
Cholangiocarcinoma: the quest for a second-line systemic treatment
Introduction
Biliary tract cancer (BTC) is the second most common primary liver malignancy (10,000 and 12,000 new cases/year in Europe and the United States, respectively) after hepatocellular carcinoma (HCC) (1,2). BTCs are a heterogeneous group of epithelial neoplasms (adenocarcinoma in 90% of cases), and are classified into four subtypes based on their anatomical origin: (I) peripheral or intrahepatic cholangiocarcinoma (iCCA), developed within the hepatic parenchyma; (II) perihilar cholangiocarcinoma, most frequent, also known as Klatskin tumors (pCCA), between the second-order bile ducts and the cystic duct; (III) distal cholangiocarcinoma (dCCA), located on the main bile duct below the bifurcation of the cystic duct, which are often grouped with pCCAs under the appellation extrahepatic cholangiocarcinoma (eCCA); (IV) and gallbladder carcinoma (3,4).
BTCs display a poor prognosis, with a 5-year overall survival (OS) rate for all stages taken together of only 18% (5). This poor prognosis is mainly due to late diagnosis, as only 30–40% of patients with BTC are amenable to surgery, which is the only treatment with curative intent (3,4). Therefore, the majority of patients are diagnosed at an advanced, unresectable stage, because of the presence of distant metastasis, vascular invasion, and/or the extent of intrahepatic involvement with insufficient remaining healthy liver parenchyma (3). In addition, the majority of those who are operated on develop tumor recurrence (3). Advanced or recurrent BTC remains a challenging, non-curable disease, for which therapeutic options are limited and mainly rely on supportive care and systemic chemotherapy to improve patient OS and quality of life (4,6). Remarkably, the prognosis of patients with advanced BTC is highly heterogeneous, with median OS ranging from three to 12 months across studies (7,8).
The gemcitabine plus cisplatin doublet (GEMCIS) became the first-line (L1) reference chemotherapy in this setting in 2010 based on the ABC-02 phase III trial (9). This study showed the superiority of GEMCIS over gemcitabine monotherapy in terms of OS (median: 11.7 vs. 8.1 months, P<0.001) and progression-free survival (PFS) (median: 8.0 vs. 5 months, P<0.001) in 410 patients with advanced BTC and Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤2 (9). A similar magnitude of benefit was found in a Japanese randomized phase II trial (10), and in a meta-analysis pooling the results from these two trials [hazard ratio (HR) for OS: 0.65, P<0.001; for PFS: 0.64, P<0.001] (11). Due to better tolerance and simpler outpatient administration, many European centers use the gemcitabine plus oxaliplatin doublet (GEMOX), which is considered as a standard equivalent to GEMCIS and backbone chemotherapy in many clinical trials, and yields a median OS of 10–12 months (12,13). Based on these data, GEMCIS or GEMOX chemotherapy doublets are well-established L1 reference therapy in patients with advanced BTC and a PS ≤2 (3,4).
Beyond failure of L1 chemotherapy (due to disease progression or limiting toxicity), available evidence to guide therapeutic decisions is scarce. Data from phase III studies are lacking and there is no validated strategy to date. In this review, we provide an overview of the systemic therapeutic options that can be proposed and unsolved questions in the management of patients with advanced BTC in the second-line (L2) setting.
Second-line systemic treatment: for which patients?
At the time of disease progression under L1 chemotherapy, between 15% and 40% of patients with advanced BTC remain in good general condition, and thus may receive subsequent line(s) of therapy (9,14-17). This relatively low proportion of patients may be explained by frequent therapeutic limitations due to jaundice and its complications (malnutrition, infection) and/or rapid deterioration of the PS. Indeed, in the pivotal ABC-02 study, only 15% of the patients included in the trial received a L2, 72% of whom were in good/excellent general condition with an ECOG PS 0–1 (9). Therefore, L2 is pragmatically administered in selected patients with preserved PS. This raises the question of the identification of advanced BTC patients who are the most likely to benefit from L2.
The benefits of L2 in routine practice remain uncertain. In a systematic review of the literature gathering 25 non-randomized prospective and retrospective studies involving 761 patients, Lamarca et al. reported median OS and PFS of 7.2 and 3.2 months, respectively, with L2 in patients with advanced BTC (18). Fornaro et al. described similar findings in a large multicenter Italian survey and pooled analysis with published data in a total of 499 patients, with median OS and PFS of 6.3 months and 3.1 months, respectively (19). The ABC-07 phase III trial [NCT01926236, modified 5-fluorouracil (5FU) plus folinic acid (FA) and oxaliplatin combination (FOLFOX) vs. best supportive care (BSC)] is ongoing to prospectively determine whether fit patients (ECOG PS 0–1) with advanced BTC benefit from L2 chemotherapy in terms of OS.
Awaiting for the results of this study, L2 decision needs to be discussed in multidisciplinary tumor board (MTB) in terms of risk/benefit ratio on an individual basis. Patients with advanced BTC are highly heterogeneous in terms of prognosis and not all of them seem to benefit from L2 administration (20). A pre-L2 estimation of OS may be useful to select patients for L2, considering that patients who are at high risk of death within 3 months should not receive chemotherapy and should be managed with BSC only. PS is a strong independent prognostic factor and a “pragmatic” parameter frequently used in MTB to estimate the potential benefit of L2. Patients with ECOG PS 2 should probably not be considered for L2 therapy due to their short life expectancy, with median OS not exceeding 3–4 months (15,17,20-22). However, this model based only on PS is simplistic, and a more comprehensive estimation of each patient’s survival is necessary. Identification of additional reliable factors for patient prognostic stratification is warranted to improve therapeutic decision-making in this setting.
Beside patient PS, disease-related factors were also associated with OS in multivariate analyses, including iCCA subtype, metastatic stage, and elevated serum carbohydrate antigen 19-9 (CA19-9) levels (17,19,22-26). Recently, our group also identified peritoneal carcinomatosis as a new independent prognostic factor (20). Data about other biological markers (e.g., albumin, bilirubin) are more limited (24). Alternatively, treatment-related parameters such as the L2 chemotherapy regimen (doublet vs. monotherapy), previous surgical resection of the primary tumor, and L1 efficacy (tumor response, duration of disease control) were also predictors of longer OS in multivariate analyses (15,17,20-22) (Table 1).
Table 1
| Author, year | No. of patients | Median PFS (months) | Median OS (months) | Prognostic factors (multivariate analysis) |
|---|---|---|---|---|
| Brieau et al., 2015 (17) | 196 | 3.2 | 6.7 | PS 0–1; PR/SD with L1; CA19-9 ≤400 UI/mL |
| Fornaro et al., 2014 (22) | 300 | 3.2 | 7.2 | PS 0; CA19-9 ≤152 UI/mL; PFS with L1 ≥6 months; surgery on primary tumor |
| Fornaro et al., 2015 (19) | 174; pooled analysis with published data: 499 | 3.0; 3.1 | 6.6; 6.3 | PS 0; CA19-9 <157 U/mL; locally advanced stage |
| Kim et al., 2017 (26) | 321 | 1.9 | 6.5 | Intrahepatic CCA; TTP with L1 >4 months; CA19-9 at diagnosis; metastatic stage at diagnosis |
CA19-9, carbohydrate antigen 19-9; CCA, cholangiocarcinoma; L1, first-line (treatment); OS, overall survival; PFS, progression-free survival; PR, partial response; PS, performance status; Ref, reference; SD, stable disease; TTP, time to tumor progression.
Nevertheless, these parameters are insufficient to fully predict the survival of patients with advanced BTC in L2. Additional variables (particularly, biological variables) could not be properly assessed in these studies because of the high rate of missing data due to the retrospective nature of the data collection. Hence, the neutrophil-to-lymphocyte ratio, which was identified as an independent prognostic factor in several cancers, may be worth exploring in BTC (27,28). Similarly, smoking status was recently suggested as a strong prognostic indicator in BTC, warranting specific assessment in L2 (29). Constitution of informative databases is necessary to allow a better comprehension of advanced BTC natural history and develop accurate tools for patient prognostic stratification (23).
A prognostic model is a useful tool for clinical management by predicting patient life expectancy. In BTC, although some scores have been proposed, they failed to complete validation process because the performance and internal validation of the final model were not assessed (22). There is no well-validated and widely accepted prognostic model for application in routine practice or in clinical trials. In this context, it is urgent to develop robust models and tools for individual estimation of patient survival (30), in a rigorous methodological framework as suggested in the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) statement (31). These prognostic models and derived tools could be useful for guiding therapeutic decisions and applied to the stratification of patient randomization in future clinical trials (32,33). In parallel, a consensus around mandatory measurements for clinical trials in BTC following the example of the COMM-PACT initiative in pancreatic cancer would be highly valuable (34).
Overall, the clinical benefit of L2 chemotherapy administration in advanced BTC has not been rigorously demonstrated so far and only patchy data are available to guide patient selection for L2. In practice, L2 is proposed to patients with preserved general condition (ECOG PS 0-1) upon failure of L1.
Second-line systemic treatment: which chemotherapy regimen?
There is insufficient evidence level to recommend a specific L2 chemotherapy regimen for BTC because of heterogeneous patient populations and small sample sizes with low statistical power in reported studies and no available phase III trial in this setting (3,4). In the above-mentioned literature reviews by Lamarca et al. and Fornaro et al., pooling together a variety of retrospective and prospective studies and chemotherapy regimens, the overall objective response rate (ORR) did not exceed 8% to 10%. The ongoing ABC-07 phase III trial (NCT01926236) will provide efficacy data about the FOLFOX combination (18,19).
In a large retrospective multicenter French study (AGEO CT2BIL cohort), 196 patients with advanced BTC who received L2 after progression under gemcitabine plus platinum L1 chemotherapy (GEMOX in 93% of patients) were analyzed (17). Three doublet chemotherapies were most represented: irinotecan plus fluoropyrimidine, either 5FU/FA (FOLFIRI) or capecitabine (XELIRI) (n=64); cisplatin plus 5FU/FA (n=38); and oxaliplatin plus fluoropyrimidine (5FU/FA, FOLFOX or capecitabine, XELOX) (n=21). As monotherapy, patients mainly received 5FU/FA or capecitabine (n=40). There was no significant survival difference between chemotherapy regimens. Noticeably, fluoropyrimidine monotherapy and doublets yielded similar PFS (median: 3.3 vs. 3.0 months, P=0.91) and OS (median: 5.6 vs. 6.3 months, P=0.93).
Similarly, another retrospective study, including 321 Korean patients treated with GEMCIS in L1, who received L2 with fluoropyrimidine monotherapy (79%; 5FU/FA, tegafur-uracil/FA, S-1, or capecitabine) or combined with platinum, also showed no significant difference between single-agent and doublet in terms of PFS (median: 1.8 vs. 2.6 months, P=0.43) and OS (median: 6.5 vs. 6.2 months, P=0.87) (26).
Conversely, the multicenter Italian survey by Fornaro et al. involving 174 patients supported an OS benefit in favor of combination chemotherapy (median: 7.1 vs. 5.0 months, P=0.006), although the benefit in PFS did not reach significance (P=0.07) (19).
Taken together, these data remain insufficient to definitively draw conclusions about the superiority of single-agent or combination chemotherapy in BTC in the L2 setting. The AGEO CT2BIL study has been recently updated and completed with European external validations (Italy, United Kingdom) including a total of 800 patients; detailed survival results according to chemotherapy regimen are pending and may provide more evidence to answer this question (20). Overall, chemotherapy shows limited efficacy and the development of new therapeutic options on one side, and the identification biomarkers predictive of response to refine the selection of patients on another side, are crucially needed (19).
Second-line systemic treatment: what place for targeted therapies?
Similar to other gastrointestinal cancers, the two classes of targeted therapies that have been the most explored in BTC are anti-epidermal growth factor receptor (EGFR) and antiangiogenic agents (35,36). In 2014, in pooled analysis in the L1 setting suggested that the addition of a targeted therapy (predominantly, agents directed against the EGFR) to a gemcitabine-based chemotherapy significantly increased the tumor control rate, PFS, and OS (37). Nevertheless, these “classical” targeted agents failed to demonstrate any significant clinical activity in subsequent randomized trials.
EGFR overexpression was described in 11–27% and 5–19% of iCCAs and eCCAs, respectively, which gave a rationale for the development of EGFR-targeted therapies in BTC (38). However, three randomized phase II studies that evaluated cetuximab or panitumumab in association with GEMOX L1 chemotherapy (16,39,40) and one phase III study using erlotinib (41) showed no PFS nor OS improvement compared to chemotherapy alone. Results in L2 studies were also negative (42,43). By analogy with colorectal cancer, KRAS status was postulated to modulate BTC tumor sensitivity to anti-EGFR therapy (44). KRAS mutations are found in 9–24% and 40% of iCCAs and pCCAs, respectively (38), but did not appear to impact survival and tumor response in post-hoc analyses (16,39). This was further confirmed by the Vecti-BIL phase II study, which enrolled only patients with wild-type KRAS BTC, and showed that panitumumab did not prolong survival even in this molecularly-selected patient population (40). Overall, these results highlight the marginal role of anti-EGFR therapy in BTC.
The vascular endothelial growth factor (VEGF) and its tyrosine kinase receptors (VEGFR) have also been explored as therapeutic targets in BTC (35,36). Several single-arm phase II studies evaluated antiangiogenic agents [bevacizumab (45,46), sorafenib (47)] in L1 in combination with chemotherapy in non-selected BTCs (i.e., regardless of the BTC anatomical subtype and molecular profile). The survival results were disappointing, with median OS ranging between 9.9 and 14.4 months, which did not compared favorably with GEMCIS historical data. Likewise, randomized phase II trials with cediranib (48), sorafenib (49), and vandetanib (50) were also negative. In L2, sunitinib provided a marginal median time to progression of 1.7 months in a single-arm phase II study (51). On another hand, antiangiogenics may be of interest in patients with iCCA. Extrahepatic CCAs (pCCAs and dCCAs) are closely anatomically and biologically related to pancreatic cancer, and are typically paucivascular tumors (35). In contrast, iCCAs have the distinction of being often hypervascular tumours displaying enhancement on imaging after intravenous contrast injection, and are biologically closer to HCC (35). Comparative immunohistochemistry studies showed an overexpression of VEGF-A and increased microvessel density in iCCA, which was not observed in extrahepatic subtype, prompting the evaluation of antiangiogenics in this specific subgroup of BTC (52,53). In small phase II studies including only patients with iCCA treated with sunitinib monotherapy (54) or bevacizumab in combination with FOLFIRI (55) in L2, the median OS reached 9.6 and 20 months, respectively. In addition, apatinib, a small-molecule inhibitor of VEGFR-2, has demonstrated encouraging anticancer activity in preclinical studies (56), and a phase III study is ongoing evaluating apatinib as L2 therapy in patients with iCCA (NCT03251443). The results of phase II studies using other antiangiogenic multikinase inhibitors (ramucirumab, lenvatinib, sulfatinib, and regorafenib) in L2 are still pending (Table 2).
Table 2
| Molecule | (Main) Targets | Type | Trial description | Key eligibility criteria | Primary outcome | ClinicalTrial.gov reference |
|---|---|---|---|---|---|---|
| Antiangiogenic therapy | ||||||
| Apatinib | VEGFR2 | MKI | Phase II, single-arm | Metastatic iCCA | PFS, ORR, DCR | NCT03251443 |
| Apatinib | VEGFR2 | MKI | Phase II, single-arm | Advanced CCA | PFS | NCT03144856 |
| Apatinib | VEGFR2 | MKI | Phase II, single-arm | Advanced CCA | PFS | NCT03427242 |
| Apatinib | VEGFR2 | MKI | Phase II, single-arm | Advanced CCA | DCR | NCT03521219 |
| Ramucirumab | VEGFR2 | mAb | Phase II, single-arm | Advanced CCA | PFS | NCT02520141 |
| Lenvatinib | VEGFR, FGFR, PDGFR | MKI | Phase II, single-arm | Advanced CCA | ORR | NCT02579616 |
| Sulfatinib | VEGFR, FGFR1, CSF1R | MKI | Phase II, single-arm | Advanced CCA | PFS | NCT02966821 |
| Regorafenib | VEGFR, FGFR, CSF1R, TIE2, RET; RAF, BRAF; PDGFR | MKI | Phase II, single-arm | Advanced CCA | OS | NCT02115542 |
| Regorafenib | MKI | Phase II, single-arm | Advanced CCA | PFS | NCT02053376 | |
| EGFR and HER2 inhibitors | ||||||
| Trastuzumab plus chemotherapy | HER2 | mAb | Phase II, single-arm | Advanced CCA, HER positive | ORR | NCT03185988 |
| Varlitinib plus capecitabine | EGFR, HER2, HER4 | MKI | Phase II, single-arm | Advanced CCA | ORR | NCT03231176 |
| Varlitinib plus capecitabine | EGFR, HER2, HER4 | MKI | Phase II–III, randomized vs. placebo plus capecitabine | Advanced CCA | AE, ORR, PFS, OS | NCT03093870 |
| FGFR inhibitors | ||||||
| Derazantinib (ARQ 087) | pan-FGFR | MKI | Phase II, single-arm | Advanced iCCA, FGFR2 gene fusion | ORR | NCT03230318 |
| BGJ398 | pan-FGFR | MKI | Phase II, single-arm | Advanced CCA, FGFR gene alteration | ORR | NCT02150967 |
| Erdafitinib | pan-FGFR | MKI | Phase II, single-arm | Advanced CCA, FGFR gene translocation or mutation | ORR | NCT02699606 |
| INCB054828 | FGFR1/2/3 | MKI | Phase II, single-arm | Advanced CCA, FGF/FGFR gene alteration | ORR | NCT02924376 |
| IDH1 inhibitor | ||||||
| Ivosidenib (AG-120) | IDH1 | SMI | Phase III, randomized vs. placebo | Advanced CCA, IDH1 gene mutation | PFS | NCT02989857 |
| TRK inhibitors | ||||||
| Larotrectinib (LOXO-101) | pan-TRK | MKI | Phase II, single-arm | Advanced CCA, NTRK gene fusion | ORR | NCT02576431 |
| Entrectinib (RXDX-101) | TRK, ROS1, ALK | MKI | Phase II, single-arm | Advanced CCA, NTRK1/2/3, ROS1, or ALK gene fusion | ORR | NCT02568267 |
| Others inhibitors | ||||||
| Olaparib | PARP | SMI | Phase II, single-arm | Advanced CCA, IDH1/2 gene mutation | ORR | NCT03212274 |
| Niraparib | PARP | SMI | Phase II, single-arm | Advanced CCA | ORR | NCT03207347 |
| Amcasertib (BBI503) | Cell stemness pathways | MKI | Phase II, single-arm | Advanced CCA | DCR | NCT02232633 |
| Bortezomib | Proteasome | SMI | Phase III trial, randomized vs. supportive care | Metastatic iCCA, PTEN gene mutation or deletion | ORR | NCT03345303 |
| ABC294640 | Sphingosine kinase 2 | MKI | Phase II, single-arm | Advanced CCA | ORR | NCT03377179 |
| RRx-001 plus cisplatin and gemcitabine | Epigenetic modulator | SMI | Phase II, single-arm | Advanced CCA | PFS | NCT02452970 |
AE, adverse events; CCA, cholangiocarcinoma; DCR, disease control rate; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; HER, human epidermal growth factor receptor; iCCA, intrahepatic cholangiocarcinoma; IDH, isocitrate dehydrogenase; mAb, monoclonal antibody; MKI, multikinase inhibitor; NTRK, neurotrophic tyrosine receptor kinase; ORR, objective response rate; OS, overall survival; PARP, poly(ADP-ribose) polymerase; PDGFR, platelet-derived growth factor receptor; PFS, progression-free survival; SMI, small molecule inhibitor; TRK, tyrosine receptor kinase; VEGFR, vascular endothelial growth factor receptor.
Human epidermal growth factor receptor 2 (HER2, encoded by ERBB2 gene) overexpression has been documented in 0–2% and 5–20% of iCCAs and pCCAs, respectively (38), and in 19% of gallbladder carcinoma (57). By analogy with breast or gastric cancers, patients with HER2-positive advanced BTC may benefit from HER2-blockade (35,58). Lapatinib failed to demonstrate any clinical activity in phase II studies in a non-molecularly selected BTC patient population (59,60). The encouraging results in case reports using trastuzumab in patients with gallbladder carcinoma (61,62) revealed the potential interest of these therapies in selected patients (Table 2). Prospective studies in HER2-positive BTC are warranted.
Overall, trials with “classical” targeted therapies (i.e., anti-EGFR, antiangiogenics, anti-HER2), alone or in combination with cytotoxic drugs, have so far yielded no or marginal benefits in the treatment of BTC (35,36). This may be explained in part by the biological and molecular heterogeneity of BTCs and the lack of predictive biomarkers to refine patient selection. The example of the antiangiogenic treatment specifically dedicated to patients with iCCA, based on the rationale of an angiogenic profile restricted to this subtype, while no activity was observed in the whole population, is an illustration of the importance of patient selection (35,36).
Second-line systemic treatment: toward a better selection of patients based on BTC molecular landscape
In recent years, knowledge about BTC molecular heterogeneity has considerably progressed with the advent of high-throughput genomic and transcriptomic analyses, opening new avenues for targeted therapies and patient therapeutic stratification (63).
Comprehensive whole-exome and transcriptome sequencing revealed multiple molecular aberrations and defined several BTC molecular profiles. In a cohort of 260 patients, Nakamura et al. (64) identified potentially targetable genetic driver alterations in 39% of tumors. Interestingly, some mutations were associated with primary tumor location, with significantly different frequencies in iCCA, eCCA, and gallbladder carcinoma (Figure 1).
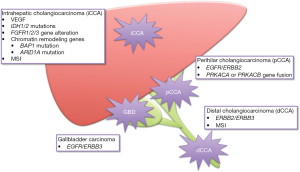
Notably, alterations in isocitrate dehydrogenase 1/2 (IDH1/2) (23–28%), fibroblast growth factor receptor 2 (FGFR2) (7–14%), BAP1 (encoding a nuclear deubiquitinase, 9–12%), and ARID1A (encoding a subunit of the SWI/SNF chromatin-remodelling complex, 15–36%) genes were identified in iCCA (65). IDH mutation leads to the production of an oncometabolite (D-2-hydroxyglutarate), responsible for epigenetic and genetic dysregulations (66). iCCAs harboring IDH mutation exhibited molecular and phenotypic similarities with other IDH mutant liver tumors (66) (high expression of mitochondrial genes, low chromatin-modifier signature) and IDH mutation had no prognostic impact (67). Alternatively, FGFR2 gene fusions drive the activation of the FGFR tyrosine-kinase receptor independent from its ligand binding, thereby promoting cellular proliferation and migration, and neoangiogenesis (64). FGFR2 alterations were associated with favorable survival outcome and could predict the response to FGFR-targeting therapy (67,68). New fusion genes have also been identified in iCCA and other subtypes, involving genes from the family of neurotrophic tyrosine receptor kinase (NTRK) (4%) (65,69,70).
Conversely, pCCAs and dCCAs presented mostly alterations in the EGFR gene family (EGFR/ERBB2 in 4–25%, and ERBB2/ERBB3 in 11–14% of tumors, respectively), as well as protein kinase A pathway aberrations (PRKACA or PRKACB gene fusions, in 10% of pCCAs) (65). These latters are notably involved in metabolic regulation, and have been described in fibrolamellar carcinomas (64,71).
Nakamura’s classification (64) also included a eCCA subtype associated with an increase in gene expression involved in the activation of antitumor immunity pathways and mutations in TP53, BRCA1/2 (DNA repair machinery), and PI3KCA genes. This cluster exhibited a higher tumor mutational burden and was associated with a poor prognosis (64).
On the other hand, other classifications have analyzed the profile of fluke-related (Opisthorchis viverrini and Clonorchis sinensis, mainly found in Asia) vs. non-infection-related BTC (72,73). Comparisons between these two groups of iCCAs demonstrated statistically significant differences in methylation patterns and genetic/transcriptomic landscape: overall, BAP1 and IDH1/2 were more frequently mutated in non-fluke-related BTC, with an enrichment in FGFR gene expression, whereas TP53 mutations and ERBB2 amplification showed the reciprocal pattern (72,73). Wnt/β-catenin pathway alterations were also described in a mixed (fluke-positive and negative samples) cluster (72). In addition, non-fluke-related BTCs displayed better prognosis (72,73).
Recently, Wardell et al. analyzed genomic features of 412 BTC samples from Japanese and Italian populations (29). A total of 32 significantly and commonly mutated genes were identified, including TP53, KRAS, SMAD4, NF1, ARID1A, PBRM1, and ATR, some of which negatively affected patient prognosis, including a novel deletion of MUC17 at 7q22.1. Moreover, deleterious germline mutations of cancer-predisposing genes such as BRCA1, BRCA2, RAD51D, MLH1, or MSH2 were detected in 11% (16/146) of BTC patients (29).
Consequently, these molecular classifications have unraveled the genomic, epigenomic, and transcriptomic diversity of BTCs and paved the way for new therapeutic options.
Second-line systemic treatment: first results and perspectives of molecular-driven therapy
The molecular heterogeneity of BTCs suggests that treatment may be tailored for each patient based on genomic and transcriptomic profiling of tumors, in the era of precision oncology (63).
The MOSCATO-01 trial provided first evidence that high-throughput molecular profiling, performed in a clinically relevant timing, was feasible and could improve outcomes, particularly in BTC patients (74,75). The panel of molecular techniques used included targeted Next Generation Sequencing, whole genome comparative genomic hybridization array, RNAseq to detect fusion transcripts, and immunohistochemistry. Among 43 BTC patients, 34 were evaluable for analysis (contributory biopsy, tumor cellularity >30%) (75). They had an ECOG PS 0-1 and 77% had iCCA; they had received a median number of two lines of treatment. Median time from biopsy to treatment decision (MTB meeting) was 21 days (range: 7–133 days). The success rate to detect at least one targetable molecular alteration was approximately 70%. Consistent with previous reports, the most frequent alterations were IDH1/2 mutations (18%), FGFR1/2 translocations or mutations (16%), and activating alterations in EGFR, ERBB2, or ERBB3 (16%). Of note, multiple molecular aberrations were detected in 87% of cases. A treatment was administered in 18 patients, with a ratio of PFS with L2 over PFS with L1 >1.3 (cut-off used to define L2 benefit) for 9 patients (80%) and an ORR of 33%. The best responders were treated with HER2, HER3, or FGFR inhibitors (75). This study suggested that patients with BTC might be particularly good candidates for biomarker-driven therapy in clinical practice. Actually, other molecular screening programs are ongoing in advanced BTC (NCT02836847; NCT02465060).
IDH and FGFR alterations are the main “modern” targets with a substantial therapeutic impact and the most advanced development in clinical trials. The favorable results observed in a phase II trial of a pan-FGFR inhibitor (BGJ398), with an ORR of 14.8% and a median PFS of 5.8 months (76), are promising, even though they should be interpreted with caution given the spontaneously more favorable outcomes in these patients (Table 2). Agents directed against the FGFR pathway are detailed in another article of this Special Issue. Targeting IDH mutations in iCCA is another promising approach. A phase I study enrolled 73 patients with IDH1-mutant iCCA upon progression after gemcitabine-based chemotherapy to receive an oral inhibitor of IDH1 (AG-120) (77). The disease control rate was 56% and median PFS was 3.8 months (77). The toxicities were acceptable [fatigue (21%), nausea (18%) and diarrhea (10%)] without dose-limiting toxicity (77). The development of this molecule continues in a phase III trial (NCT02989857, Table 2).
An evaluation of TRK inhibitors [larotrectinib (NCT02576431), entrectinib (NCT02568267)] is also ongoing in phase II studies, for patients with advanced BTC and NTRK1/2/3 gene fusion (69). Others small molecule inhibitors may be effective in BTCs and are currently evaluated in several phase II trials in pre-treated patients (Table 2).
In summary, new therapeutic perspectives in L2 are emerging from a better understanding of the biological and molecular mechanisms underlying the heterogeneity of BTCs.
Second-line systemic treatment: what about immunotherapies?
Immune therapy has opened new therapeutic opportunities in cancer. BTCs are no exception, and the links between inflammation and biliary carcinogenesis have led to the development of strategies to modulate anti-tumor immunity of the host, through vaccines, adoptive cell therapies, or immune checkpoint inhibitors (ICI) (78,79).
Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) are receptors expressed on the surface of T-cells that regulate the duration and the amplitude of immune responses in physiological conditions (80). The hijacking of these immunological “checkpoints” by cancer cells is a major mechanism of immune evasion, a better understanding of which led to the clinical development of anti-CTLA-4 and anti-PD-1/PD-L1 monoclonal antibodies (also known as ICIs) with striking efficacy and clinical approval in several malignancies (81).
BTCs are good candidates for ICIs for several reasons. Previously described molecular classifications revealed a subgroup of patients with BTC with a high mutational load (64). Moreover, a subset of BTCs (5% of pCCAs and gallbladder carcinomas; 10% of iCCAs and dCCAs) are associated with DNA mismatch repair (MMR) deficiency and/or microsatellite instability (MSI) (82,83), resulting in abundant tumor-specific neoantigens. The expression of inhibitory immune-checkpoint proteins such as programmed death ligand-1 (PD-L1) has been reported both in iCCAs and eCCAs (72,84,85), and PD-L1 is mainly expressed in tumors with a high density of tumor-infiltrating lymphocytes (TIL) (85). All these factors (high mutation burden/neoantigen load, PD-L1 expression, TILs) have been associated with response to ICIs (86).
Knowledge about BTC immune microenvironment is increasing (87). Several works reported the presence and prognostic impact of immune features and cell infiltrates in resected BTCs: CD8-positive TILs, natural killer lymphocytes, and major histocompatibility complex (MHC) class I expression were associated with prolonged survival, while neutrophils and M2-macrophages were associated with early recurrence and death, and immunohistochemistry studies of T regulatory cells (FOXP3-positive) produced inconsistent results (85,88-94).
ICIs have been tested in early studies in BTC with promising results. Pembrolizumab, a humanized monoclonal antibody against PD-1, showed encouraging activity in pre-treated patients with PD-L1-positive (>1% positive cells) BTC (95). In the KEYNOTE-028 phase Ib study, among 89 patients, 37 (42%) had PD-L1-positive tumors and 24 patients received pembrolizumab monotherapy (96). The ORR was 17%, with 5 patients with prolonged response (>40 weeks), and low rates of immune-mediated toxicity (95). Various phase II trials are ongoing in L2 evaluating anti-PD-1/PD-L1 antibodies as monotherapy or in combination with anti-CTLA-4, chemotherapy, or other therapy (e.g., GM-CSF, MEK inhibitor, TGFβ inhibitor) (Table 3). Vaccines (96-98) and cellular therapies (99,100) have also been explored in phase I/II studies with encouraging preliminary results as monotherapy. Targeted antigens are mostly Wilms tumor 1 (WT1) and mucin 1 (MUC1) (65). More broadly, and similar to pancreatic cancer, strategies targeting tumor microenvironment (e.g., fibroblasts and other components the abundant desmoplastic stroma of BTCs) are emerging in BTC (35,101,102).
Table 3
| Molecule | Targets | Type | Trial description | Key eligibility criteria | Primary outcome | ClinicalTrial.gov reference |
|---|---|---|---|---|---|---|
| Immunotherapies in monotherapy | ||||||
| Pembrolizumab | PD-1 | mAb | Phase II, single-arm | Advanced CCA | ORR | NCT02628067 |
| Pembrolizumab | PD-1 | mAb | Phase II, single-arm | Advanced CCA | ORR, PFS, OS | NCT03110328 |
| Nivolumab | PD-1 | mAb | Phase II, single-arm | Advanced CCA | ORR | NCT02829918 |
| Immunotherapies in combotherapy | ||||||
| Nivolumab and ipilimumab | PD-1 and CTLA-4 | mAb | Phase II, single-arm | Advanced CCA | ORR | NCT02834013 |
| Durvalumab and tremelimumab with or without paclitaxel | PD-L1 and CTLA-4 | mAb | Phase II, randomized | Advanced CCA | PFS | NCT in process (PRODIGE 57-IMMUNOBIL study) |
| Durvalumab and tremelimumab plus radiation therapy | PD-L1 and CTLA-4 | mAb | Phase II, single-arm | Advanced CCA | ORR | NCT03482102 |
| Durvalumab and tremelimumab plus TACE/RFA/cryoablation | PD-L1 and CTLA-4 | mAb | Phase II, single-arm | Advanced CCA | PFS | NCT02821754 |
| Immunotherapies in association with another therapy | ||||||
| Pembrolizumab plus capecitabine and oxaliplatin | PD-1 | mAb | Phase II, single-arm | Advanced CCA | PFS | NCT03111732 |
| Pembrolizumab plus sargramostim (GM-CSF) | PD-1 | mAb | Phase II, single-arm | Advanced CCA | ORR | NCT02703714 |
| Pembrolizumab plus sylatron (Peg-interferon α2b) | PD-1 | mAb | Phase II, single-arm | Advanced CCA | ORR | NCT02982720 |
| Nivolumab plus entinostat (HDAC inhibitor) | PD-1 | mAb | Phase II, single-arm | Advanced CCA | ORR | NCT03250273 |
| Atezolizumab with or without cobimetinib (MEK inhibitor) | PD-L1 | mAb | Phase II, randomized | Metastatic CCA | PFS | NCT03201458 |
CCA, cholangiocarcinoma; CTLA-4, cytotoxic T-lymphocyte–associated antigen 4; GM-CSF, granulocyte-macrophage colony stimulating factor; HDAC, histone deacetylase inhibitor; mAb, monoclonal antibody; ORR, objective response rate; OS, overall survival; PD-1, programmed death 1; PD-L1, programmed death ligand-1; PFS, progression-free survival; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Overall, immunotherapies are under clinical development in BTC, including in the L2 setting. Similarly to other cancers, biomarkers to predict the response to ICIs in BTC still remain to be identified (103,104).
Conclusions
BTCs are a heterogeneous group of epithelial neoplasms, with a poor prognosis. Advanced BTC remains a challenging, non-curable disease. There is no standard therapy in L2 beyond failure of gemcitabine plus platinum L1 standard due to the lack of evidence from prospective randomized phase III trials. Chemotherapy yields modest survival results and no targeted therapy has been validated for this indication.
The identification of prognostic and predictive biomarkers to better stratify patients with BTC and guide therapeutic decisions has become a major research area in recent years. Understanding molecular alterations, their mechanisms of action, and how they can be exploited in a therapeutic perspective is a major challenge in BTC. Among the ongoing developments, targeting FGFR and IDH mutations in iCCA and immune therapies hold many promises for the next future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giovanni Brandi; Francesco Tovoli) for the series “Primary Liver Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: A Vienot has no conflicts of interest to declare; C Neuzillet is the PI of a clinical trial funded by AstraZeneca.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lepage C, Capocaccia R, Hackl M, et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999-2007: Results of EUROCARE-5. Eur J Cancer Oxf Engl 1990 2015;51:2169-78.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma — evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2018;15:95-111. [Crossref] [PubMed]
- Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v28-37. [Crossref] [PubMed]
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271-89. [Crossref] [PubMed]
- Hepatobiliary Cancers - version 3.2017. National Comprehensive Cancer Network Guidelines.
- Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 2010;28:4581-6. [Crossref] [PubMed]
- Glimelius B, Hoffman K, Sjödén PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 1996;7:593-600. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469-74. [Crossref] [PubMed]
- Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol 2014;25:391-8. [Crossref] [PubMed]
- André T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 2004;15:1339-43. [Crossref] [PubMed]
- Fiteni F, Nguyen T, Vernerey D, et al. Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: a systematic review. Cancer Med 2014;3:1502-11. [Crossref] [PubMed]
- Bridgewater J, Palmer D, Cunningham D, et al. Outcome of second-line chemotherapy for biliary tract cancer. Eur J Cancer Oxf Engl 1990 2013;49:1511.
- Walter T, Horgan AM, McNamara M, et al. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer Oxf Engl 1990 2013;49:329-35.
- Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 2014;15:819-28. [Crossref] [PubMed]
- Brieau B, Dahan L, De Rycke Y, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Entérologues Oncologues: Chemotherapy for Advanced Biliary Tract Cancer. Cancer 2015;121:3290-7. [Crossref] [PubMed]
- Lamarca A, Hubner RA, David Ryder W, et al. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 2014;25:2328-38. [Crossref] [PubMed]
- Fornaro L, Vivaldi C, Cereda S, et al. Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: a multicenter survey and pooled analysis with published data. J Exp Clin Cancer Res 2015;34:156. [Crossref] [PubMed]
- Neuzillet C, Casadei Gardini A, Brieau B, et al. Prediction of overall survival with 2nd-line (L2OS) chemotherapy (CT) in patients (Pts) with advanced biliary tract cancer (aBTC): AGEO CT2BIL cohort update and international multicenter external validations. J Clin Oncol 2018;36:abstr e16119.
- Lee SC, Kim K, Kim H, et al. Prognostic factor analysis of second-line chemotherapy in advanced biliary tract cancer. J Clin Oncol 2012;30:e14688.
- Fornaro L, Cereda S, Aprile G, et al. Multivariate prognostic factors analysis for second-line chemotherapy in advanced biliary tract cancer. Br J Cancer 2014;110:2165-9. [Crossref] [PubMed]
- Cereda S, Belli C, Rognone A, et al. Second-line therapy in advanced biliary tract cancer: What should be the standard? Crit Rev Oncol Hematol 2013;88:368-74. [Crossref] [PubMed]
- Kang EJ, Choi YJ, Kim JS, et al. Prognostic Factors for the Selection of Patients Eligible for Second-Line Chemotherapy in Advanced Biliary Tract Cancer. Chemotherapy 2014;60:91-8. [Crossref] [PubMed]
- Zheng Y, Tu X, Zhao P, et al. A randomised phase II study of second-line XELIRI regimen versus irinotecan monotherapy in advanced biliary tract cancer patients progressed on gemcitabine and cisplatin. Br J Cancer 2018;119:291-5. [Crossref] [PubMed]
- Kim BJ, Hyung J, Yoo C, et al. Prognostic factors in patients with advanced biliary tract cancer treated with first-line gemcitabine plus cisplatin: retrospective analysis of 740 patients. Cancer Chemother Pharmacol 2017;80:209-15. [Crossref] [PubMed]
- Tang H, Lu W, Li B, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in biliary tract cancers: a systematic review and meta-analysis. Oncotarget 2017;8:36857-68. [PubMed]
- Tan DW, Fu Y, Su Q, et al. Prognostic Significance of Neutrophil to Lymphocyte Ratio in Oncologic Outcomes of Cholangiocarcinoma: A Meta-analysis. Sci Rep 2016;6:33789. [Crossref] [PubMed]
- Wardell CP, Fujita M, Yamada T, et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J Hepatol 2018;68:959-69. [Crossref] [PubMed]
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. Br J Cancer 2015;112:251-9. [Crossref] [PubMed]
- Vienot A, Beinse G, Louvet C, et al. Overall Survival Prediction and Usefulness of Second-Line Chemotherapy in Advanced Pancreatic Adenocarcinoma. J Natl Cancer Inst 2017;109:djx037. [Crossref] [PubMed]
- Vernerey D, Huguet F, Vienot A, et al. Prognostic nomogram and score to predict overall survival in locally advanced untreated pancreatic cancer (PROLAP). Br J Cancer 2016;115:281-9. [Crossref] [PubMed]
- Ter Veer E, van Rijssen LB, Besselink MG, et al. Consensus statement on mandatory measurements in pancreatic cancer trials (COMM-PACT) for systemic treatment of unresectable disease. Lancet Oncol 2018;19:e151-60. [Crossref] [PubMed]
- Neuzillet C, Rousseau B, Kocher H, et al. Unravelling the pharmacologic opportunities and future directions for targeted therapies in gastro-intestinal cancers Part 1: GI carcinomas. Pharmacol Ther 2017;174:145-72. [Crossref] [PubMed]
- Marret G, Neuzillet C, Rousseau B, et al. Medical management of cholangiocarcinomas in 2015. Bulletin du Cancer 2016;103:389-99. [Crossref] [PubMed]
- Eckel F, Schmid RM. Chemotherapy and targeted therapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Chemotherapy 2014;60:13-23. [Crossref] [PubMed]
- Chong DQ, Zhu AX. The landscape of targeted therapies for cholangiocarcinoma: current status and emerging targets. Oncotarget 2016;7:46750-67. [Crossref] [PubMed]
- Chen JS, Hsu C, Chiang NJ, et al. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann Oncol 2015;26:943-9. [Crossref] [PubMed]
- Leone F, Marino D, Cereda S, et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: A randomized phase 2 trial (Vecti-BIL study). Cancer 2016;122:574-81. [Crossref] [PubMed]
- Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2012;13:181-8. [Crossref] [PubMed]
- Philip PA, Mahoney MR, Allmer C, et al. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol 2006;24:3069-74. [Crossref] [PubMed]
- Chiorean EG, Ramasubbaiah R, Yu M, et al. Phase II trial of erlotinib and docetaxel in advanced and refractory hepatocellular and biliary cancers: Hoosier Oncology Group GI06-101. Oncologist 2012;17:13. [Crossref] [PubMed]
- Jensen LH, Lindebjerg J, Ploen J, et al. Phase II marker-driven trial of panitumumab and chemotherapy in KRAS wild-type biliary tract cancer. Ann Oncol 2012;23:2341-6. [Crossref] [PubMed]
- Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol 2010;11:48-54. [Crossref] [PubMed]
- Lubner SJ, Mahoney MR, Kolesar JL, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol 2010;28:3491-7. [Crossref] [PubMed]
- Lee JK, Capanu M, O’Reilly EM, et al. A phase II study of gemcitabine and cisplatin plus sorafenib in patients with advanced biliary adenocarcinomas. Br J Cancer 2013;109:915-9. [Crossref] [PubMed]
- Valle JW, Wasan H, Lopes A, et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. Lancet Oncol 2015;16:967-78. [Crossref] [PubMed]
- Moehler M, Maderer A, Schimanski C, et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: a double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur J Cancer Oxf Engl 1990 2014;50:3125-35.
- Santoro A, Gebbia V, Pressiani T, et al. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: the VanGogh study. Ann Oncol 2015;26:542-7. [Crossref] [PubMed]
- Yi JH, Thongprasert S, Lee J, et al. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: a multicentre, multinational study. Eur J Cancer 2012;48:196-201. [Crossref] [PubMed]
- Guedj N, Zhan Q, Perigny M, et al. Comparative protein expression profiles of hilar and peripheral hepatic cholangiocarcinomas. J Hepatol 2009;51:93-101. [Crossref] [PubMed]
- Wiggers JK, Ruys AT, Groot Koerkamp B, et al. Differences in immunohistochemical biomarkers between intra- and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol 2014;29:1582-94. [Crossref] [PubMed]
- Neuzillet C, Seitz JF, Fartoux L. Second line therapy with sunitinib as single agent in patients with advanced intrahepatic cholangiocarcinoma (update on SUN-CK phase II trial). Ann Oncol 2014;25 suppl 4:abstr 720P.
- Guion-Dusserre JF, Lorgis V, Vincent J, et al. FOLFIRI plus bevacizumab as a second-line therapy for metastatic intrahepatic cholangiocarcinoma. World J Gastroenterol 2015;21:2096-101. [Crossref] [PubMed]
- Peng H, Zhang Q, Li J, et al. Apatinib inhibits VEGF signaling and promotes apoptosis in intrahepatic cholangiocarcinoma. Oncotarget 2016;7:17220-9. [PubMed]
- Galdy S, Lamarca A, McNamara MG, et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastasis Rev 2017;36:141-57. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Ramanathan RK, Belani CP, Singh DA, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol 2009;64:777-83. [Crossref] [PubMed]
- Peck J, Wei L, Zalupski M, et al. HER2/neu may not be an interesting target in biliary cancers: results of an early phase II study with lapatinib. Oncology 2012;82:175-9. [Crossref] [PubMed]
- Sorscher S. Marked radiographic response of a HER-2-overexpressing biliary cancer to trastuzumab. Cancer Manag Res 2013;9:1-3. [Crossref] [PubMed]
- Roa I, de Toro G, Schalper K, et al. Overexpression of the HER2/neu Gene: A New Therapeutic Possibility for Patients With Advanced Gallbladder Cancer. Gastrointest Cancer Res GCR 2014;7:42-8. [PubMed]
- Goldstein D, Lemech C, Valle J. New molecular and immunotherapeutic approaches in biliary cancer. ESMO Open 2017;2:e000152. [Crossref] [PubMed]
- Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003-10. [Crossref] [PubMed]
- Valle JW, Lamarca A, Goyal L, et al. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov 2017;7:943-62. [Crossref] [PubMed]
- Farshidfar F, Zheng S, Gingras MC, et al. Integrative Genomic Analysis of Cholangiocarcinoma Identifies Distinct IDH-Mutant Molecular Profiles. Cell Rep 2017;18:2780-94. [Crossref] [PubMed]
- Javle M, Bekaii-Saab T, Jain A, et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016;122:3838-47. [Crossref] [PubMed]
- Jain A, Borad MJ, Kelley RK, et al. Cholangiocarcinoma With FGFR Genetic Aberrations: A Unique Clinical Phenotype. JCO Precision Oncology - published online January 17, 2018.
- Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 2016;1:e000023. [Crossref] [PubMed]
- Ross JS, Wang K, Gay L, et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist 2014;19:235-42. [Crossref] [PubMed]
- Honeyman JN, Simon EP, Robine N, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 2014;343:1010-4. [Crossref] [PubMed]
- Jusakul A, Cutcutache I, Yong CH, et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov 2017;7:1116-35. [Crossref] [PubMed]
- Chan-On W, Nairismägi ML, Ong CK, et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat Genet 2013;45:1474-8. [Crossref] [PubMed]
- Massard C, Michiels S, Ferté C, et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov 2017;7:586-95. [Crossref] [PubMed]
- Verlingue L, Malka D, Allorant A, et al. Precision medicine for patients with advanced biliary tract cancers: An effective strategy within the prospective MOSCATO-01 trial. Eur J Cancer 2017;87:122-30. [Crossref] [PubMed]
- Javle M, Lowery M, Shroff RT, et al. Phase II Study of BGJ398 in Patients With FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol 2018;36:276-82. [Crossref] [PubMed]
- Lowery MA, Abou-Alfa GK, Burris HA, et al. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: Results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol 2017;35:4015. [Crossref]
- Shah UA, Nandikolla AG, Rajdev L. Immunotherapeutic Approaches to Biliary Cancer. Curr Treat Options Oncol 2017;18:44. [Crossref] [PubMed]
- Duffy AG, Makarova-Rusher OV, Greten TF. The case for immune-based approaches in biliary tract carcinoma. Hepatology 2016;64:1785-91. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Hilmi M, Bartholin L, Neuzillet C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now? World J Gastroenterol 2018;24:2137-51. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Silva VW, Askan G, Daniel TD, et al. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol 2016;5:62. [Crossref] [PubMed]
- Sabbatino F, Villani V, Yearley JH, et al. PD-L1 and HLA Class I Antigen Expression and Clinical Course of the Disease in Intrahepatic Cholangiocarcinoma. Clin Cancer Res 2016;22:470-8. [Crossref] [PubMed]
- Fontugne J, Augustin J, Pujals A, et al. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget 2017;8:24644-51. [Crossref] [PubMed]
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321-30. [Crossref] [PubMed]
- Wang Y, Ding M, Zhang Q, et al. Activation or suppression of the immune response mediators in biliary tract cancer (BTC) patients: a systematic review and meta-analysis. J Cancer 2017;8:74-84. [Crossref] [PubMed]
- Kitano Y, Okabe H, Yamashita YI, et al. Tumour-infiltrating inflammatory and immune cells in patients with extrahepatic cholangiocarcinoma. Br J Cancer 2018;118:171-80. [Crossref] [PubMed]
- Gu FM, Gao Q, Shi GM, et al. Intratumoral IL-17+ cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol 2012;19:2506-14. [Crossref] [PubMed]
- Goeppert B, Frauenschuh L, Zucknick M, et al. Major histocompatibility complex class I expression impacts on patient survival and type and density of immune cells in biliary tract cancer. Br J Cancer 2015;113:1343-9. [Crossref] [PubMed]
- Goeppert B, Frauenschuh L, Zucknick M, et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br J Cancer 2013;109:2665-74. [Crossref] [PubMed]
- Tsukagoshi M, Wada S, Yokobori T, et al. Overexpression of natural killer group 2 member D ligands predicts favorable prognosis in cholangiocarcinoma. Cancer Sci 2016;107:116-22. [Crossref] [PubMed]
- Oshikiri T, Miyamoto M, Shichinohe T, et al. Prognostic value of intratumoral CD8+ T lymphocyte in extrahepatic bile duct carcinoma as essential immune response. J Surg Oncol 2003;84:224-8. [Crossref] [PubMed]
- Lim YJ, Koh J, Kim K, et al. High ratio of programmed cell death protein 1 (PD-1)(+)/CD8(+) tumor-infiltrating lymphocytes identifies a poor prognostic subset of extrahepatic bile duct cancer undergoing surgery plus adjuvant chemoradiotherapy. Radiother Oncol 2015;117:165-70. [Crossref] [PubMed]
- Bang YJ, Doi T, Piha-Paul S, et al. Safety and efficacy of pembrolizumab in patients with advanced biliary tract cancers. Eur J Cancer 2015;51:S112. [Crossref]
- Aruga A, Takeshita N, Kotera Y, et al. Long-term Vaccination with Multiple Peptides Derived from Cancer-Testis Antigens Can Maintain a Specific T-cell Response and Achieve Disease Stability in Advanced Biliary Tract Cancer. Clin Cancer Res 2013;19:2224-31. [Crossref] [PubMed]
- Aruga A, Takeshita N, Kotera Y, et al. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J Transl Med 2014;12:61. [Crossref] [PubMed]
- Shirahama T, Muroya D, Matsueda S, et al. A randomized phase II trial of personalized peptide vaccine with low dose cyclophosphamide in biliary tract cancer. Cancer Sci 2017;108:838-45. [Crossref] [PubMed]
- Guo Y, Feng K, Liu Y, et al. Phase I Study of Chimeric Antigen Receptor-Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin Cancer Res 2018;24:1277-86. [Crossref] [PubMed]
- Kobayashi M, Sakabe T, Abe H, et al. Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J Gastrointest Surg 2013;17:1609-17. [Crossref] [PubMed]
- Mertens JC, Rizvi S, Gores GJ. Targeting cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis 2018;1864:1454-60. [Crossref] [PubMed]
- Sirica AE, Campbell DJ, Dumur CI. Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol 2011;27:276-84. [Crossref] [PubMed]
- Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016;17:e542-51. [Crossref] [PubMed]
- Hegde PS, Karanikas V, Evers S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin Cancer Res 2016;22:1865-74. [Crossref] [PubMed]


