Adherence to Mediterranean diet and the risk of breast cancer: a meta-analysis
Introduction
Breast cancer is the most common cancer in women, but the incidence of breast cancer varies widely among different countries (1). Many reproductive factors are involved in the generation of breast cancer, such as the woman’s age at full-term pregnancy, the number of births, menarche, and menopause (2). Physical activity is also involved in breast cancer incidence and recurrence (3-5). However, these factors cannot adequately explain the high incidence of breast cancer, suggesting that environmental factors such as nutrition might also be involved.
Previous studies have shown that diet influences the risk of breast cancer, but these studies are mainly focused on investigating the impact of a single nutrient or a certain food group; thus, the results are not conclusive (6-8).
For a long period of time, the Mediterranean diet (MD) has been regarded as a balanced and healthy daily diet. Although no gold standard exists, the MD is characterized by many conspicuous features, such as high intake of olive oil, which contains a large amount of monounsaturated fatty acids, vegetables, legumes, fruits, nuts, and unrefined cereals; low-to-moderate intake of dairy products (principally cheese and yogurt), seafood, and poultry; low intake of red meat; zero to four eggs weekly; and regular but moderate intake of alcohol (9).
According to a previous meta-analysis (10), the MD could reduce the overall risk of the incidence/mortality of many types of cancer by 10%; the risk of colorectal cancer could be reduced by 14%, and the risk of prostate cancer could be reduced by 4%. However, findings from studies of the MD and breast cancer risk have been inconsistent. A study that included 4,282 women aged 60–80 years old who underwent follow-up for 4.8 years showed that adherence to the MD was inversely associated with the risk of breast cancer [hazard ratio (HR) =0.43, 95% confidence interval (95% CI): 0.21, 0.88] (11). Similar results were reported in the cohort study by Cottet et al. (HR =0.85, 95% CI: 0.75, 0.95) (12), especially when tumors were estrogen receptor positive/progesterone receptor-negative. Numerous other cohort studies and case-control studies have reached a similar conclusion (13-15). Another study concluded that adherence to the MD was not associated with the risk of breast cancer. In February 2016, an epidemiology study that included 100,643 women who underwent follow-up from 1984 to 2006 did not observe any significant associations between the MD and the risk of breast cancer according to the molecular subtype (16). A case-control study from the UK that included 610 patients and 1,891 matched controls did not find that the MD was related to breast cancer (17). A similar observation was found in many other studies (18-20).
However, in a cohort study from Greece (21), conformity to the MD was not associated with breast cancer risk in the entire cohort (HR =0.88, 95% CI: 0.75, 1.03) or in premenopausal women (HR =1.01, 95% CI: 0.80, 1.28) but was inversely associated with breast cancer risk in postmenopausal women (HR =0.78, 95% CI: 0.62, 0.98), suggesting that menopausal status differentially impacts the relationship between the MD and breast cancer. Nevertheless, a cohort study conducted by British researchers (22) found a nonsignificant inverse association of adherence to the MD and breast cancer risk in both premenopausal women (HR =0.65, 95% CI: 0.42, 1.02) and postmenopausal women (HR =1.30, 95% CI: 0.83, 2.05). Thus, the correlation among the MD, breast cancer risk and menopausal status has not been determined.
Therefore, a meta-analysis was conducted to reassess the correlation between the MD and breast cancer risk by reviewing all of the inconsistent results that were collected from previously published articles. Additionally, we evaluated the influence of menopausal status on breast cancer risk in women who adhered to the MD through subgroup analysis. We attempted to produce the best possible evidence regarding the correlation between the MD and breast cancer risk in all and pre- or postmenopausal women.
Methods
Literature search strategy
We searched PubMed, Medline, and EMBASE for relevant articles published earlier than May 2017. The following search terms were used: “Mediterranean diet” combined with “breast cancer” and “breast carcinoma”. The search terms were used in all fields. Moreover, we manually searched the references of relevant articles. When necessary, we contacted the authors of the original articles for useful information.
Study selection
We included studies that met the following criteria: (I) the study design was a cohort or case-control study; (II) the exposure was the MD or an MD-style dietary pattern, and the assessment method of the dietary pattern was validated by the Food Frequency Questionnaire (FFQ) or factor analysis posteriori; (III) the outcome was the incidence or risk of breast cancer; (IV) the diagnosis of breast cancer was performed by pathological biopsy or other standard methods; (V) the specific relative risk (RR), odds ratio (OR) or HR and corresponding 95% CI were reported; (VI) studies were written in English.
First, relevant studies were screened by searching the titles and abstracts. If the relevance was in doubt, the whole text of the paper was assessed, and any disagreements were discussed.
Data extraction and quality assessment
Two independent investigators extracted key data, including: the last name of the first author, geographic area, publication year, study design, number of cases for participants or controls, age range or average age, menopausal status, hormone receptor status, follow-up time, dietary assessment method, end point of observation, diagnostic criteria/grade of cancer, results, RR/OR/HR (95% CI) for the highest vs. the lowest score for the MD and variables used in a multivariate model.
The quality of the involved studies was assessed using the Newcastle-Ottawa Scale (NOS), for which the scores range from 0–9 stars. Any studies that were ranked 4 stars or lower were excluded, while studies that were graded 6 stars or higher were regarded as good quality.
Data analysis
We conducted the meta-analysis by combining the multivariable adjusted RRs, HRs or ORs of the highest compared with the lowest MD adherence category based on a random-effects model using the Der Simonian-Laird method. This method consisted of both within and between study variabilities. To assess the weighting of each study, we calculated the standard errors for the logarithm of the RR/OR/HR of every study; these were regarded as the estimated variance of the logarithm of the RR/OR/HR, and an inverse variance method was used accordingly (23). In addition, we used the STATA version 12.0 software to analyze the data. A random-effects model was used to compute the combined RR and the 95% CI to assess the association between the MD and the risk of breast cancer. The Q statistic and I2 statistic were used to determine the heterogeneity among the studies. Subgroup analysis was used to identify the association between the risk of breast cancer and menopausal status or study design. A sensitivity analysis was conducted to assess the influence of a single study on the overall risk estimate. Begg’s and Egger’s tests were used to detect the publication bias. A P value less than 0.05 was considered statistically significant.
Results
Literature search and study characteristics
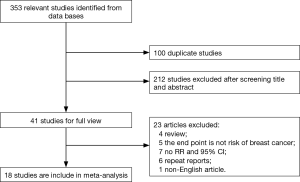
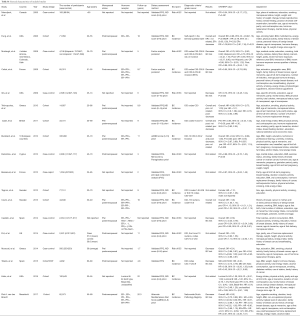
Initially, we collected 353 related articles from the databases, including 100 duplicate studies. After the titles and abstracts were screened, 212 of the 253 remaining articles were excluded. Then, we reviewed the full texts of the remaining 41 articles. Finally, a total of 18 articles (11-22,24-29) were included in our meta-analysis. Figure 1 shows a flow chart of the study selection.
The NOS scores of the 18 included studies were greater than 6 stars, and most studies were performed in Europe. The studies spanned 11 years, from 2006 to May 2017. Ten cohort and eight case-control studies were included. Seven studies analyzed breast cancer incidence separately in premenopausal and postmenopausal women, whereas 6 studies were performed only in postmenopausal women, for a total of 13 analyses in postmenopausal women and 7 analyses of premenopausal women. Table S1 summarizes the basic characteristics of the 18 included studies.
Main analysis
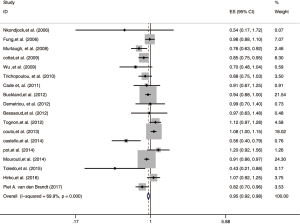
A random-effects model was used to analyze the data of the 18 studies to assess the association between the MD and the risk of breast cancer. We found that the MD could markedly reduce the risk of breast cancer (RR: 0.92, 95% CI: 0.86, 0.99). Strong evidence of heterogeneity was detected among these studies (I2=69.9%, P=0.000). Figure 2 shows the results of our meta-analysis.
Subgroup analysis
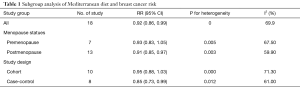
We conducted a subgroup analysis to identify the source of heterogeneity according to menopausal status and study design. Adherence to the MD significantly decreased the risk of breast cancer, showing an inverse association between the MD and breast cancer in postmenopausal women (RR 0.91, 95% CI: 0.85, 0.97) but not in premenopausal women (RR 0.91, 95% CI: 0.85, 0.97). Study design analysis showed that adherence to the MD significantly decreased the risk of breast cancer in case-control studies (RR 0.85, 95% CI: 0.73, 0.99) but not in cohort studies (RR 0.85, 95% CI: 0.73, 0.99). Nevertheless, evidence of heterogeneity was also found across the four subgroups (premenopausal: I2=67.5%, P=0.005; postmenopausal: I2=59.9%, P=0.003; case-control: I2=61.0%, P=0.012; cohort studies: I2=71.3%, P=0.000), suggesting that other factors were involved. Table 1 shows the results of the subgroup analysis.
Full table
Sensitivity analysis and publication bias
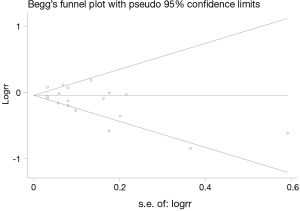
Removal of each study individually showed that the combined RRs were similar, and no single study obviously modified the combined results, which implied that our results were statistically stable and reliable. Figure 3 shows the results of the sensitivity analysis. In addition, Begg’s and Egger’s regression tests showed a low probability of publication bias (P=0.121) (Figure 4).
Discussion
Many studies have investigated the association between the MD and breast cancer risk in the past 10 years (11-16), including prospective cohort studies and retrospective case-control studies. However, those studies did not reach a precise conclusion regarding whether the MD could reduce the risk of breast cancer. Our meta-analysis included 18 studies on the association of the MD and breast cancer published from 2006 to May 2017 and found a statistically significant inverse association, i.e., the MD could reduce the risk of breast cancer. This conclusion is consistent with that of a previous meta-analysis (30), in which adherence to the MD was shown to reduce the risk, incidence and mortality of many cancers, including breast cancer. Moreover, stratification analysis by menopausal status revealed a significant inverse association between the MD and breast cancer risk in postmenopausal women but not in premenopausal women, which is consistent with the results of many other studies (31).
The MD is characterized by increased consumption of vegetables, fruits, nuts, and cereals. The flavonoids contained in these types of food may contribute to cancer prevention through multiple biological effects, including antioxidant activity, inhibition of inflammation, and antimutagenic and antiproliferative properties (32).
The main source of fat of the MD is olive oil, which contains abundant oleic acid, a monounsaturated fatty acid, and squalene. Oleic acid can suppress overexpression of the HER2 oncogene and in turn promotes apoptosis in tumor cells (33). Squalene can play an anticancer role by reducing oxidative DNA damage and inhibiting beta-hydroxy-beta methylglutaryl-CoA-reductase, which affects cellular signal transduction and proliferation in human mammary cells (34).
Seafood, such as fish and shrimp, is an important component of the MD. The anticarcinogenic effects of seafood are mainly due to n-3 polyunsaturated fatty acid, which inhibits the transformation of arachidonic acid into eicosanoids, which are proinflammatory signaling molecules, and regulates gene expression, transcription and activities of molecules related to the signal transduction for cell growth (35).
The reduced consumption of red meat, dairy products and alcohol in the MD may also contribute to its anticarcinogenic effect. Red meat and dairy products contain abundant saturated fat, which has been demonstrated to be an independent carcinogenetic factor likely due to increased energy balance and insulin resistance. Red meat is also a source of some known mutagenic compounds, such as heterocyclic amines and polycyclic aromatic hydrocarbons, which are related to the breast cancer etiology (36,37). The MD can be classified as a traditional or an adapted pattern. The former, but not the latter, includes regular but moderate intake of alcohol (38). It is well known that alcohol consumption is a risk factor for breast cancer (39). However, a case-control study (40) conducted in France reported a lower risk of breast cancer among women consuming 10–12 g/d of wine compared with non-wine drinkers. This result was explained because red and white wine contain resveratrol, an anticarcinogenic polyphenol (41). In addition, regardless of the health benefits of the traditional MD, the total daily calorie content must be controlled to avoid obesity, which is a risk factor for breast cancer (42).
Our results showed that adherence to the MD is associated with a significant reduction of breast cancer risk in postmenopausal women but not in premenopausal women. One of the possible causes may be that with age, postmenopausal women focus more on their diet than premenopausal women to maintain their health.
Because strong evidence of heterogeneity was observed in our meta-analysis, we used a random-effects model to calculate the combined RR and maintain the stability of the results.
Although our statistical data showed that an MD could reduce the risk of breast cancer in women, especially in postmenopausal women, three limitations of our meta-analysis should be addressed. First, the contents of the MD were not identical in different studies, and the methods used to assess the MD were also not identical. Some studies used the validated FFQ a priori, and the others used the factor analysis posteriori. Second, the heterogeneity was still high after subgroup analysis by study design and menopausal status, indicating that other factors also contribute to the heterogeneity, such as hormone receptor status, age of menarche, oral contraceptive use, full-term pregnancy, number of births, family history, related genetic mutations and the determination of menopausal status. Third, the studies included in this meta-analysis were mainly conducted in Europe; therefore, further validation is required for the conclusions to be applied to other populations.
Conclusions
This meta-analysis revealed that the MD is significantly associated with a reduction of breast cancer risk in women, especially in postmenopausal women. The MD can be suggested to women, especially postmenopausal women, as a healthy dietary pattern to reduce breast cancer risk.
Full table
Acknowledgments
Funding: This work is supported by the Science and Technology Innovation Plan of Southwest Hospital (SWH2016JCYB-04, SWH2016YSCXZD-10), Third Military Medical University Foundation (2016XPY12).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.10.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Potentas E, Witkowska AM, Zujko ME. Mediterranean diet for breast cancer prevention and treatment in postmenopausal women. Prz Menopauzalny 2015;14:247-53. [Crossref] [PubMed]
- Lahart IM, Metsios GS, Nevill AM, et al. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol 2015;54:635-54. [Crossref] [PubMed]
- Vardar-Yagli N, Sener G, Saglam M, et al. Associations among physical activity, comorbidity, functional capacity, peripheral muscle strength and depression in breast cancer survivors. Asian Pac J Cancer Prev 2015;16:585-9. [Crossref] [PubMed]
- Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results Cancer Res 2011;186:13-42. [Crossref] [PubMed]
- Jung S, Spiegelman D, Baglietto L, et al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst 2013;105:219-36. [Crossref] [PubMed]
- Lagiou P, Olsen J, Trichopoulos D. Consumption of vegetables and fruits and risk of breast cancer. JAMA 2005;293:2209-author reply 10. [PubMed]
- Michels KB, Mohllajee AP, Roset-Bahmanyar E, et al. Diet and breast cancer: a review of the prospective observational studies. Cancer 2007;109:2712-49. [Crossref] [PubMed]
- La Vecchia C. Mediterranean diet and cancer. Public Health Nutr 2004;7:965-8. [Crossref] [PubMed]
- Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: a systematic review and meta-analysis of observational studies. Int J Cancer 2014;135:1884-97. [Crossref] [PubMed]
- Toledo E, Salas-Salvado J, Donat-Vargas C, et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern Med 2015;175:1752-60. [Crossref] [PubMed]
- Cottet V, Touvier M, Fournier A, et al. Postmenopausal breast cancer risk and dietary patterns in the E3N-EPIC prospective cohort study. Am J Epidemiol 2009;170:1257-67. [Crossref] [PubMed]
- Fung TT, Hu FB, McCullough ML, et al. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr 2006;136:466-72. [Crossref] [PubMed]
- Mourouti N, Kontogianni MD, Papavagelis C, et al. Adherence to the Mediterranean diet is associated with lower likelihood of breast cancer: a case-control study. Nutr Cancer 2014;66:810-7. [Crossref] [PubMed]
- Wu AH, Yu MC, Tseng CC, et al. Dietary patterns and breast cancer risk in Asian American women. Am J Clin Nutr 2009;89:1145-54. [Crossref] [PubMed]
- Hirko KA, Willett WC, Hankinson SE, et al. Healthy dietary patterns and risk of breast cancer by molecular subtype. Breast Cancer Res Treat 2016;155:579-88. [Crossref] [PubMed]
- Pot GK, Stephen AM, Dahm CC, et al. Dietary patterns derived with multiple methods from food diaries and breast cancer risk in the UK Dietary Cohort Consortium. Eur J Clin Nutr 2014;68:1353-8. [Crossref] [PubMed]
- Bessaoud F, Tretarre B, Daures JP, et al. Identification of dietary patterns using two statistical approaches and their association with breast cancer risk: a case-control study in Southern France. Ann Epidemiol 2012;22:499-510. [Crossref] [PubMed]
- Couto E, Sandin S, Lof M, et al. Mediterranean dietary pattern and risk of breast cancer. PLoS One 2013;8:e55374 [Crossref] [PubMed]
- Tognon G, Nilsson LM, Lissner L, et al. The Mediterranean diet score and mortality are inversely associated in adults living in the subarctic region. J Nutr 2012;142:1547-53. [Crossref] [PubMed]
- Trichopoulou A, Bamia C, Lagiou P, et al. Conformity to traditional Mediterranean diet and breast cancer risk in the Greek EPIC (European Prospective Investigation into Cancer and Nutrition) cohort. Am J Clin Nutr 2010;92:620-5. [Crossref] [PubMed]
- Cade JE, Taylor EF, Burley VJ, et al. Does the Mediterranean dietary pattern or the Healthy Diet Index influence the risk of breast cancer in a large British cohort of women? Eur J Clin Nutr 2011;65:920-8. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Buckland G, Travier N, Cottet V, et al. Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int J Cancer 2013;132:2918-27. [Crossref] [PubMed]
- Castelló A, Pollan M, Buijsse B, et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case-control EpiGEICAM study. Br J Cancer 2014;111:1454-62. [Crossref] [PubMed]
- Demetriou CA, Hadjisavvas A, Loizidou MA, et al. The mediterranean dietary pattern and breast cancer risk in Greek-Cypriot women: a case-control study. BMC Cancer 2012;12:113. [Crossref] [PubMed]
- Murtaugh MA, Sweeney C, Giuliano AR, et al. Diet patterns and breast cancer risk in Hispanic and non-Hispanic white women: the Four-Corners Breast Cancer Study. Am J Clin Nutr 2008;87:978-84. [Crossref] [PubMed]
- Nkondjock A, Ghadirian P. Diet quality and BRCA-associated breast cancer risk. Breast Cancer Res Treat 2007;103:361-9. [Crossref] [PubMed]
- van den Brandt PA, Schulpen M. Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. Int J Cancer 2017;140:2220-31. [Crossref] [PubMed]
- Schwingshackl L, Hoffmann G. Adherence to Mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med 2015;4:1933-47. [Crossref] [PubMed]
- Farsinejad-Marj M, Talebi S, Ghiyasvand R, et al. Adherence to Mediterranean diet and risk of breast cancer in premenopausal and postmenopausal women. Arch Iran Med 2015;18:786-92. [PubMed]
- Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr 2005;81:317S-25S. [Crossref] [PubMed]
- Menendez JA, Vellon L, Colomer R, et al. Oleic acid, the main monounsaturated fatty acid of olive oil, suppresses Her-2/neu (erbB-2) expression and synergistically enhances the growth inhibitory effects of trastuzumab (Herceptin) in breast cancer cells with Her-2/neu oncogene amplification. Ann Oncol 2005;16:359-71. [Crossref] [PubMed]
- Warleta F, Campos M, Allouche Y, et al. Squalene protects against oxidative DNA damage in MCF10A human mammary epithelial cells but not in MCF7 and MDA-MB-231 human breast cancer cells. Food Chem Toxicol 2010;48:1092-100. [Crossref] [PubMed]
- Zheng JS, Hu XJ, Zhao YM, et al. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ 2013;346:f3706. [Crossref] [PubMed]
- Khodarahmi M, Azadbakht L. The association between different kinds of fat intake and breast cancer risk in women. Int J Prev Med 2014;5:6-15. [PubMed]
- Seitz HK, Pelucchi C, Bagnardi V, et al. Epidemiology and pathophysiology of alcohol and breast cancer: Update 2012. Alcohol Alcohol 2012;47:204-12. [Crossref] [PubMed]
- Trichopoulou A, Costacou T, Bamia C, et al. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599-608. [Crossref] [PubMed]
- Brennan SF, Cantwell MM, Cardwell CR, et al. Dietary patterns and breast cancer risk: a systematic review and meta-analysis. Am J Clin Nutr 2010;91:1294-302. [Crossref] [PubMed]
- Bessaoud F, Daures JP. Patterns of alcohol (especially wine) consumption and breast cancer risk: a case-control study among a population in Southern France. Ann Epidemiol 2008;18:467-75. [Crossref] [PubMed]
- Whitsett T, Carpenter M, Lamartiniere CA. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats. J Carcinog 2006;5:15. [Crossref] [PubMed]
- Argolo DF, Hudis CA, Iyengar NM. The Impact of Obesity on Breast Cancer. Curr Oncol Rep 2018;20:47. [Crossref] [PubMed]