Prognostic factors in advanced pancreatic cancer patients receiving second-line chemotherapy: a single institution experience
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth highest cause of cancer-related death among men and women in the US and continues to have the worst prognosis of all the gastrointestinal malignancies. Because of its aggressive growth and early metastatic dissemination, the overall 5-year survival rate for patients with pancreatic cancer remains around 3–5%; in fact, death rates from 2010 to 2014 increased in particular for men (1). The use of palliative first-line chemotherapy has been shown to improve the survival and quality of life compared with best supportive care (BSC) in patients with good performance status, although the survival gain was modest (2). The FOLFIRINOX regimen, including 5-fluorouracil (5-FU), oxaliplatin and irinotecan (3), and the combination of nab-paclitaxel with gemcitabine (4) in first-line setting provide more effective treatment options, in particular for the patients with a good performance status but there is currently no consensus concerning the role and the optimal regimen for second-line chemotherapy after first-line chemotherapy treatment failure. In the light of such bleak statistics, it is important to select subgroups of patients with metastatic or recurrent pancreatic adenocarcinoma that could benefit from second-line chemotherapy in order to maximize benefit and avoid over-treatment in frail patients. The aim of our study was to identify prognostic factors that could predict which patients may receive the maximum benefit of second-line treatment.
Methods
The study included patients with a cytological or histological diagnosis of ductal pancreatic adenocarcinoma who received second-line chemotherapy at the Department of Oncology of AOU Ospedali Riuniti–Università Politecnica delle Marche from January 2002 to December 2016 and who were then followed on a regular basis in a specific follow-up program, based on the evaluation of routine blood tests, CEA and carbohydrate antigen 19-9 (CA 19-9) biomarkers for each chemotherapy cycle and 3-month-cyclic instrumental re-evaluation (by TC Chest/Abdomen with contrast or TC Chest without contrast + RMN abdomen with contrast). The inclusion criteria for the study included progressive disease after first-line chemotherapy and presence of measurable or evaluable disease. Recorded patient characteristics and clinical features included: gender, age, sex, weight, risk factors (smoking status), symptoms (pain, jaundice), Eastern Cooperative Oncology Group Performance Status (ECOG PS), type of surgery (when performed), histological type, grading, pathological stage of disease (T, N, M), presence or absence of peritoneal involvement, value of tumor markers CA19-9 (NL <5 ng/mL) and CEA (NL <37 U/mL), dates of chemotherapy and radiotherapy, response to first line chemotherapy and clinical benefit, and time to progression, hemoglobin levels (Hb), neutrophil-lymphocyte ratio (NLR), CA 19-9, lactate dehydrogenase (LDH), sodium (Na+) levels, mono-chemotherapy vs. combination therapy and PFS after first line chemotherapy (more vs. less than 4 months). The upper limit of normal (ULN) was 250 UI/L for LDH and 35 UI/mL for CA 19-9. The cut-off chosen to determine high NLR was 5, as already tested in similar studies on advanced pancreatic cancer (5). Data were retrieved from institutional database and patients’ clinical records. The study was approved by local ethics committee (AOU Ospedali Riuniti, No. 214341).
Statistical analysis
Primary endpoint of this study was to evaluate the prognostic role of clinical and biological factors in patients with advanced pancreatic cancer who received second-line chemotherapy. Overall survival (OS) was defined as the time between the start of second-line chemotherapy and the date of death; progression-free survival (PFS) was defined as the time from the date of second-line chemotherapy to the date of disease progression or death from any cause. The association between categorical variables was estimated by χ2 test. Survival distribution was estimated by the Kaplan-Meier method. Significant differences in probability of surviving between strata were evaluated by log-rank test. Variables that achieved statistical significance (P<0.05) for univariate analysis were used for multivariate analysis by Cox’s multiple regression to identify independent prognostic factors. The hazard ratio (HR) was also calculated. The statistical analysis was conducted using the MedCalc version 14.10.2 for Windows software.
Results
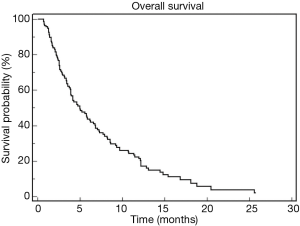
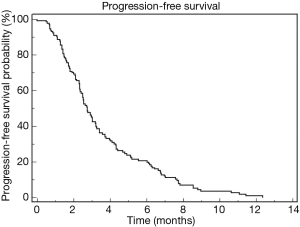
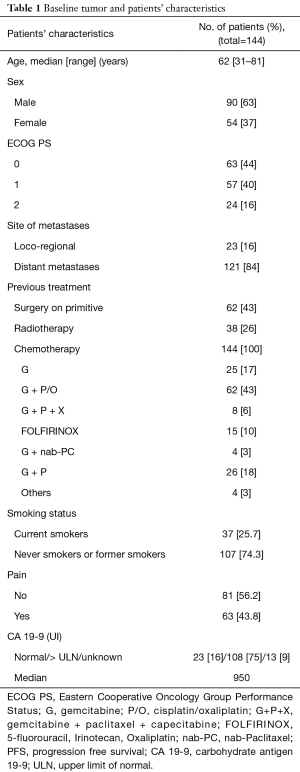
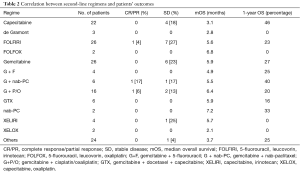
Three hundred and thirty-three advanced PDAC patients were treated with first-line chemotherapy from January 2002 to December 2016. One hundred and forty-four patients (43.2%) received second-line chemotherapy and were included in this retrospective study. Median age of the patients was 62 years (range, 31–81 years). Ninety patients (63%) were male and 54 (37%) female. ECOG performance status was 0 in 63 patients (44%) and 1 or 2 in the remaining 81 patients. Table 1 summarize patients’ clinical and pathological characteristics. Most of the patients received first-line treatment with gemcitabine-based regimens (70%) while 10% received FOLFIRINOX chemotherapy. Second line chemotherapy regimens included capecitabine, 5-FU or 5-FU based combinations, as FOLFOX (5-FU, leucovorin and oxaliplatin) or FOLFIRI (5-FU, leucovorin and irinotecan) regimens. A significant proportion of patients, in particular those treated with FOLFIRINOX or with a long PFS to first-line gemcitabine-based treatment, received gemcitabine or gemcitabine-based combinations. A small percentage of patients were treated with nab-paclitaxel or taxanes. Median PFS for second line treatment was 2.76 months while median OS was 5.26 months. Table 2 summarizes second line regimens used and patients’ outcome. The median OS was 5.26 months (95% CI, 4.01–6.84) (Figure 1) while median PFS was 2.76 (95% CI, 2.50–3.22) (Figure 2).
Full table
Full table
Prognostic variables were selected based on those identified in previous studies on the same setting (6-8) and their prognostic role was analyzed. The variables examined were gender, ECOG PS, stage of disease, metastatic localization, presence or absence of peritoneal involvement, surgery on the primary tumor, age, smoking status, pain, hemoglobin levels (Hb), NLR, CA 19-9, LDH, sodium (Na+) levels, mono-chemotherapy vs. combination therapy and PFS after first line chemotherapy (more vs. less than 4 months). The ULN was 250 UI/L for LDH and 35 UI/mL for CA 19-9. The optimal cut-off value for NLR was determined using time-dependent receiver operating curve (ROC) analysis. The NLR value was categorized in two groups, NLR ≤2.5 and NLR >2.5. At univariate analysis, 6 out of these 16 clinical-laboratory features showed a significant correlation with OS. In particular ECOG PS was found to be a significant prognostic factor (ECOG 0 vs. 1–2; median OS =8.55 vs. 3.42 months, respectively; HR 0.47; 95% CI, 0.28–0.64; P<0.0001). Also, PFS to first line chemotherapy was significantly related to OS (median OS of 6.84 vs. 4.14 months in patients with first-line PFS more than 4 months vs. less than 4 months respectively, HR 1.48; 95% CI, 1.02–2.16; P=0.035). Furthermore, patients with a single metastatic site had median OS of 6.77 vs. 3.91 months for multiple site of metastases (HR 0.65; 95% CI, 0.39–0.96; P=0.032). Again, NL ratio showed a correlation with OS (NL ≤2.5 ratio vs. NL >2.5; median OS =7.33 vs. 4.14 months, respectively; HR 0.55; 95% CI, 0.34–0.81; P=0.003), as well as CA 19-9 (CA 19-9 <10 vs. >10 ULN; median OS =10.72 vs. 3.91 months, respectively; HR 0.39; 95% CI, 0.24–0.55; P<0.0001). LDH level also was significantly associated with prognosis (LDH < ULN vs. LDH > ULN, 10.72 vs. 4.77 months; HR 0.49; 95% CI, 0.33–0.87; P=0.011). Conversely, no significant impact on prognosis was observed for age, serum levels of Na+ and Hb. Patients who underwent surgical resection of primary tumor did not have a better survival than patients with non-resectable disease at diagnosis. At multivariate analysis, three clinical-laboratoristic features, in particular ECOG PS (HR =1.94; 95% CI, 1.18–3.19; P=0.009), CA 19-9 value (HR =2.99; 95% CI, 1.37–6.54; P=0.006) and LDH value (HR =2.10; 95% CI, 1.08–4.04; P=0.029), resulted significant independent prognostic factors for OS (Table 3).
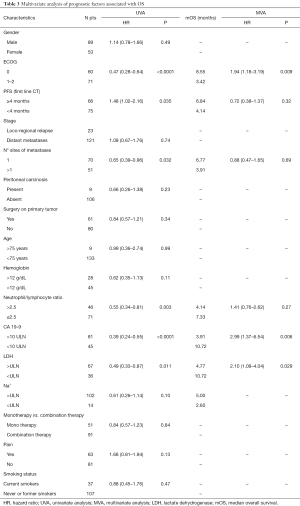
Full table
Discussion
In therapeutic guidelines for the treatment of advanced PDAC, the administration of second line chemotherapy after disease progression under first line treatment is currently recommended (9). However, the real benefit of second line chemotherapy in the palliative treatment of PDAC remains controversial and the choice of second line treatment is still a matter of debate (10). According to literature data, the benefit of second line chemotherapy in terms of OS seems to be marginal and there is no consensus on an optimal regimen to be administered (11-13). A phase III study from CONKO group, published in 2011, was the first to demonstrate a benefit for second line treatment with a regimen based on 5-FU, oxaliplatin and leucovorin (OFF regimen) vs. BSC with a median OS of 4.8 months for chemotherapy vs. 2.3 months for BSC (12). Recently the phase III NAPOLI-1 trial evaluating a combination of 5-FU and nanoliposomal irinotecan (nal-IRI) in second line showed interesting results, demonstrating a median OS of 6.1 months, introducing a new treatment option in this setting (14). Overall, these results indicate that second-line treatment may provide a clinical benefit in patients with advanced pancreatic cancer as confirmed in a systemic analysis of 34 second-line studies, including over 1,500 patients who had progressed on gemcitabine, reporting a median OS of 2.8 months for patients who received BSC and 6 months for patients who received second-line treatment (13). The choice of second-line regimen in clinical practice depends upon the first-line regimen used, the patients’ ECOG PS, residual toxicities and comorbidities. Moreover, in this very palliative setting it must be remembered that toxicities and quality of life are of paramount importance. In this scenario, prognostic factors able to help in selection of patients who are more likely to receive a benefit from second line chemotherapy is really important in daily practice. This retrospective analysis aims to identify prognostic factors related to the patient and the disease for the choice of second line chemotherapy of advanced PDAC. Indeed, as mentioned above, identification of prognostic variables can be an important aid to the clinician in the choice of patients to treat after progression to first-line chemotherapy. At the same time, validated prognostic factors to stratify patients in clinical trials in the setting of second line chemotherapy may be particularly useful. In our study ECOG PS, CA 19-9 and LDH value were demonstrated to be significant prognostic factors for OS in this setting.
A similar study by Sinn et al. (15) on 208 advanced PDAC patients who received second line chemotherapy showed that poor KPS (Karnofsky Performance Status), CA 19-9 and duration of first-line treatment were prognostic factors associated with OS. These results are comparable to what observed in our analysis. However, in our study the prognostic value of duration of first-line chemotherapy was not confirmed as an independent prognostic factor at multivariate analysis. In a large retrospective analysis by Vienot et al. on 261 advanced PDAC patients treated with second line chemotherapy, age, smoking status, liver metastases, performance status, pain, jaundice, ascites, duration of first-line, and type of chemotherapy regimen were identified as prognostic factors for OS. Interestingly, the authors developed a prognostic model which allowed to identify three risk groups (low, intermediate and high risk) with different survival (16). Performance status and duration of first-line chemotherapy were confirmed as prognostic factor also in our study, while age, pain and smoking status were not found to be significantly associated with prognosis in our analysis.
Another study by Kasuga et al. (17) assessed prognostic factors in second line treatment in 61 patients gemcitabine refractory patients. Interestingly, the study showed significant prognostic value for ECOG PS and CA 19-9 as in our analysis. The prognostic role of ECOG PS in this setting has been confirmed also in a recent systematic review and meta-analysis of randomized controlled trials in PDAC (18). In addition, the study also demonstrated prognostic value of modified Glasgow prognostic score (mGPS), an inflammation-based prognostic score based on C-reactive protein and albumin. In our study, a different inflammation biomarker, namely NLR, was found to be related to poor prognosis at univariate analysis but this was not confirmed at multivariate analysis.
Inflammation response in tumor microenvironment has several tumor promoting effects including inhibition of apoptosis or enhancement of angiogenesis and multiple clinical studies have demonstrated negative prognostic factors for inflammation-related biomarkers in different tumors, including advanced chemotherapy refractory PDAC (19).
It is to notice that the variables analyzed in our study can be easily determined and, in particular, LDH and CA 19-9, are part of routine laboratory evaluations. CA 19-9 is the most extensively studied and validated biomarker in PDAC. In a large meta-analysis, Ballehaninna et al. (20) concluded that normal (<37 U/mL) or moderately elevated pre-operative CA 19-9 serum levels (<100 U/mL) independently predict improved OS, whereas elevated Ca19-9 serum levels (>100 U/mL) were associated with a poor prognosis. Moreover, CA 19-9 has already been identified as a potential independent prognostic factor also in PDAC patients receiving second-line chemotherapy (6,21).
With regard to the LDH, the upper limit of the standard (250 U/L) was defined as cut-off for this study and was found to be significantly associated to median OS in both univariate and multivariate analysis. To date there are only a few reports that describe the potential prognostic role of LDH in patients with pancreatic cancer. A retrospective analysis on 127 pancreatic cancer patients, including 56 patients with metastatic disease, demonstrated a significant prognostic value of LDH levels in metastatic setting. In particular LDH high value was associated with shorter OS when compared to normal values (10 vs. 39 months, P=0.0001) in this study and prognostic value was also confirmed at multivariate analysis. However, in this study no data about second line chemotherapy were available (22). In our study ECOG PS, duration of PFS to first-line chemotherapy, single metastatic site, NLR, CA 19-9 and LDH showed significant correlation with the median OS at univariate analysis. High levels of LDH and CA 19-9 as well as ECOG PS remained independent prognostic factors for OS also at multivariate analysis. Meta-analysis (23) of two international phase III studies in which 34 prognostic factors, including CA 19-9 and LDH, evaluated in 436 metastatic patients treated with gemcitabine-based chemotherapy showed that serum CA 19-9 (HR =1.38; 95% CI, 1.12–1.70, P=0.028) and LDH levels (HR =2.08; 95% CI, 1.50–2.88, P<0.001) were highly significant prognostic factors. However even in this study there were no data about second line chemotherapy. Contrary to other prospective studies (7,8,21,24) NLR demonstrated no independent prognostic value in our analysis. Also, Maréchal et al. (25) reported that ECOG performance status and albumin level were independent prognostic factors in chemo-naive and gemcitabine refractory patients with advanced pancreatic cancer. Indeed, a poor performance status associated with high CA 19-9 value and LDH may reflect an inherent aggressiveness of pancreatic cancer, an increased disease burden and may also be related to the inability to complete the prescribed treatment. Moreover, association between high LDH serum levels and tumour angiogenesis may also explain the prognostic role of LDH in PDAC such as in different solid tumors including for example biliary tract cancer (26) considering that tumor angiogenesis and tumour-induced hypoxia are in fact usually related to poor prognosis and clinical outcome. This monocentric study cannot be exempt from the limits derived mainly from his retrospective nature. Another limit is the heterogeneity of the chemotherapy regimens used, that were chosen by the treating oncologist on the basis of clinical factors, as well as the lack of patients treated with innovative cytotoxic agents that showed promising results in second-line setting, for example irinotecan nanoliposomal, which has been shown to significantly prolong median OS in combination with 5-FU in pretreated advanced pancreatic cancer patients. Moreover, not all potential prognostic factors emerging from scientific literature such as reactive protein C, uric acid (27), and coagulation factors (28) have been taken into account. However, this retrospective study, despite its inherent intrinsic limitations, confirms the important role of CA 19-9 as biomarker in advanced pancreatic cancer also in second line setting and highlights the potential role of another serum marker, LDH, as a prognostically relevant factor in this disease. It is therefore a further confirmation of how prognostic and predictive factors can play a crucial role in improving outcome in second line setting after the failure of first-line chemotherapy. It can also be a valuable tool for future stratification procedures in clinical trials and for selection of high and low risk patients in different therapeutic strategies. Patient selection could be the key to maximizing the benefits of available therapeutic options. However, these results should be confirmed in prospective trial of second-line chemotherapy for advanced PDAC. Such prospective studies should be large-scale, with homogeneous disease stages and standardized cutoff levels to facilitate comparative analysis of results, determine the exact role of these variables in terms of clinical significance and facilitate an appropriate evaluation of new therapeutic strategies.
Conclusions
Our study shows that three clinical-laboratoristic features (ECOG PS, CA 19-9 value and LDH value) may be significant independent prognostic factors for OS in evaluation of second-line chemotherapy in pancreatic cancer. No significant impact on prognosis was observed for the other variables evaluated [gender, stage of disease, metastatic localization, presence or absence of peritoneal involvement, surgery on the primary tumor, age, hemoglobin levels, NLR, sodium (Na+) levels, mono-chemotherapy vs. combination therapy and PFS after first line chemotherapy].
Acknowledgments
Funding: The study was realized with solely funding from Università Politecnica delle Marche, Ancona Italy.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.08.34). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Data were retrieved from institutional database and patients’ clinical records. The study was approved by local ethics committee (AOU Ospedali Riuniti, No. 214341). Informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst 2017;109: [Crossref] [PubMed]
- Sultana A, Smith CT, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol 2007;25:2607-15. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- An X, Ding PR, Li YH, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 2010;15:516-22. [Crossref] [PubMed]
- Reni M, Berardi R, Mambrini A, et al. A multi-centre retrospective review of second-line therapy in advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol 2008;62:673-8. [Crossref] [PubMed]
- Kadokura M, Ishida Y, Tatsumi A, et al. Performance status and neutrophil-lymphocyte ratio are important prognostic factors in elderly patients with unresectable pancreatic cancer. J Gastrointest Oncol 2016;7:982-8. [Crossref] [PubMed]
- Caparello C, Vivaldi C, Fornaro L, et al. Second-line therapy for advanced pancreatic cancer: evaluation of prognostic factors and review of current literature. Future Oncol 2016;12:901-8. [Crossref] [PubMed]
- Seufferlein T, Bachet JB, Van Cutsem E, et al. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii33-40. [Crossref] [PubMed]
- Walker EJ, Ko AH. Beyond first-line chemotherapy for advanced pancreatic cancer: an expanding array of therapeutic options? World J Gastroenterol 2014;20:2224-36. [Crossref] [PubMed]
- Ciuleanu TE, Pavlovsky AV, Bodoky G, et al. A randomised Phase III trial of glufosfamide compared with best supportive care in metastatic pancreatic adenocarcinoma previously treated with gemcitabine. Eur J Cancer 2009;45:1589-96. [Crossref] [PubMed]
- Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 2011;47:1676-81. [Crossref] [PubMed]
- Rahma OE, Duffy A, Liewehr DJ, et al. Second-line treatment in advanced pancreatic cancer: a comprehensive analysis of published clinical trials. Ann Oncol 2013;24:1972-9. [Crossref] [PubMed]
- Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016;387:545-57. [Crossref] [PubMed]
- Sinn M, Dälken L, Striefler JK, et al. Second-Line Treatment in Pancreatic Cancer Patients: Who Profits?--Results From the CONKO Study Group. Pancreas 2016;45:601-5. [Crossref] [PubMed]
- Vienot A, Beinse G, Louvet C, et al. Overall Survival Prediction and Usefulness of Second-Line Chemotherapy in Advanced Pancreatic Adenocarcinoma. J Natl Cancer Inst 2017;109. [PubMed]
- Kasuga A, Okano N, Naruge D, et al. Retrospective analysis of fixed dose rate infusion of gemcitabine and S-1 combination therapy (FGS) as salvage chemotherapy in patients with gemcitabine-refractory advanced pancreatic cancer: inflammation-based prognostic score predicts survival. Cancer Chemother Pharmacol 2015;75:457-64. [Crossref] [PubMed]
- Kasuga A, Hamamoto Y, Takeuchi A, et al. Post-progression survival following second-line chemotherapy in patients with advanced pancreatic cancer previously treated with gemcitabine: a meta-analysis. Invest New Drugs 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Nakachi K, Furuse J, Ishii H, et al. Prognostic factors in patients with gemcitabine-refractory pancreatic cancer. Jpn J Clin Oncol 2007;37:114-20. [Crossref] [PubMed]
- Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol 2012;3:105-19. [PubMed]
- Tanaka T, Ikeda M, Okusaka T, et al. Prognostic factors in Japanese patients with advanced pancreatic cancer treated with single-agent gemcitabine as first-line therapy. Jpn J Clin Oncol 2008;38:755-61. [Crossref] [PubMed]
- Tas F, Aykan F, Alici S, et al. Prognostic factors in pancreatic carcinoma: serum LDH levels predict survival in metastatic disease. Am J Clin Oncol 2001;24:547-50. [Crossref] [PubMed]
- Stocken DD, Hassan AB, Altman DG, et al. Modelling prognostic factors in advanced pancreatic cancer. Br J Cancer 2008;99:883-93. [Crossref] [PubMed]
- Ben Q, An W, Wang L, et al. Validation of the pretreatment neutrophil-lymphocyte ratio as a predictor of overall survival in a cohort of patients with pancreatic ductal adenocarcinoma. Pancreas 2015;44:471-7. [PubMed]
- Maréchal R, Demols A, Gay F, et al. Prognostic factors and prognostic index for chemonaïve and gemcitabine-refractory patients with advanced pancreatic cancer. Oncology 2007;73:41-51. [Crossref] [PubMed]
- Faloppi L, Del Prete M, Casadei Gardini A, et al. The correlation between LDH serum levels and clinical outcome in advanced biliary tract cancer patients treated with first line chemotherapy. Sci Rep 2016;6:24136. [Crossref] [PubMed]
- Stotz M, Szkandera J, Seidel J, et al. Evaluation of uric acid as a prognostic blood-based marker in a large cohort of pancreatic cancer patients. PLoS One 2014;9:e104730 [Crossref] [PubMed]
- Tas F, Karabulut S, Bilgin E, et al. Clinical significance of coagulation assays in metastatic pancreatic adenocarcinoma. J Gastrointest Cancer 2013;44:404-9. [Crossref] [PubMed]