Effect of 17β-estradiol on the migration ability of human osteosarcoma U2OS cells
Introduction
Osteosarcoma is the most common bone malignancy in children and adolescents. Moreover, 74% of patients with pulmonary metastasis exhibit poor response to chemotherapy and poor prognosis (1). Therefore, it is vital to explore new treatment options to improve the prognosis of osteosarcoma patients with lung metastasis. Osteosarcoma is a disease exhibiting hyperactivity and abnormal proliferation of the osteo blasts and occurs in children and adults between ages 10 and 20 years old, during which period sex hormones are the most active, suggesting that this disease could be related to the in vivo estrogen levels and the abnormal expression of related receptors. Currently, molecular mechanisms of the effect of estrogen and estrogen receptors on tumor metastasis are mainly reported in breast cancer. According to previous studies, estrogen plays a dual role in the occurrence and development of breast cancer; it promotes the growth of breast cancer cells at low concentrations but its long-term deficiency mediates apoptosis of breast cancer cells (2). Studies have reported that through interaction with ERα-Sp1, estradiol (E2) can increase the expression of integrinα5, thereby reducing cell viability and invasiveness (3). Similarly, in special cases, E2 can activate the adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK) pathway and c-Jun N-terminal kinase (JNK) pathway or inhibit the nuclear factor (NF-kB) pathway, thus regulating the proliferation and invasion of tumor cells (4,5). Certain studies have also confirmed that the occurrence and development of osteosarcoma is closely related to estrogen and ER subtypes. There have been other reports on estrogen promoting the growth of osteosarcoma cells (6-9); it has been recently found that high concentrations of estrogen can inhibit the proliferation of osteosarcoma cells (10). However, there is no research on its effect on the migration ability of osteosarcoma cells. Estrogen and its receptors are closely related to the growth of osteosarcoma cells (11,12). Therefore, investigating the mechanisms of estrogen, through estrogen receptors, in regulating the growth and metastasis of osteosarcoma cells is of great significance, and this can lay the basis for endocrine therapies of osteosarcoma. We investigated the effect of high-concentration 17β-estradiol on the migration ability of U2OS cells, hoping to unveil the effect of different concentrations of estrogen on the migration ability of osteosarcoma cells and lay a theoretical foundation for the treatment of metastatic osteosarcoma.
Methods
Growth test (CCK-8)
The cell counting kit-8 (CCK-8) test was conducted to show surviving cells is widely used in the determination of the activity bioactive factors, antitumor drug screening, cell proliferation test, cytotoxicity test and drug sensitivity test (13). U2OS cells (ATCC cell library, purchased from Tongpaibio, Shanghai, China) at a good growth stage were plated at a density of 1×104 cells per well in 96-well plates. 17β-estradiol [Cayman (No.50-28-2) was added at six concentrations: 0, 10−12, 10−10, 10−8, 10−6, 10−4 M]. After incubation for 72 h at 37 °C and 5% CO2, the plate was stained with CCK-8 and absorbance was measured at λ =450 nm. Meanwhile, the cell proliferation rate of each group was measured according to the following equation: cell proliferation rate (%) = (absorbance of experiment group − absorbance of blank group)/(absorbance of control group − absorbance of blank group) × 100%.
Based on results from the above experiment, five different concentrations of 17β-estradiol were further established together with one blank control group (solvent control): 0, 1/125×10−10, 1/25×10−10, 1/5×10−10, and 10−10 M. Absorbance values were determined by the same method so as to calculate the cell proliferation in each group.
Cell scratch test
Cells were treated with different concentrations of 17β-estradiol: 0, 1/125×10−10, 1/25×10−10, 1/5×10−10, 10−10 M. A line crossing the well behind the 6-well plates was drawn and 500 µL of the cell suspension was cultured in 6-well plates. Next day, another line perpendicular to the line in the wells was drawn. The cells were washed with phosphate-buffered saline (PBS) thrice, the medium was changed, and cells were cultured in an incubator at 37 °C and 5% CO2. Samples were prepared for observation at 0 and 24 h.
According to the above results, five different concentrations of 17β-estradiol were further established together with one blank control group (solvent control): 0, 1/125×10−10, 1/25×10−10, 1/5×10−10, and 10−10 M. The cells were sampled and photographed at 0 and 24hby the same method.
Transwell invasion assay
Cells were treated with different concentrations of 17β-estradiol: 0, 1/125×10−10, 1/25×10−10, 1/5×10−10, 10−10 M. Eighty microliters of diluted Matrigel was added in the Transwell room (4 µg/µL), and Matrigel gel was formed at 37 °C for 30 min; 200 µL 1×105 cells/mL suspension was added in transwell (corning). After incubation for 24 h, the medium was discarded, eliminating the layer of cells that did not cross the membrane. Cells were washed with PBS twice, fixed with methanol for 10 min and stained with 0.1% crystal violet. The cells were dehydrated with 0.1 mL 33% acetic acid, and absorbance values were measured at 570 nm, which could reflect the number of cells.
Morphological observation
Cells were treated with different concentrations of 17β-estradiol: 0, 1/125×10−10, 1/25×10−10, 1/5 × 10−10, 10−10 M. The supernatant was discarded after the treatment period, and cells washed with PBS twice. Next, the cells were fixed with 4% paraformaldehyde at room temperature for 20 min, and washed with PBS three times. Crystal violet staining was performed at room temperature for 3–5 min followed by removal of the supernatant and the cells were then washed with PBS twice.
Statistical analysis
All the data were analyzed for normality and those that were normally distributed were subjected to an independent sample t-test, with P<0.05 considered to indicate statistically significant differences. All statistical analyses were performed using Prism5.
Results
Growth detection (CCK-8)
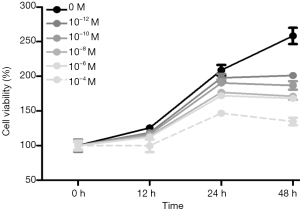
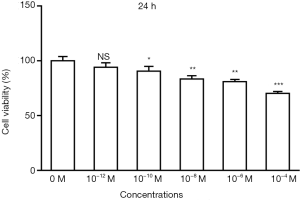
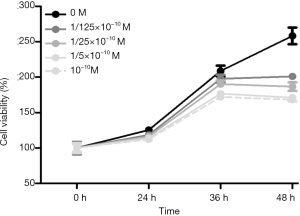
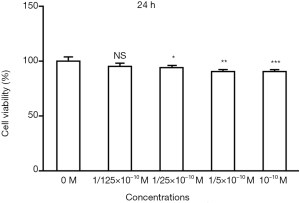
It can be seen from Figures 1,2 that with increase in time, the survival rate of the cells exhibited an initial increasing trend and stabilized or slightly decreased after a period of 24 h at a concentration. The results (Figures 3,4) showed that under the same concentration of 10−10 M, the survival rate of the cells exhibited a similar trend in a concentration-dependent manner. At 24 h, the cell viability decreased with the increase in 17β-estradiol concentration, and exhibited significant changes at 1/25×10−10 M (P<0.05).
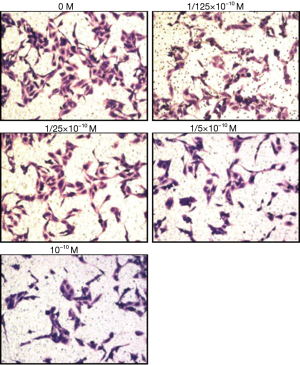
Cell scratch test
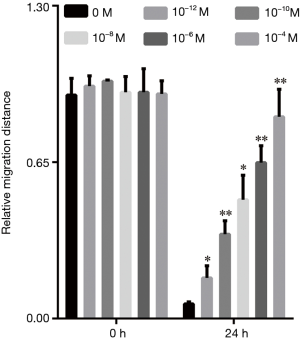
It can be seen from Figures 5,6 that compared to that at 0 h, the cell migration distance at 24 h decreased gradually with the increase in17β-estradiol concentration.
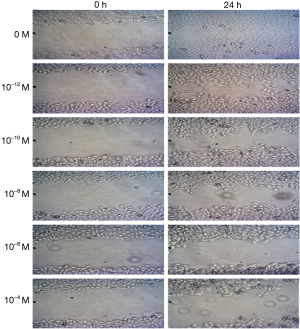
It can be seen from Figures 7,8 that compared to that at 0 h, the cell migration distance at 24 h decreased gradually with the increase in 17β-estradiol concentration; the changes at the concentration of 1/125×10−1 to 10−10 M17β-estradiol were not obvious.
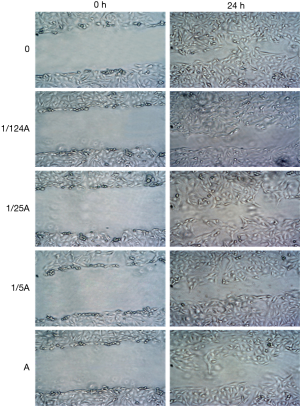
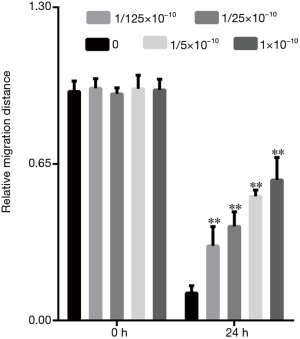
Transwell invasion assay
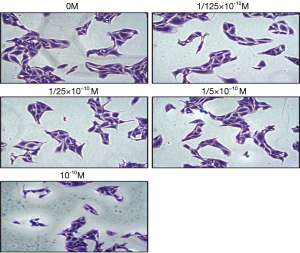
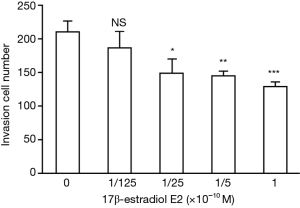
As can be seen from Figures 9,10 (×200), after treatment with 17β-estradiol (0, 1/125×10−10, 1/25×10−10, 1/5×10−10, and 10−10 M), the number of invasive cells significantly decreased with the increase in 17β-estradiol concentration, starting from the concentration of 1/25×10−10 M, indicating that 17β-estradiol can inhibit U2OS cell invasion, and this inhibition effect was positively correlated with its concentration.
Morphological observation
As can be seen from Figure 11, after treatment with 17β-estradiol (0, 1/125×10−10, 1/25×10−10, 1/5×10−10, and 10−10 M), the number of spindle cells decreased with increase in 17β-estradiol concentration; the changes at the concentration of 1/125×10−10 to 1/5×10−10 M were not conspicuous. The results showed that 17β-estradiol can inhibit the EMT transformation of U2OS cells, decreasing the number of spindle-shaped cells while increasing the number of round cells.
Discussion
Osteosarcoma is the most common primary bone malignancy in children and adolescents, and pulmonary metastasis of the tumors is associated with poor prognosis. Chemotherapy is the most important critical method to control lung metastasis, and high-dose methotrexate, doxorubicin, cisplatin, and ifosfamide are considered the most effective chemotherapy drug combination for the treatment of osteosarcoma (14). However, there exist many side effects of chemotherapy, such as bone marrow suppression, gastrointestinal reactions, and liver and kidney dysfunction. These have become important factors that can affect treatment processes and treatment doses (15). Particularly, 74% of patients with lung metastasis respond poorly to chemotherapy, but enhanced chemotherapy is more likely to induce side effects, and even threaten the lives of patients (1). At present, treatment protocols targeting patients with pulmonary metastasis of osteosarcoma have been positively explored, such as monoclonal antibody immunotherapy of tumor-associated antigens (16) or anti-angiogenesis therapy (17), but none have achieved satisfactory results. Therefore, exploring new treatment options to improve the prognosis of patients with lung metastasis of osteosarcoma has become urgent. Since osteosarcoma is a disease with hyperactivity and abnormal proliferation of osteoblasts, which affects age groups of 10–20 years, and this period is also the period when sex hormones are in the most active state, suggesting that this disease is related to the in vivo estrogen level and abnormal expression of corresponding receptors. Currently, the molecular mechanisms of the impact of estrogen and estrogen receptors on tumor metastasis are mainly reported in breast cancer, and according to previous studies, estrogen plays a dual role in the occurrence and development of breast cancer, wherein it promotes the growth of breast cancer cells at low concentrations but its long-term deficiency mediates apoptosis of breast cancer cells (2). Endocrine therapy for osteosarcoma is worthy of further studies and discussions. There have been many reports on the promotive effects of estrogen toward the growth of osteosarcoma cells (6-9), and it has been reported recently that high-concentration estrogen can inhibit the proliferation osteosarcoma cells (10). However, no related research has been focused on the migration ability of osteosarcoma cells. This study explored the impact of high concentrations of estrogen on the migration ability of osteosarcoma U2OS cells.
It can be seen from the results of this study that cell survival was decreased with increase in 17β-estradiol concentration at 24 h, and exhibited significant changes at 1/25×10−10 M (P<0.05). The results of cell scratch test and crystal violet staining also showed that 17β-estradiol (10−10 M) can significantly inhibited the migration ability of U2OS cells. The transwell test proved that with increase in 17β-estradiol concentration, the number of invasive cells began to decrease at 1/25×10−10 M, indicating that 17β-estradiol has a significant inhibitory effect on the migration of U2OS cells, and the degree of inhibition was positively correlated with its concentration. In short, high concentrations of 17β-estradiol can significantly inhibit the migration ability of U2OS cells, the concentration of 10−10 M can significantly inhibit this ability, and this concentration is the lowest for inhibiting the proliferation of U2OS cells. This shows that the inhibitory ability of estrogen toward the migration of osteosarcoma cells is through inhibiting the migration rather than the proliferation of U2OS cells. Furthermore, the inhibition was positively correlated with the concentration of 17β-estradiol. This study has laid the foundation for further exploring estrogen receptor-mediated migration ability of osteosarcoma cells. Currently it has been found that the occurrence and development of osteosarcoma is closely related to estrogen and its ER subtypes. Solakidi et al. (18) reported that ERα is mainly located in the nuclei of osteosarcoma U2OS cells, but ERβ is abundantly expressed in the mitochondria, and its actions are related to the receptors. Dohi et al. (19) found that in MG-63 cells, ERβ accounts for the main part. Monroe et al. (20) reported that the ratio of ERα and ERβ in human osteosarcoma U2OS cells is 1:4. By in vitro experiments of osteosarcoma U2OS cell lines, estradiol-mediated anti-osteoblast apoptosis effects are mainly mediated by ERα and ERβ (21). Our study found that high concentrations of estrogen can inhibit the migration ability of osteosarcoma cells, so it can be speculated that estrogen is also most likely to exhibit its effects through estrogen receptors. The roles of classic estrogen receptor pathways (ERα and ERβ) are the worthiest of investigation. In future, we will further research the roles of estrogen receptors in the inhibitory effects of high concentrations of estrogen toward the migration ability of osteosarcoma cells.
Conclusions
In summary, high concentrations of 17β-estradiol can inhibit the migration ability of osteosarcoma U2OS cells in a concentration-dependent manner beginning from 10−10 M. We will further explore the related mechanisms and relationships of estrogen and the expressions of corresponding receptors with the migration ability of osteosarcoma cells.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.09.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethical approval and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lewis VO, Raymond K, Mirza AN, et al. Outcome of postradiation osteosarcoma does not correlate with chemotherapy response. Clin Orthop Relat Res 2006;60-6. [Crossref] [PubMed]
- Lipovka Y, Konhilas JP. The complex nature of estrogen signaling in breast cancer: enemy or ally? Biosci Rep 2016;36:e00352 [Crossref] [PubMed]
- Sisci D, Middea E, Morelli C, et al. 17β-Estradiol enhances α5 integrin subunit gene expression through ERα-Sp1 interaction and reduces cell motility and invasion of ERα-positive breast cancer cells. Breast Cancer Res Treat 2010;124:63-77. [Crossref] [PubMed]
- Chen H, Wang JP, Santen RJ, et al. Adenosine monophosphate activated protein kinase (AMPK), a mediator of estradiol-induced apoptosis in long-term estrogen deprived breast cancer cells. Apoptosis 2015;20:821-30. [Crossref] [PubMed]
- Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res 2009;11:206. [Crossref] [PubMed]
- Walker MJ, Chaudhuri PK, Beattie CW, et al. Steroid receptors in malignant skeletal tumors. Cancer 1980;45:3004-7. [Crossref] [PubMed]
- Stedman KE, Moore GE, Morgan RT. Estrogen receptor proteins in diverse human tumors. Arch Surg 1980;115:244-8. [Crossref] [PubMed]
- Rajabalian S, Hajializadeh Z, Pooraboli I, et al. Establishment characterization and drug sensitivity of a new Ewing sarcoma cell line. J Pediatr Hematol Oncol 2010;32:e331-7. [Crossref] [PubMed]
- Saraiva PP, Teixeira SS, Conde SJ, et al. The importance of hormone receptor analysis in osteosarcoma cells growth submitted to treatment with estrogen in association with thyroid hormone. Cell Biochem Funct 2008;26:107-10. [Crossref] [PubMed]
- Fang D, Yang H, Lin J, et al. 17β-eEstradiol regulates cell proliferation, colony formation, migration, invasion and promotes apoptosis by upregulating MiR-9 and thus degrades MALAT-1 in osteosarcoma cell MG-63 in an estrogen receptor independent manner. Biochem Biophys Res Commun 2015;457:500-6. [Crossref] [PubMed]
- Chen FP, Hsu T, Hu CH, et al. Expression of estrogen receptors alfa and beta mRNA and alkaline phosphatase in the differentiation of osteoblasts from elderly posmenopausal women comparison with osteoblasts from osteosarcoma cell lines. Taiwanese J Obstet Gynecol 2006;45:307-12. [Crossref] [PubMed]
- Sayeed A, Konduri SD, Liu W, et al. Estrogen receptor A inhibits p53- mediated transcriptional repression Implications for the regulation of apoptosis. Cancer Res 2007;67:7746-55. [Crossref] [PubMed]
- Tominaga H, Ishiyama M, Ohseto F, et al. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal Commun 1999;36:47-50. [Crossref]
- Kalifa C, Laurence B, Le Deley MC. Neoadjuvant treatment in osteosarcomas. Bull Cancer 2006;93:1115-20. [PubMed]
- Bruland OS, Pihl A. On the current management of osteosarcoma. A critical evaluation and a proposal for a modified treatment strategy. Eur J Cancer 1997;33:1725-31. [Crossref] [PubMed]
- Mori K, Rédini F, Gouin F, et al. Osteosarcoma: current status of immunotherapy and future trends Oncol Rep 2006;15:693-700. (Review). [PubMed]
- Quan GM, Choong PF. Anti-angiogenic therapy for osteosarcoma. Cancer Metastasis Rev 2006;25:707-13. [Crossref] [PubMed]
- Solakidi S, Psarra AM, Sekeris CE. Differential subcellular distribution of estrogen receptor isoforms: localization of ERalpha in the nucleoli and ERbeta in the mitochondria of human osteosarcoma SaOS-2 and hepatocarcinoma HepG2 cell lines. Biochim Biophys Acta 2005;1745:382-92. [Crossref] [PubMed]
- Dohi O, Hatori M, Suzuki T, et al. Sex steroid receptors and hormone induced cell proliferation in human osteosarcoma. Cancer Sci 2008;99:518-23. [Crossref] [PubMed]
- Monroe DG, Secreto FJ, Subramaniam M, et al. Estrogen receptor alpha and beta heterodimers exert unique effects on estrogen and tamoxifen dependent gene expression in human U2OS osteosarcoma cells. Mol Endocrinol 2005;19:1555-68. [Crossref] [PubMed]
- Kallio A, Guo T, Lamminen E, et al. Estrogen and the selective estrogen receptor modulator protection against cell death in estrogen receptor alpha and beta expressing U2OS cells. Mol Cell Endocrinol 2008;289:38-48. [Crossref] [PubMed]