
Immuno-targeted combinations in oncogene-addicted non-small cell lung cancer
Introduction
The identification of molecular networks underlying tumor cell growth and proliferation marked a new era in the treatment of lung cancer. Tumor genotyping has been incorporated into therapeutic decision making of advanced non-small cell lung cancer (NSCLC) (1), since has become clear that individuals with actionable molecular alterations receiving a matched targeted agent certainly live longer and better (2,3). The impressive tumor response and clinical benefit observed with tyrosine kinase inhibitors (TKIs) targeting epidermal growth factor receptor (EGFR) (4-6) and anaplastic lymphoma kinase (ALK) (7-9) generated growing emphasis on personalized treatment leading to increasing inclusion of molecular biomarkers in clinical trials. Beyond EGFR and ALK, new tailored drugs effectively targeting both ROS-1 rearrangements and BRAF mutations have been recently approved for clinical use, with several other molecules under different stages of clinical development. The College of American Pathologists (CAP) and the International Association for the Study of Lung Cancer (IASLC) updated recommendations in 2018 include upfront testing for EGFR, ALK, and ROS-1 mutations in all patients with advanced non-squamous NSCLC (10). Routine testing for BRAF, RET, HER2, KRAS, and MET genes by using multiplex gene panels is recommended as part of a broader testing panel or if EGFR, ALK, and ROS-1 testing are negative (11), with the final goal of identifying rare molecular drivers for which effective drugs may be available in the context of clinical trials. The application of precision medicine is leading to a significant improvement of life expectancy in a subset of patients with advanced NSCLC. However intra-tumor heterogeneity and acquired resistance are well-known biological phenomena which might significantly impact sensitivity to specific molecular targeted agents. A deeper understanding of the immune landscape of tumors and immune-evasion process has recently led to breakthrough therapeutic advances for patients with advanced NSCLC. Although the great benefit observed with PD-1/PD-L1 inhibitors regardless of tumor subtype and PD-L1 expression status (12-15), randomized studies suggested lack of efficacy for single agent checkpoint inhibitors in patients with oncogene-addiction (16), thus creating a platform for alternative approaches including combination strategies. The addition of pembrolizumab to standard first-line platinum-chemotherapy resulted in a significant survival benefit for patients with EGFR/ALK wild-type NSCLC (17), while patients with high tumor mutation burden (TMB) seem to derive most benefit from PD-1 plus CTLA-4 checkpoint inhibitors combinations (18). The analysis of available evidence suggested that targeted therapy and immunotherapy have different therapeutic effects with potential complementary and synergistic role in cancer treatment. It’s known that targeted therapy is able to induce dramatic tumor response, thus favouring the release of large amounts of antigens upon tumor cell death. Furthermore it may directly enhance the immune response by modulating activation, effector function, and differentiation of specific subsets of immune cells (19,20). As result of this complex and multifactorial interactions, immunotherapy could consolidate the transient tumor responses achieved with targeted therapy into long-term survival benefit, due to the generation of potent anti-tumor memory. This review briefly summarizes the basis of immunogenicity and immune escape in oncogene addicted NSCLC, providing an updated overview of clinical trials, with the final aim of defining the current unmet needs of immuno-targeted combinations in clinical practice.
EGFR-mutations
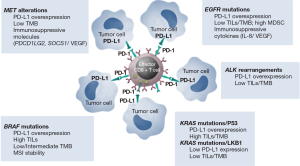
The biological background supporting the use of checkpoint inhibitors in EGFR-mutant NSCLC come from pre-clinical studies showing that EGFR oncogenic signaling could directly induce PD-L1 expression in lung cancer cell lines, thus enhancing sensitivity to PD-1 blockade in pre-clinical models (21,22). However the very low rate of tumor infiltrating lymphocytes (TILs) along with the low TMB and consequent reduced number of “neo-antigens” featuring EGFR-mutated tumors, makes the chances that immunotherapy could trigger an effective immune response rather negligible (23,24). In addition to that, EGFR-mutant NSCLC displayed significant myeloid cell recruitment, while failed to activate a CD8+ immune response (25). Gainor et al. showed that high tumor PD-L1 expression and TILs rate were simultaneously detected only in 1/57 TKI-naïve and 5/57 TKI-resistant EGFR-mutated NSCLC patients with objective response rate (ORR) around 3% (26), suggesting that very few, highly selected patients could gain benefit from immunotherapy. In line with these evidences subgroup analysis of randomized studies included in a recent meta-analysis (16), showed no survival benefit with checkpoint inhibitors in EGFR mutated subset. These data were confirmed in the real-word setting of the Italian expanded access program (EAP), showing ORR of 9% and median OS of 8.3 months in about 150 pre-treated patients with advanced NSCLC harboring EGFR-activating mutations (27). ATLANTIC represented the first prospective trial investigating activity and safety of the PD-L1 inhibitor durvalumab in a cohort of oncogene addicted, pre-treated NSCLC patients. Consistently with previous results the activity of durvalumab was modest in the overall population, with 4% and 12% ORR observed in patients with low and high tumor PD-L1 expression, respectively (28). Considering that EGFR-TKI downregulate PD-L1 expression in a lab setting, clinical studies explored immunotherapy activity also in TKI-naïve, PD-L1 positive, EGFR-mutant NSCLC patients. Modest but encouraging activity was initially observed with atezolizumab in the phase II BIRCH study (29), while pembrolizumab has recently shown lack of efficacy in this setting (30), regardless of tumor PD-L1 expression levels, suggesting that single agent PD-1/PD-L1 inhibitor should not be considered as an appropriate treatment. Since the majority of patients with EGFR-mutant NSCLC do not respond to PD-1 blockade, the development of immune-based combinations remains a crucial unmet need. Pre-clinical studies showed that combination of EGFR-TKIs and PD-1 inhibitors did not induce synergistic tumor cell killing effects in co-culture systems, suggesting that targeting both molecular pathways may have similar but not additional effects on PD-1 mediated antitumor immune response (22). Despite this evidence early clinical trials investigated activity and safety of checkpoint inhibitors and EGFR-TKIs combinations in EGFR-mutant NSCLC and for some of them preliminary results have been recently reported. Nivolumab was combined with erlotinib in a cohort of 20 patients with chemotherapy-naïve, TKI-resistant, EGFR-mutant advanced NSCLC included in the multi-arm phase I CheckMate 012 study, showing ORR: 15%, 2-year PFS rate: 48%, grade 3 adverse events (AEs): 25%, with no grade 4 toxicities. The combination of erlotinib and atezolizumab reached 75% ORR, with 9.7 months of response duration and about 40% grade 3–4 AEs, in a phase Ib study including 20 TKI-naïve EGFR-mutant NSCLC patients (31). Similar activity and safety profiles were observed in 10 TKI-naïve EGFR-mutant NSCLC patients included in a phase I trial of durvalumab plus gefitinib, showing ORR: 78% and any grade AEs: 80%, with no reported grade 3–4 AEs leading to treatment discontinuation (32). Finally the phase Ib multi-arm TATTON trial investigated durvalumab and osimertinib combination in both TKI naïve and pre-treated NSCLC patients. Despite encouraging activity with combination reaching an ORR nearly to 70%, recruitment was early stopped because of high incidence of grade 3–4 AEs and pulmonary toxicity, with interstitial lung disease occurring in 26% of EGFR TKI-pretreated and 64% of EGFR TKI-naïve patients (33). These studies overall suggested that the addition of checkpoint inhibitors to EGFR-TKI do not significantly enhance clinical activity observed with TKI alone, confirming data emerging from pre-clinical models. Furthermore, the high incidence of severe AEs and pulmonary toxicities raise relevant concerns about the use of this kind of combinations in clinical practice.
ALK-rearrangements
Patients with ALK-rearranged NSCLC have higher frequency of tumor PD-L1 overexpression as compared to EGFR or KRAS-mutant disease (25,34). ALK rearrangements upregulated PD-L1 expression by activating PI3K-AKT and MEK-ERK signaling pathways in NSCLC cell lines (35). However, the very low rate of associated TILs along with the lack of an inflammatory microenvironment limited the efficacy of immunotherapy in this tumor subset. Gainor et al. showed that tumor PD-L1 overexpression and high TILs rate were simultaneously detected in none of ALK-rearranged NSCLC patients, with objective response to PD-1 inhibitors of 3.6% as compared to 23.3% reported in those with wild type/unknown EGFR/ALK status (26). In line with these retrospective evidences the preliminary results of the phase II prospective ATLANTIC trial showed no clinical responses to durvalumab in a small cohort of 15 patients with pre-treated ALK-rearranged NSCLC (28). Pre-clinical studies showed that ALK targeted inhibition promoted T-cells interactions with monocytes and tumor cells, enhanced T-cell proliferation as well as cytokine production, and increased T-cell tumor infiltration (36,37), providing biological rationale for combination strategies. The CheckMate 370 phase 1/2 study investigated activity and tolerability of crizotinib plus nivolumab in patients with previously untreated, ALK-rearranged advanced NSCLC. Similarly to TATTON trial, recruitment was early stopped due to the occurrence of severe hepatic toxicities leading to treatment discontinuation in 5/13 (38%) of patients (38). Likewise, combination of ceritinib and nivolumab was associated with high rate of AEs (83%), in a cohort of ALK-positive NSCLC patients, leading to protocol amendment to address observed toxicities (39). The JAVELIN Lung 101 is a phase 1b/2 dose finding trial evaluating two different combinations: crizotinib plus avelumab in 12 pre-treated patients with ALK-negative advanced NSCLC, and lorlatinib plus avelumab in 28 patients with ALK-rearranged NSCLC (40). The preliminary results of this trial revealed that crizotinib plus avelumab was not well tolerated due to the high incidence of dose limiting toxicities (DLT), leading to discontinuation of this combination regimen. Conversely lorlatinib plus avelumab showed manageable safety profile (no DLT observed) along with great antitumor activity (ORR: 46.4%), in a heavily pre-treated population with ALK-rearranged NSCLC, ensuring further development in clinical trials. Similarly alectinib plus atezolizumab also showed an acceptable tolerability profile with no DLT and grade 4–5 AEs in TKI-naïve patients with ALK-positive disease. Early efficacy data were also very promising with ORR of 85% and median duration of response of 20.3 months (41). However additional follow-up is needed to confirm the potential survival benefit of these combinations including checkpoint inhibitors and second/third generation ALK-TKIs in these molecular selected NSCLC patients.
KRAS mutations
KRAS represents the most common oncogene driver detected in about 30% of non-squamous NSCLC (42) with no effective targeted therapy available yet for clinical use. Pre-clinical and clinical evidences suggested that KRAS-mutant tumors are characterized by high PD-L1 expression and the presence of CD8+ TILs (43). Beyond PD-L1, the cross-talk between cancer cells intrinsic RAS signaling and tumor microenvironment has different other potential immune-modulating effects, including regulation of immune T-cells and myeloid cells density, cancer associated fibroblasts and endothelial cells properties, and extra-cellular matrix (ECM) composition, with significant impact on tumor immune-escape, growth and metastatic process (44). Clinical trials and recent meta-analysis showed a greater benefit of PD-1 inhibitors in KRAS-mutant subgroups, however lack of significant difference precluded any definitive conclusion (45). More recently has become clear that KRAS-mutant NSCLC is a heterogeneous disease, with patients harboring simultaneous KRAS/P53 mutations deriving the greatest and durable benefit from PD-1 blockade, because of the high PD-L1 expression, CD8+ T-cells and TMB associated with this molecular subtype (46). Conversely co-occurring inactivation of LKB1 was associated with lack of tumor response and survival benefit in patients with KRAS-mutant lung adenocarcinoma, likely due to the low PD-L1 expression and paucity of infiltrating CD8+ TILs, suggesting LKB1-loss as a genomic biomarker of innate resistance to PD-1 blockade (47). The molecular subtyping of KRAS-mutant NSCLC supports the development of immuno-targeted combinations strategies which require further investigation in prospective clinical trials including larger cohorts of patients.
BRAF mutations
Recent evidences revealed that v-Raf murine sarcoma viral oncogene homolog B (BRAF)-mutant NSCLC is characterized by high tumor PD-L1 expression, low TMB and microsatellite-stable status (48), thus questioning the efficacy of immunotherapy in this subgroup of patients. Since none of randomized trials with checkpoint inhibitors reported survival outcomes in this specific subset, preliminary data emerging from retrospective series revealed that immunotherapy has a favorable clinical activity with ORR of 28% and median PFS of 3.9 months, which is comparable to that observed in the unselected population (48). These data have been recently confirmed by a retrospective multicenter study including patients with advanced NSCLC harboring different genomic alterations, showing ORR of 24%, PFS: 2.9 months, and OS: 17.2 months for BRAF- mutant subgroup (49). Immunotherapy activity observed in BRAF-mutant NSCLC was somewhat higher than that observed in other specific molecular subtypes, with no significant association to the tumor PD-L1 expression status. An extensive immunogenomic analysis of more than 9,000 tumors showed that BRAF mutations are correlated with high leucocyte levels across 33 different cancer types analyzed by The Cancer Genome Atlas (TCGA) (50). Furthermore, both RAS and BRAF V600 mutations are among the most frequently predicted neoantigens in cancer and could thus be directly steering immune response (51). The PanCancer Atlas analysis has recently revealed the complex relationship between molecular signaling and immune-cell composition of tumor microenvironment. These data revealed that BRAF-driven cancers are associated with an inflammatory immune subtype and are characterized by higher CD8+ TILs than NRAS-driven tumors, identifying a signaling loop where simultaneous targeting of BRAF and PD-L1 might have synergistic effects (50). Taken together these evidences support the design of prospective clinical trials of immuno-targeted combinations in BRAF-mutant advanced NSCLC.
MET alterations
MET-driven NSCLC include both high-level MET amplification and MET exon 14 skipping mutations (METex14), overall accounting for 3–7% of this molecular subtype (3). Pre-clinical studies showed that MET-positive lung cancer cell lines were characterized by high PD-L1-expression levels regardless from the IFNγ-mediated pathway. However the oncogenic activation of MET signaling induced an immunosuppressive tumor microenvironment through the transcriptional control of immunosuppressive molecules (i.e., PDCD1LG2, SOCS1) and pro-angiogenic factors (VEGFA and NRP1) (52). Although PD-L1 expression ≥50% has been detected in about half of patients with advanced NSCLC harboring MET exon 14 skipping mutations, clinical responses to immunotherapy were generally poor, with ORR of 13% in the overall population and 30% in selected patients with high PD-L1 levels (53). The lack of efficacy observed in this subset of patients could be partially ascribed to the low TMB found in the majority of METex14-positive tumor samples analyzed within large retrospective series. Interestingly the average TMB observed in patients with METex14-driven NSCLC was 6.9 mutations per MB, thus significantly lower than 10.7 mutations per MB reported for all lung cancer cases (54) but somewhat higher than the average of 4.5 and 2.8 mutations per MB, respectively observed with EGFR-mutated and ALK-rearranged NSCLC patients (23). Conversely high MET expression, defined as MET IHC 3+ or MET H-Score in the upper quartile, was associated with favorable survival outcomes in patients with advanced NSCLC receiving checkpoint-inhibitors, regardless of smoking history, PD-L1 expression or KRAS mutations (55). Overall these data suggest that MET-driven NSCLC is a heterogeneous disease with different tumor biology, supporting the development of biomarker driven combination strategies.
Rare oncogenic drivers
The evidence regarding immunotherapy efficacy in advanced NSCLC patients with rare oncogene drivers, including c-Ros oncogene 1 (ROS1), erythroblastic leukemia viral oncogene homolog 2 (Her2), “rearranged during transfection” proto-oncogene (RET), and neurotrophic tyrosine kinase receptor (NTRK) is very limited. Preliminary results of retrospective studies have recently shown very low activity of single agent PD-1 inhibitors in a limited cohort of pre-treated NSCLC patients harboring Her2 alterations and RET rearrangements, with an ORR of 6–7% and a median PFS: 2.1–2.4 months in both oncogene addicted cohorts (49). NSCLC harboring rare oncogenic drivers is usually characterized by never-smoking status and low TMB, however future analysis is needed to further clarify the role of immuno-targeted combinations in these NSCLC subtypes.
Conclusions
The recent sequencing of human genome revealed that lung cancer is the product of dynamic molecular networks, including complex cross-talk between cancer cells intrinsic molecular pathways and tumour microenvironment with significant impact on immunomodulating process. The majority of lung cancer immunotherapies act to re-invigorate pre-existing immunity that has been suppressed in the tumor microenvironment by therapeutic blocking of immune-checkpoints PD-1/PD-L1, providing a relatively anti-tumor-specific immune response. Pre-clinical studies indicated that oncogenic signaling may directly induce PD-L1 expression in lung cancer models providing biological rational to combine different treatment modalities such as targeted therapy and immunotherapy in oncogene addicted NSCLC (Figure 1). The complementary therapeutic effects of these two different approaches suggested intriguing potential for therapeutic synergy with combination strategies. However, the great emphasis and expectations linked to immune-targeted combinations have been mostly disappointed by preliminary results emerging from early-phase trials. The addition of checkpoint inhibitors to both first and third generation EGFR-TKIs showed to not significantly enhance clinical activity observed with EGFR-TKI alone in TKI naïve and pre-treated NSCLC patients, at cost of unexpected high incidence of AEs, resulting in the limitation of further active investigation. In line with pre-clinical evidence these data suggest that targeting both molecular pathways may have similar but not additional/synergistic effects on PD-1 mediated antitumor immune response, raising critical concerns about clinical development of such combination. Similarly phase 1/2 clinical studies in ALK-positive NSCLC showed that combining PD-1/PD-L1 inhibitors with first-generation ALK-TKI was not well tolerated. Conversely the addition of PD-L1 blockers to second-third generation TKIs has recently shown manageable safety profile along with great antitumor activity, ensuring further investigation in larger clinical trials. The clinical evaluation of immunotherapy activity in NSCLC harboring oncogenic drivers other than EGFR and ALK has primarily involved single agent PD-1/PD-L1 inhibitors. The few available evidences emerging from subgroup analysis and retrospective series overall suggested that oncogene addicted NSCLC should be considered a heterogeneous disease with different immunological background and clinical response to immunotherapy according to the specific molecular subtype. Tumors with both KRAS/P53 and BRAF mutations seem to derive major benefit from immunotherapy as compared to LKB1, cMET, HER2 or RET-driven disease. However, these data are still immature and additional long-term follow-up are warranted. Unfortunately, the low prevalence of oncogenic events in advanced NSCLC limits the possibility to address this question in a prospective manner, making the design of clinical trials a real challenge. Other opened questions to be addressed in the near future include timing, dosage, and sequence of combined treatments, in order to optimize the balance between overall anti-tumor effects and toxicity profiles. A deeper understanding of the complex interplay between oncogenic tumor cell signaling, immune cells and tumor microenvironment, identification of reliable predictive biomarkers of response, and characterization of the immune-modulating effects induced by targeted inhibition, will likely help to determine the best ways to combine targeted agents and immunotherapy in the treatment of oncogene addicted NSCLC patients. Technological and computational innovations will be instrumental to overcome existing challenges and to develop a comprehensive model integrating all these parameters to accurately predict patients’ response and tolerability to immuno-targeted combinations.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Umberto Malapelle, Christian Rolfo) for the series “Targeted Therapy and Non-Small Cell Lung Cancer: A New Era?” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.10.04). The series “Targeted Therapy and Non-Small Cell Lung Cancer: A New Era?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1-27. [Crossref] [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]
- Sholl LM, Aisner DL, Varella-Garcia M, et al. Multi-institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: The Lung Cancer Mutation Consortium Experience. J Thorac Oncol 2015;10:768-77. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:841-9. [Crossref] [PubMed]
- Mok T, Kim DW, Wu YL, et al. First-line Crizotinib versus Pemetrexed Cisplatin or Pemetrexed Carboplatin in Patients with Advanced ALK-Positive Non-Squamous Non Small-Cell Lung Cancer: Results of a Phase III Study (PROFILE 1014). J Clin Oncol 2014;32:abstr 8002.
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn 2018;20:129-59. [Crossref] [PubMed]
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw 2018;16:807-21. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 2017;12:403-7. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Nefedova Y, Cheng P, Gilkes D, et al. Activation of dendritic cells via inhibition of Jak2/STAT3 signaling. J Immunol 2005;175:4338-46. [Crossref] [PubMed]
- Nefedova Y, Nagaraj S, Rosenbauer A, et al. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res 2005;65:9525-35. [Crossref] [PubMed]
- Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63. [Crossref] [PubMed]
- Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol 2015;10:910-23. [Crossref] [PubMed]
- Spigel DR. et al. Total mutation burden (TMB) in lung cancer (LC) and relationship with response to PD-1/PD-L1 targeted therapies. J Clin Oncol 2016;34: abstr 9017.
- Offin M, Rizvi H, Tenet M, et al. Tumor Mutation Burden and Efficacy of EGFR-Tyrosine Kinase Inhibitors in Patients with EGFR-Mutant Lung Cancers. Clin Cancer Res 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Busch SE, Hanke ML, Kargl J, et al. Lung Cancer Subtypes Generate Unique Immune Responses. J Immunol 2016;197:4493-503. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Garassino MC, Gelibter AJ, Grossi F, et al. Italian Nivolumab Expanded Access Program in Nonsquamous Non-Small Cell Lung Cancer Patients: Results in Never-Smokers and EGFR-Mutant Patients. J Thorac Oncol 2018;13:1146-55. [Crossref] [PubMed]
- Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018;19:521-36. [Crossref] [PubMed]
- Peters S, Gettinger S, Johnson ML, et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J Clin Oncol 2017;35:2781-9. [Crossref] [PubMed]
- Lisberg A, Cummings A, Goldman JW, et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naïve Patients With Advanced NSCLC. J Thorac Oncol 2018;13:1138-45. [Crossref] [PubMed]
- Gettinger S, Hellmann MD, Chow LQM, et al. Nivolumab Plus Erlotinib in Patients With EGFR-Mutant Advanced NSCLC. J Thorac Oncol 2018;13:1363-72. [Crossref] [PubMed]
- Gibbons DL, Chow LQ, Kim DW, et al. 57O Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): A phase I expansion in TKI-naïve patients (pts) with EGFR mutant NSCLC. J Thorac Oncol 2016;11:S79. [Crossref]
- Ahn MJ, Yang J, Yu H, et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol 2016;11:S115. [Crossref]
- Koh J, Jang JY, Keam B, et al. EML4-ALK enhances programmed cell death-ligand 1 expression in pulmonary adenocarcinoma via hypoxia-inducible factor (HIF)-1α and STAT3. Oncoimmunology 2015;5:e1108514. [Crossref] [PubMed]
- Ota K, Azuma K, Kawahara A, et al. Induction of PD-L1 Expression by the EML4-ALK Oncoprotein and Downstream Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res 2015;21:4014-21. [Crossref] [PubMed]
- Zhou P, Shaffer DR, Alvarez Arias DA, et al. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature 2014;506:52-7. [Crossref] [PubMed]
- Vladimer GI, Snijder B, Krall N, et al. Global survey of the immunomodulatory potential of common drugs. Nat Chem Biol 2017;13:681-90. [Crossref] [PubMed]
- Spigel DR, Reynolds C, Waterhouse D, et al. Phase 1/2 Study of the Safety and Tolerability of Nivolumab Plus Crizotinib for the First-Line Treatment of Anaplastic Lymphoma Kinase Translocation - Positive Advanced Non-Small Cell Lung Cancer (CheckMate 370). J Thorac Oncol 2018;13:682-8. [Crossref] [PubMed]
- Felip E, De Braud FG, Maur M, et al. Ceritinib plus nivolumab (NIVO) in patients (pts) with anaplastic lymphoma kinase positive (ALK+) advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2017;35:abstr 2502.
- Shaw AT, Lee SH, Ramalingam SS, et al. Avelumab (anti–PD-L1) in combination with crizotinib or lorlatinib in patients with previously treated advanced NSCLC: Phase 1b results from JAVELIN Lung 101. J Clin Oncol 2018;36:abstr 9008.
- Kim DW, Gadgeel SM, Gettinger SN, et al. Safety and clinical activity results from a phase Ib study of alectinib plus atezolizumab in ALK+ advanced NSCLC (aNSCLC). J Clin Oncol 2018;36:abstr 9009.
- Sholl LM, Aisner DL, Varella-Garcia M, et al. Multi-institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: The Lung Cancer Mutation Consortium Experience. J Thorac Oncol 2015;10:768-77. [Crossref] [PubMed]
- Kim DW, Gadgeel SM, Gettinger SN, et al. Immune marker profiling and PD-L1, PD-L2 expression mechanisms across non–small cell lung cancer mutations. J Clin Oncol 2017;35:abstr 9076.
- Dias Carvalho P, Guimarães CF, Cardoso AP, et al. KRAS Oncogenic Signaling Extends beyond Cancer Cells to Orchestrate the Microenvironment. Cancer Res 2018;78:7-14. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Clinical and Molecular Characteristics Associated With Survival Among Patients Treated With Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:210-6. [Crossref] [PubMed]
- Dong ZY, Zhong WZ, Zhang XC, et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res 2017;23:3012-24. [Crossref] [PubMed]
- Skoulidis F, Byers LA, Diao L, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov 2015;5:860-77. [Crossref] [PubMed]
- Dudnik E, Peled N, Nechushtan H, et al. BRAF Mutant Lung Cancer: Programmed Death Ligand 1 Expression, Tumor Mutational Burden, Microsatellite Instability Status, and Response to Immune Check-Point Inhibitors. J Thorac Oncol 2018;13:1128-37. [Crossref] [PubMed]
- Mhanna L, Milia J, Lusque A, et al. Efficacy of immune-checkpoint inhibitors (ICI) in non-small cell lung cancer (NSCLC) patients harboring activating molecular alterations (ImmunoTarget). J Clin Oncol 2018;36:abstr 9010.
- Sanchez-Vega F, Mina M, Armenia J, et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018;173:321-337.e10. [Crossref] [PubMed]
- Thorsson V, Gibbs DL, Brown SD, et al. The Immune Landscape of Cancer. Immunity 2018;48:812-830.e14. [Crossref] [PubMed]
- Saigi M, Alburquerque-Bejar JJ, Mc Leer-Florin A, et al. MET-Oncogenic and JAK2-Inactivating Alterations Are Independent Factors That Affect Regulation of PD-L1 Expression in Lung Cancer. Clin Cancer Res 2018;24:4579-87. [Crossref] [PubMed]
- Sabari JK, Montecalvo J, Chen R, et al. PD-L1 expression and response to immunotherapy in patients with MET exon 14-altered non-small cell lung cancers (NSCLC). J Clin Oncol 2017;35:abstr 8512.
- Schrock AB, Frampton GM, Suh J, et al. Characterization of 298 Patients with Lung Cancer Harboring MET Exon 14 Skipping Alterations. J Thorac Oncol 2016;11:1493-502. [Crossref] [PubMed]
- Reis H, Metzenmacher M, Goetz M, et al. MET Expression in Advanced Non-Small-Cell Lung Cancer: Effect on Clinical Outcomes of Chemotherapy, Targeted Therapy, and Immunotherapy. Clin Lung Cancer 2018;19:e441-63. [Crossref] [PubMed]


