer Res 2000;60:6935-41. [PubMed]
- . Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: can testing of tumor tissue for mutations in EGFR pathway downstream effector genes in patients with metastatic colorectal cancer improve health outcomes by guiding decisions regarding anti-EGFR therapy? Genet Med 2013;15:517-27. [PubMed]
- Kumar K, Brim H, Giardiello F, et al. Distinct BRAF (V600E) and KRAS mutations in high microsatellite instability sporadic colorectal cancer in African Americans. Clin Cancer Res 2009;15:1155-61. [PubMed]


