
Serum VEGF during chemo-radiotherapy and its clinical significance in esophageal squamous cell carcinoma
Introduction
Esophageal squamous cell carcinoma (ESCC), a malignant tumor of the digestive system (1,2), accounts for approximately 80% of all esophageal cancer (EC) cases worldwide. More than 60% of patients with EC cannot undergo surgical treatment because they are diagnosed at advanced stages (3). Recently, chemo-radiotherapy (CRT) has been commonly used for the treatment of patients with EC (4). Unfortunately, a majority of EC patients (approximately 60–70%) show poor response after treatment due to local recurrence and/or metastasis with a 5-yr survival rate of 20–42.5% (5-7).
Vascular endothelial growth factor (VEGF), an independent prognostic factor for EC, plays an important role in recurrence and metastasis (8). Our previous studies indicated that increased VEGF in EC correlated with poor prognosis in those receiving radiotherapy. Meanwhile, decreased VEGF levels during/post radiotherapy were associated with satisfactory responses (3,9). Antiangiogenic drugs (e.g., thalidomide) combined with radiotherapy reduced VEGF levels in patients with EC and may improve the prognosis (10). In clinical practice, the alternation of serum VEGF in patients undergoing radiotherapy is usually reflected in the comparison between serum VEGF in the fourth week during radiotherapy or post-radiotherapy and the level determined pre-radiotherapy. However, few studies have been focused on whether the timing of serum VEGF determination is appropriate, as well as whether it reflects VEGF changes in a timely manner.
In the present study, serum VEGF was determined before, during (per 10 Gy) and after radiotherapy. Changes in serum VEGF levels were analyzed, and the correlation between serum VEGF changes and prognosis was explored.
Methods
Clinical data
A total of 76 patients [male: 57, female: 19; Karnofsky (KPS) score: ≥80; age: 44–86 y, median age: 67 y] with ESCC treated for the first time in the Department of radiotherapy of Changzhou No. 2 People’s Hospital between March 2012 and November 2014 were included in this study. Among these patients, 2 had lesions in cervical segments, 17 had lesions in the upper thoracic portion, 27 had lesions in the mid-thoracic portion, and 30 had lesions in the lower thoracic portion. The pathological types were medullary type (n=71), ulcerative type (n=2) and mushroom type (n=3). According to the AJCC esophageal cancer staging system (the 7th Edition) (11), 2 were T1 stage, 4 were T2 stage, 58 were T3 stage, 12 were T4 stage, 10 were N0 stage, 59 were N1 stage, 7 were N2 stage, 2 were TNM I stage, 64 were TNM II stage and 10 were TNM III stage. Thirty healthy individuals (male: 18, female: 12, age: 26–45 y, average: 33.3 y) were selected as controls. Written informed consent was obtained from each patient. The study protocols were approved by the Ethical Committee of Changzhou No. 2 People’s Hospital (No. 2012-S002-01).
Treatment
Patients received CT simulation in the supine position, and CT images were obtained at 5-mm thickness throughout the entire neck and thorax. Treatment plans were generated using a three-dimensional planning system (Pinnacle 3, version 9.3). Radiation was given with 6-MV photon energy using a three-dimensional conformal technique. The gross tumor volume (GTV) included primary tumor (GTVp) and metastatic lymph nodes (GTVn). Clinical target volume (CTV) consisted of GTVp plus 3–5 cm of proximal and distal normal esophagus without lateral margins and GTVn. Planning target volume (PTV) was determined by adding a 1-cm margin around the CTV. Conventional dose fractionation was used to ensure PTV with a radiation dose of 60–66 Gy (30–33 fractions/6–6.6 weeks). Twenty-three patients received single radiotherapy. Fifty-three patients received concurrent chemotherapy (1–2 cycles) using a regimen including paclitaxel (Lvye Pharma, Nanjing, China; d1, 135 mg/m2) and cisplatin (Hanson Pharma, Lianyungang, China; d2–5, 20 mg/m2). Patients received maintenance chemotherapy (1–3 cycles, 21–28 days for each cycle) after radiotherapy.
Measurement of serum VEGF
Peripheral venous blood was collected to determine serum VEGF using a commercial ELISA kit (Pierce Biotech, Rockford, IL, USA), according to the manufacturer’s instructions. Blood sample collection was performed one week before radiotherapy, as well as during (per 10 Gy) and after radiotherapy. Blood samples (2 mL) were centrifuged at 3,000 r/min for 10 min. The obtained sera were stored at −70 °C until use.
Follow-up
The primary lesions in the esophagus were evaluated by barium enema. Treatment efficiency of the lymph node metastatic lesions was evaluated based on the response evaluation criteria in the solid tumors (RECIST 1.1) guidelines (12). Follow-up was carried out every 3 months among those with an overall survival (OS) of 2 y and every 6 months among those with an OS of >2 y. For each visit, data collection including case history, physical examination, complete peripheral blood tests, electrocardiography, ultrasonic examination of the abdomen, esophageal barium radiography and chest CT were performed. Outcomes included OS, progression-free survival (PFS) and local control (LC).
Statistical analysis
All data were analyzed by SPSS 19.0 (SPSS version19.0, Inc., Chicago, IL, USA). The measurement data were presented as the mean ± standard deviation (SD). The Chi-square test was used to compare several groups. Variance analysis was used to compare the means among the multi-group measurement data. The same index measured at various time points was included in the one-way ANOVA with repeated measures. The determination was performed at least in triplicate. Prognosis analysis was carried out using the Kaplan-Meier method and the Log Rank test. Multivariate analysis was performed using the Cox proportional hazards model. P<0.05 was considered statistically significant.
Results
Results of follow-up and survival
All 76 patients completed the therapy. Six patients dropped out from the group due to absence of serum VEGF drawn on time. All patients were followed-up for 12–47 months until December 31, 2015. During follow-up, 48 patients died. The 1-yr OS rate and 1-yr PFS rates were 55.7% and 51.4%, respectively. The median OS and PFS were 15.8 months (95% CI: 10.4–21.2 months) and 12.5 months (95% CI: 7.6–17.5 months), respectively. Thirteen patients showed recurrence within 2 years, and the median recurrence time was 7.7 months (95% CI: 4.9–10.4 months).
Effects of CRT on serum VEGF level
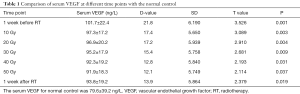
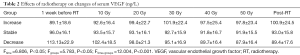
The levels of serum VEGF before, during (10–50 Gy) and after treatment were 101.7±22.4, 97.3±17.2, 96.9±20.2, 95.2±17.9, 92.3±19.2, 91.9±18.3 and 93.8±19.2 ng/L. Compared with the normal control, serum VEGF was substantially higher during and after radiotherapy (P<0.05, Table 1). The standard deviation (SD) of serum VEGF was 8.1 ng/L, and 2 SD was 16.2 ng/L. VEGF with an increase or decrease of 2 SD compared to the baseline level was defined as increased or decreased, respectively. A VEGF change of less than 2 SD was defined as stable. The patient numbers with increased, stable or decreased VEGF were 19, 30 and 21, respectively. One-way ANOVA with repeated measures indicated that the serum VEGF during radiotherapy showed significant decreases compared with those of baseline levels that showed gradual decreases from week 1 to week 5 during radiotherapy (Ftime =6.806, P<0.05; Fgroup =5.783, P<0.05; Ftime×group =12.004, P<0.001, Table 2).
Full table
Full table
Correlation between serum VEGF change and EC prognosis
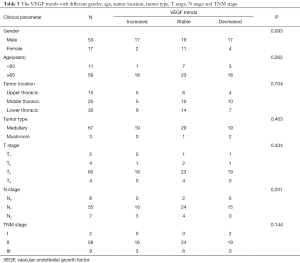
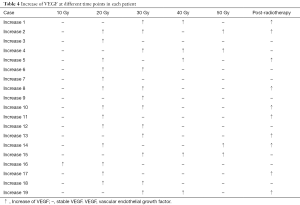
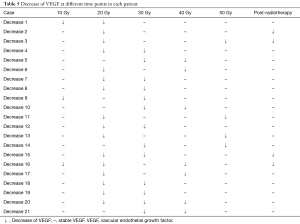
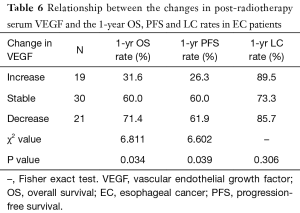
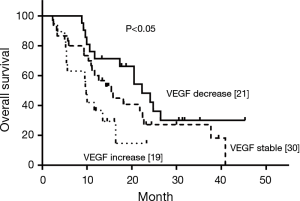
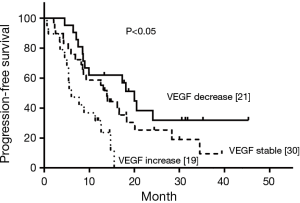
Pre-radiotherapy serum VEGF showed no correlations with OS (r= –0.033, P=0.789) or PFS (r= –0.056, P=0.645). The VEGF changes (increased, stable or decreased) in patients with different gender, ages, tumor locations, tumor types, T stages, N stages, TNM stages and treatment regimens are shown in Table 3. Among the patients with increased VEGF, serum VEGF increased from 10 to 50 Gy during the radiotherapy in 1, 13, 12, 5 and 4 patients, respectively. Ten patients showed decreased serum VEGF post-radiotherapy. Patients with increased VEGF at 10, 40 and 50 Gy also showed increases at 20, 30 Gy and post-radiotherapy (Table 4). Among the patients with decreased VEGF, decreases were observed per 10 Gy during radiotherapy and post-radiotherapy in 3, 16, 13, 7, 4 and 4 patients, respectively. Cases with decreased VEGF at 10, 40, 50 Gy and post-radiotherapy also showed decreases at 20, 30 Gy (Table 5). The 1-yr OS rate, 1-yr PFS rate and 1-yr LC rate of patients are shown in Table 6. Comparison of the OS and PFS are shown in Figures 1 and 2, respectively.
Full table
Full table
Full table
Full table
Prognostic factor analysis
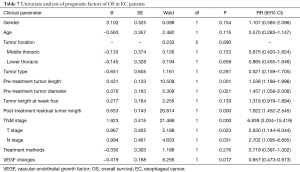
Univariate analysis showed that age, gender, tumor sites and types showed no substantial effects on survival time, while tumor length before and after radiotherapy, tumor diameter, T stage, N stage, TNM stage and VEGF levels had substantial effects on survival time (Table 7). Multivariate analysis showed that TNM stages, VEGF levels, and tumor length after the radiotherapy were prognostic factors for patients (Table 8).
Full table

Full table
Discussion
VEGF, an important cytokine secreted by vascular endothelial cells (VSCs), specifically promotes endothelial cell division and increases capillary permeability (13). In normal cells such as VSCs, esophageal mucosal cells and macrophages, VEGF is involved in vascular density and basic capillary permeability (14,15). In cancer tissues, VEGF is over-expressed and is essential for tumor progression and metastasis (16-18). Studies showed that VEGF was over-expressed in several tumors including ESCC, and high VEGF expression in ESCC was related with progression (19) and/or poor prognosis (13,19-23). Serum VEGF level was positively correlated with tumor load, depth of invasion and lymph node metastasis (20-22).
Our previous study suggested that VEGF levels in patients undergoing CRT were related to radiosensitivity and prognosis (3). Serum VEGF showed significant decreases during radiotherapy in patients with good prognosis, while levels showed significant increases during radiotherapy in those with poor prognosis. This outcome may be related to the self-protection of cancer cells against vascular toxicities induced by irradiation. In the present study, the clinical value of VEGF for patients undergoing CRT was evaluated, showing that high expression levels of VEGF were associated with poor prognosis and OS.
VEGF expression in tumor tissues is closely related with radiotherapy sensitivity, manifested by higher serum VEGF levels in those with poor radiosensitivity (24). To date, several studies have been conducted to investigate the relationship between pre-radiotherapy serum VEGF level and prognosis, however, the results remain controversial. For example, Rades et al. showed that pre-radiotherapy VEGF levels were negatively correlated with the prognosis in local advanced ESCC (25). Our previous study showed that pre-radiotherapy serum VEGF levels had no correlation with prognosis (3). Cheng et al. (26) found that pre-radiotherapy VEGF levels were negatively correlated with PFS and had no correlation with OS. Yoon et al. (27) reported that high VEGF levels before radiotherapy were positively correlated with complete response after radiotherapy. Our data showed that pre-radiotherapy serum VEGF levels showed no correlation with OS or PFS. Pre-radiotherapy VEGF levels had no correlation with LC rate.
The combination of anti-angiogenic agent(s) and radiotherapy contributed to the decrease in VEGF, possibly being promising for the treatment of cancer patients with VEGF increases (28-32). The prognosis of patients with decreased serum VEGF during the radiotherapy showed more satisfactory responses than those without. For these patients, anti-angiogenic therapy caused no additional benefits (10). To date, there remains no consensus regarding the selection of patients with increased VEGF level during radiotherapy, as well as the appropriate time for the administration of anti-angiogenic agent(s) over time. In our study, VEGF levels were determined before, after, and every 10 Gy during radiotherapy, demonstrating that the VEGF increased at 20 and 30 Gy during radiotherapy and post-radiotherapy. Patients with decreased VEGF showed decreased VEGF at 20 and 30 Gy during radiotherapy. Thus, 20 and 30 Gy during radiotherapy or post-radiotherapy may be the suitable time for determination of VEGF levels based on our data. Future studies are needed to confirm this aspect.
In conclusion, changes in VEGF levels were associated with prognosis in ESCC receiving CRT. VEGF levels during radiotherapy may serve as a factor for evaluating radiosensitivity and prognosis. Doses of 20 and 30 Gy during radiotherapy or post-radiotherapy may be the suitable times for the determination of VEGF levels. Our study provided a theoretical basis for the management of ESCC in clinical practice.
Acknowledgments
Funding: This study was supported by National Natural Science Foundation of China (11705095); Key Science & Technology Program of Changzhou Municipal Commission of Health and family planning (ZD200710); Changzhou High Level Health Personnel Training Program (2016C2BJ007); Changzhou Scientific and Technological Support Social Development Project (CE20165024) and Changzhou Municipal Science and Technology Bureau Basic Research Project (CJ20159050).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.08.32). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocols were approved by the Ethical Committee of Changzhou No. 2 People’s Hospital (No. 2012-S002-01) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Yu JP, Lu WB, Wang JL, et al. Pathologic response during chemo-radiotherapy and variation of serum VEGF levels could predict effects of chemo-radiotherapy in patients with esophageal cancer. Asian Pac J Cancer Prev 2015;16:1111-6. [Crossref] [PubMed]
- Yu J, Liu F, Sun Z, et al. The enhancement of radiosensitivity in human esophageal carcinoma cells by thalidomide and its potential mechanism. Cancer Biother Radiopharm 2011;26:219-27. [Crossref] [PubMed]
- Clavier JB, Antoni D, Atlani D, et al. Baseline nutritional status is prognostic factor after definitive radiochemotherapy for esophageal cancer. Dis Esophagus 2014;27:560-7. [Crossref] [PubMed]
- Jing Z, Chen T, Zhang X, et al. Long-term outcome of concurrent chemoradiotherapy with elective nodal irradiation for inoperable esophageal cancer. Cancer Sci 2017;108:1828-33. [Crossref] [PubMed]
- Xi M, Xu C, Liao Z, et al. Comparative Outcomes After Definitive Chemoradiotherapy Using Proton Beam Therapy Versus Intensity Modulated Radiation Therapy for Esophageal Cancer: A Retrospective, Single-Institutional Analysis. Int J Radiat Oncol Biol Phys 2017;99:667-76. [Crossref] [PubMed]
- Cellini F, Valentini V. Targeted therapies in combination with radiotherapy in oesophageal and gastroesophageal carcinoma. Curr Med Chem 2014;21:990-1004. [Crossref] [PubMed]
- Wang J, Yu JP, Wang JL, et al. Pathologic response and changes of serum VEGF during chemoradiotherapy may predict prognosis in non-surgical patients with esophageal carcinoma. Zhonghua Zhong Liu Za Zhi 2016;38:589-95. [PubMed]
- Yu JP, Sun SP, Sun ZQ, et al. Clinical trial of thalidomide combined with radiotherapy in patients with esophageal cancer. World J Gastroenterol 2014;20:5098-103. [Crossref] [PubMed]
- Huang Y, Guo W, Shi S, et al. Evaluation of the 7(th) edition of the UICC-AJCC tumor, node, metastasis classification for esophageal cancer in a Chinese cohort. J Thorac Dis 2016;8:1672-80. [Crossref] [PubMed]
- Odawara S, Kitajima K, Katsuura T, et al. Tumor response to neoadjuvant chemotherapy in patients with esophageal cancer assessed with CT and FDG-PET/CT - RECIST 1.1 vs. PERCIST 1.0. Eur J Radiol 2018;101:65-71. [Crossref] [PubMed]
- Thomas KA. Vascular endothelial growth factor, a potent and selective angiogenic agent. J Biol Chem 1996;271:603-6. [Crossref] [PubMed]
- Bedoya F, Meneu JC, Macias MI, et al. Mutation in CNR1 gene and VEGF expression in esophageal cancer. Tumori 2009;95:68-75. [Crossref] [PubMed]
- Lu JJ, Ma J, Miao R, et al. Expression of vascular endothelial growth factor D in human esophageal squamous cell carcinoma tissue and its significance. Zhonghua Wei Chang Wai Ke Za Zhi 2013;16:1191-4. [PubMed]
- Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist 2004;9:2-10. [Crossref] [PubMed]
- Kimura S, Kitadai Y, Tanaka S, et al. Expression of hypoxia-inducible factor (HIF)-1alpha is associated with vascular endothelial growth factor expression and tumour angiogenesis in human oesophageal squamous cell carcinoma. Eur J Cancer 2004;40:1904-12. [Crossref] [PubMed]
- Möbius C, Stein HJ, Becker I, et al. Vascular endothelial growth factor expression and neovascularization in Barrett's carcinoma. World J Surg 2004;28:675-9. [Crossref] [PubMed]
- Yoon MS, Nam TK, Lee JS, et al. VEGF as a predictor for response to definitive chemoradiotherapy and COX-2 as a prognosticator for survival in esophageal squamous cell carcinoma. J Korean Med Sci 2011;26:513-20. [Crossref] [PubMed]
- Ogata Y, Fujita H, Yamana H, et al. Expression of vascular endothelial growth factor as a prognostic factor in node-positive squamous cell carcinoma in the thoracic esophagus: long-term follow-up study. World J Surg 2003;27:584-9. [Crossref] [PubMed]
- Shih CH, Ozawa S, Ando N, et al. Vascular endothelial growth factor expression predicts outcome and lymph node metastasis in squamous cell carcinoma of the esophagus. Clin Cancer Res 2000;6:1161-8. [PubMed]
- Srivastava VK, Gara RK, Rastogi N, et al. Serum vascular endothelial growth factor-A (VEGF-A) as a biomarker in squamous cell carcinoma of head and neck patients undergoing chemoradiotherapy. Asian Pac J Cancer Prev 2014;15:3261-5. [Crossref] [PubMed]
- Tzao C, Lee SC, Tung HJ, et al. Expression of hypoxia-inducible factor (HIF)-1alpha and vascular endothelial growth factor (VEGF)-D as outcome predictors in resected esophageal squamous cell carcinoma. Dis Markers 2008;25:141-8. [Crossref] [PubMed]
- Yoshikawa R, Fujiwara Y, Koishi K, et al. Cyclooxygenase-2 expression after preoperative chemoradiotherapy correlates with more frequent esophageal cancer recurrence. World J Gastroenterol 2007;13:2283-8. [Crossref] [PubMed]
- Rades D, Golke H, Schild SE, et al. Impact of VEGF and VEGF receptor 1 (FLT1) expression on the prognosis of stage III esophageal cancer patients after radiochemotherapy. Strahlenther Onkol 2008;184:416-20. [Crossref] [PubMed]
- Cheng JC, Graber MS, Hsu FM, et al. High serum levels of vascular endothelial growth factor-A and transforming growth factor-beta1 before neoadjuvant chemoradiotherapy predict poor outcomes in patients with esophageal squamous cell carcinoma receiving combined modality therapy. Ann Surg Oncol 2014;21:2361-8. [Crossref] [PubMed]
- Yoon HH, Catalano PJ, Murphy KM, et al. Genetic variation in DNA-repair pathways and response to radiochemotherapy in esophageal adenocarcinoma: a retrospective cohort study of the Eastern Cooperative Oncology Group. BMC Cancer 2011;11:176. [Crossref] [PubMed]
- Sekis I, Gerner W, Willmann M, et al. Effect of radiation on vascular endothelial growth factor expression in the C2 canine mastocytoma cell line. Am J Vet Res 2009;70:1141-50. [Crossref] [PubMed]
- Yu J, Liu F, Sun M, et al. Enhancement of radiosensitivity and the potential mechanism on human esophageal carcinoma cells by tetrandrine. Cancer Biother Radiopharm 2011;26:437-42. [Crossref] [PubMed]
- Karar J, Maity A. Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol Ther 2009;8:1994-2001. [Crossref] [PubMed]
- Zhou R, Xiao Z, Liao Y, et al. Enhancement of radio sensitivity in nasopharyngeal cancer cells by the own regulation of VEGF expression after adenovirus-E1A gene therapy. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2008;22:933-6. [PubMed]
- Xueguan L, Xiaoshen W, Yongsheng Z, et al. Hypoxia inducible factor-1 alpha and vascular endothelial growth factor expression are associated with a poor prognosis in patients with nasopharyngeal carcinoma receiving radiotherapy with carbogen and nicotinamide. Clin Oncol (R Coll Radiol) 2008;20:606-12. [Crossref] [PubMed]


