
EGFR first- and second-generation TKIs—there is still place for them in EGFR-mutant NSCLC patients
Introduction
The discovery of somatic mutations in the tyrosine kinase (TK) domain of epidermal growth factor receptor (EGFR) was a paradigm shift in the understanding of the relevance of lung cancer molecular biology to therapeutic strategy and identified a subset of patients with a unique susceptibility to EGFR tyrosine kinase inhibitors (TKIs) (1). Protein TK gene families are regulators of cell proliferation, metabolism, migration, differentiation, and survival. Receptor tyrosine kinases (RTKs) are transmembrane proteins known to have a ligand-controlled TK activity. The EGFR family consists of four members: EGFR (HER1, ErbB1), ErbB2 (HER2), ErbB3 (HER3) and ErbB4 (HER4) (2). Under physiological conditions, the EGFR family members bind ligands on their extracellular ligand-binding domains, promoting homo- and hetero-dimerization among them, and consequently activating their intracellular TK domains (3). This process induces activation of signaling pathways, such as phosphatidylinositol 3-kinase (PI3K)/AKT, Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3), and Ras/mitogen-activated protein kinase (MAPK) and stimulates downstream components involved in cell proliferation, cell cycle progression, survival and motility (4,5).
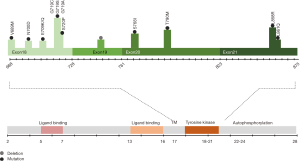
EGFR is a frequently over-expressed and aberrantly activated trans-membrane protein in non-small cell lung cancer (NSCLC) patients, described for the first time in 2004 (4,6,7). EGFR mutations are more often detected in females and never-smokers (1). Activation of EGFR can occur through mutations, deletions, and amplifications, in sequences that code for the TK domain. Furthermore, altered EGFR protein or ligand expression can lead to constitutively active downstream signaling (8). Mutations in the kinase domain of EGFR cluster in exons 18–21, around the adenosine triphosphate (ATP)-binding pocket of the enzyme (Figure 1). Of all patients with EGFR mutations, 90% have in-frame exon 19 deletions or exon 21 missense mutations (9,10). First-generation and second-generation EGFR TKIs have more than doubled progression-free survival (PFS) for patients with NSCLC with actionable EGFR mutations, in comparison with chemotherapy. Also, they have improved objective response rates (ORRs), duration of response, quality of life and decreased treatment-related toxicity.
In the present review, we will summarise the results of the most critical studies with first- and second-generation EGFR inhibitors, highlighting those who have led to their regulatory approval. Mechanisms of resistance, activity on brain metastases, combinatorial therapies as well as the efficacy of these compounds in early-stage disease will be thoroughly reviewed.
First-generation EGFR TKIs
First-generation EGFR TKIs (gefitinib, erlotinib and icotinib) reversibly bind to EGFR and inhibit the binding of ATP to the TK domain. This block hampers cell proliferation, ultimately leading to cell death (11). Gefitinib and erlotinib, are globally approved for the treatment of EGFR-mutant NSCLC patients while icotinib is approved in China.
Gefitinib
The drug development history of gefitinib (ZD1839, Iressa, AstraZeneca Inc.; London, UK) has been complicated. It became available as the first small TKI against EGFR, in 2002 in Japan for the treatment of advanced NSCLC, before the discovery of activating mutations in the EGFR kinase domain. In May 2003, gefitinib received accelerated U.S. Food and Drug Administration (FDA) approval as monotherapy for advanced stage NSCLC patients after failure of both platinum-based and docetaxel chemotherapies (12) (Figure 2). The approval was based on an ORR of around 15% in this setting, as shown in two phase II clinical trials, the IRESSA Dose Evaluation in Advanced NSCLC (IDEAL)-1 and 2 (13,14). However, in June 2005, FDA restricted the use of gefitinib only for NSCLC patients who were already receiving and benefited from the drug or for new patients included in clinical trials with an Institutional Review Board approval before June 2005. Finally, FDA withdrew gefitinib approval in April 2012 (Figure 2). These regulatory approval changes were due to the negative results of three phase III clinical trials (IBREESE, ISEL, and INTEREST) (12,15,16), after IDEAL-1 and 2, in which gefitinib failed to demonstrate its benefit in previously treated NSCLC patients. In 2004 the IRESSA NSCLC Trial Assessing Combination Treatment (INTACT-1 and 2) phase III clinical trials were carried out (17,18). INTACT-1 and 2 evaluated the effect of gefitinib in the first-line setting combined with chemotherapy. Both studies were negative.
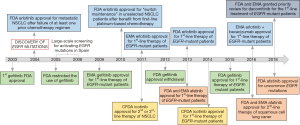
After the discovery of activating EGFR mutations in 2004 (4,6,7), gefitinib was tested in many phase III clinical trials until receiving FDA approval on 13th of July 2015 for the first-line treatment of EGFR-mutant patients. Earlier, in 2009, the European Medicines Agency (EMA) approved gefitinib for the same indication (Figure 2). At the beginning of the discovery of EGFR mutations, the scientific community was confused, and studies were claiming that high EGFR expression, rather than EGFR mutations, is relevant for the efficacy of EGFR TKIs (19,20). We were among the first to perform a large-scale screening for activating EGFR mutations in Spain, from 2005 to 2008 (1). Rosell’s group described that EGFR mutations are more frequent in women, never smokers and lung adenocarcinoma patients, while median PFS and OS to first-line erlotinib, the other first-generation EGFR TKI, were 14 and 27 months, respectively (1). From 2006 to 2007, the first line iressa versus carboplatin/paclitaxel in Asia [Iressa Pan-Asia Study (IPASS)] study was conducted and evaluated gefitinib versus platinum-based chemotherapy as first-line therapy in a clinically selected Asiatic population (21). The study demonstrated the superiority of gefitinib especially for the population carrying EGFR mutations (21,22) (Table 1). Similar results were shown in two phase III Japanese studies (NEJ002 and WJTOG3405) (23,25,35) and a phase III Korean study (36), all conducted in NSCLC patients carrying EGFR mutations (Table 1). Together with the IPASS, NEJ002 and WJTOG3405 are considered as key clinical trials for the FDA and EMA approval of gefitinib for the first-line therapy of EGFR-mutant NSCLC (12). In the European Union, the Iressa Follow-Up Measure (IFUM) study, which was conducted in order to address the efficacy of gefitinib in non-Asian patients, supported the results of the previous studies (37).
Table 1
| Study name | Design | Population (N of patients) | Median PFS (months) | Median OS (months) | ORR (%) | Ref. |
|---|---|---|---|---|---|---|
| IPASS | Gefitinib vs. carboplatin-paclitaxel in pulmonary adenocarcinoma; phase 3 | 261 EGFR mutant (1,217 total) | 9.5 (gefitinib) vs. 6.3 (chemotherapy); P<0.001 | 21.6 (gefitinib) vs. 21.9 (chemotherapy); ns | 71.2% (gefitinib) vs. 47.3% (chemotherapy); P<0.001 | (21,22) |
| WJTOG3405 | Gefitinib vs. cisplatin plus docetaxel in EGFR mutant NSCLC patients; phase 3 | 117 | 9.2 (gefitinib) vs. 6.3 (chemotherapy); P<0.0001 | 34.8 (gefitinib) vs. 37.3 (chemotherapy); ns | 62.1% (gefitinib) vs. 32.2% (chemotherapy); P<0.0001 | (23,24) |
| NEJ002 | Gefitinib vs. chemotherapy in EGFR mutant NSCLC patients; phase 3 | 230 | 10.8 (gefitinib) vs. 5.4 (chemotherapy); P<0.001 | 30.5 (gefitinib) vs. 23.6 (chemotherapy); ns | 73.7% (gefitinib) vs. 30.7% (chemotherapy); P<0.001 | (25) |
| NEJ009 | Gefitinib plus chemotherapy vs. gefitinib in EGFR mutant NSCLC patients; phase 3 |
334 | 20.9 (gefitinib plus chemotherapy) vs. 11.2 (gefitinib); P<0.001 PFS21, 20.9 (gefitinib plus chemotherapy) vs. 20.7 (gefitinib); P=0.0774 |
52.2 (gefitinib plus chemotherapy) vs. 38.8 (gefitinib); P=0.013 | 84.0 (gefitinib plus chemotherapy) vs. 76.4 (gefitinib) | (26) |
| EURTAC | Erlotinib vs. chemotherapy in EGFR mutant NSCLC patients; phase 3 | 173 | 9.4 (erlotinib) vs. 5.2 (chemotherapy); P<0.0001 | 19.3 (erlotinib) vs. 19.5 (chemotherapy); ns | 64% (erlotinib) vs. 18% (chemotherapy); P<0.0001 | (27) |
| OPTIMAL | Erlotinib vs. chemotherapy in EGFR mutant NSCLC patients; phase 3 | 165 | 13.1 (erlotinib) vs. 4.6 (chemotherapy); P<0.0001 | 22.8 (erlotinib) vs. 27.2 (chemotherapy); ns | 83% (erlotinib) vs. 36% (chemotherapy); P<0.0001 | (28,29) |
| ENSURE | Erlotinib vs. chemotherapy in EGFR mutant NSCLC patients; phase 3 | 217 | 11 (erlotinib) vs. 5.5 (chemotherapy); P<0.0001 | 26.3 (erlotinib) vs. 22.5 (chemotherapy); ns | 62.7% (erlotinib) vs. 33.6% (chemotherapy); P<0.0001 | (30) |
| CONVINCE | Icotinib vs. chemotherapy in EGFR mutant NSCLC patients; phase 3 | 217 | 11.2 (icotinib) vs. 7.9 (chemotherapy); P=0.006 | 30.5 (icotinib) vs. 32.1 (chemotherapy); ns | – | (31) |
| LUX-Lung 3 | Afatinib vs. chemotherapy in EGFR mutant NSCLC patients; phase 3 | 345 | 11.1 (afatinib) vs. 6.9 (chemotherapy); P=0.001 | Overall: 28.2 (afatinib) vs. 28.2 (chemotherapy); ns. Exon 19 deletion: 33.3 (afatinib) vs. 21.1 months; P=0.0015. L858R mutation: 27.6 (afatinib) vs. 40.3 months; ns | 56% (afatinib) vs. 23% (chemotherapy); P=0.001 | (32,33) |
| LUX-Lung 6 | Afatinib vs. chemotherapy in EGFR mutant NSCLC patients; phase 3 | 345 (Asiatic) | 11.0 (afatinib) vs. 5.6 (chemotherapy); P<0.0001 | Overall: 23.1 (afatinib) vs. 23.5 (chemotherapy); ns Exon 19 deletion: 31.4 (afatinib) vs. 18.4 (chemotherapy); P=0.023 L858R mutation: 19.6 (afatinib) vs. 24.3 (chemotherapy); ns |
66.9% (afatinib) vs. 23% (chemotherapy); P<0.0001 | (33,34) |
| LUX-Lung 3 and LUX-Lung 6 | – | – | – | Exon 19 deletion: 31.7 (afatinib) vs. 20.7 (chemotherapy); P<0.0001 L858R mutation: 22.1 (afatinib) vs. 26.9 (chemotherapy); ns |
– | (33) |
ns, not significant; 1PFS2, progression after the next line of therapy; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PFS, progression-free survival; OS, overall survival; ORR, objective response rate.
Finally, the results of the phase 3 NEJ009 study (26) (Table 1) were presented at the 2018 ASCO Annual Meeting. The results of the NEJ009 study were among the most impressive results at the meeting, at least for EGFR-mutant patients. Although one of the primary endpoints of the study, time to second objective disease progression (PFS2), was not achieved, since patients who started on gefitinib and received chemotherapy at progression had similar PFS as those who received the concurrent therapy upfront (20.9 versus 20.7), the patients who started with gefitinib plus chemotherapy combination had a significantly longer overall survival (OS) of 52.2 versus 38.8 months (P=0.013) (26). Despite these results, it is not at all likely that gefitinib plus standard chemotherapy will be favored over osimertinib, considering its good tolerability and activity in the central nervous system (CNS) (38). However, maybe the results can lead us to pursue clinical trials combining osimertinib with chemotherapy in the first-line setting.
Erlotinib
Erlotinib (OSI-774, CP-358774, Tarceva; OSI Pharmaceuticals, Inc., Melville, NY, USA and Genentech, Inc., South San Francisco, CA, USA) was discovered in 1997 (39) and was initially FDA approved in November 2004 for the treatment of metastatic NSCLC after the failure of at least one prior chemotherapy regimen (19,40). In 2010, erlotinib was approved for “switch maintenance” in unselected NSCLC patients who had benefited from first-line platinum-based chemotherapy based on statistically significant OS improvement of 1 month in the SATURN trial (41,42).
Randomized trials have demonstrated the superiority of erlotinib compared to chemotherapy regarding response and PFS for the first-line therapy of EGFR-mutant NSCLC patients (27,28). The EURTAC (European Randomized Trial of Tarceva versus Chemotherapy), the OPTIMAL (CTONG0802) and the ENSURE phase III clinical trials have shown that erlotinib is better than platinum-based chemotherapy, concerning PFS and responses, as first-line treatment in EGFR-mutant European and Asiatic patients (27-30) (Table 1). Based on the results of the pivotal EURTAC study, in May, 2013, the FDA approved erlotinib for the first-line therapy of EGFR-mutant NSCLC patients (Figure 2). The FDA also approved the cobas® EGFR Mutation Test, which was developed by Roche and validated in the EURTAC study (43). Based on the results of the same study, EMA approved erlotinib in 2011 for the first-line therapy of EGFR-mutant NSCLC patients (Figure 2).
After the abovementioned studies of gefitinib and erlotinib, the scientific community should have gained a much better appreciation of the clinical efficacy of EGFR TKIs and the design of clinical trials, however, other phase III clinical trials that evaluated the efficacy of erlotinib in different settings had rather disappointing results. For instance, the TRIBUTE (44) and the Tarceva Lung Cancer Investigation Trial (45) were two phase III clinical trials in which erlotinib or placebo was combined with platinum-based chemotherapy for the first-line therapy of NSCLC patients unselected for EGFR mutations. In both studies, no survival benefit was found for the combination of erlotinib with chemotherapy (44,45). Perhaps the TOPICAL phase III clinical trial was the last study with erlotinib as first-line therapy in unselected advanced stage NSCLC, which demonstrated the futility of EGFR inhibitors in patients without EGFR mutations (46,47). The EMPHASIS trial was another negative trial comparing erlotinib versus docetaxel in squamous cell lung cancer after failure to platinum-based chemotherapy (48). Two randomized trials (TaiLOR, DELTA) comparing single-agent chemotherapy to erlotinib as second-line treatment of unselected NSCLC reported that, statistically, chemotherapy significantly improved PFS compared with erlotinib (49,50). All these futile studies reinforce the backdrop of the standard of care with EGFR TKIs. Erlotinib is also approved but rarely used in combination with gemcitabine in metastatic pancreatic cancer (51).
Icotinib
Icotinib hydrochloride is the first self-developed small molecular drug in China, synthesized in 2002, that has a similar structure to erlotinib and gefitinib. It is designed and patented by Beta Pharma (Zhejiang, People’s Republic of China). In the phase III ICOGEN trial, icotinib was compared with gefitinib in NSCLC patients who have progressed to one or two lines of chemotherapy, and it was found to be equivalent regarding efficacy (Table 2). Icotinib was less toxic and better tolerated than gefitinib (52). The overall incidence of adverse events was 61% with icotinib versus 70% with gefitinib (P=0.046) (52). Based on the results of the ICOGEN study, icotinib was approved by the China Food and Drug Administration (CFDA) in June 2011 for the second- or third-line therapy of metastatic NSCLC (Figure 2). Some years later followed the open-label randomized phase III CONVINCE trial, in which first-line icotinib was compared with platinum-pemetrexed in EGFR-mutant NSCLC patients. Icotinib significantly improved PFS compared to chemotherapy (31) (Table 1). On the 13th of November 2014, icotinib was approved in China for the first-line treatment of EGFR-mutant NSCLC patients (59) (Figure 2).
Table 2
| Study name | Details of the study | PFS (months) | OS (months) | ORR (%) | Ref. |
|---|---|---|---|---|---|
| ICOGEN | Icotinib [200] vs. gefitinib [199]; 51% EGFR-mutant; previously treated; phase III | 4.6 (icotinib) vs. 3.4 (gefitinib); ns | 13.3 (icotinib) vs. 13.9 (gefitinib); ns | 27.6 (icotinib) vs. 27.2 (gefitinib); ns | (52) |
| LUX-Lung 8 | Afatinib [398] vs. erlotinib [397]; squamous-cell lung cancer; previously treated; phase III | 2.4 (afatinib) vs. 1.9 (erlotinib); P=0.0103 | 7.9 (afatinib) vs. 6.8 (erlotinib); P=0.0077 | 6.0 (afatinib) vs. 3.0 (erlotinib); ns | (53) |
| CTONG 0901 | Erlotinib [128] vs. gefitinib [128]; EGFR-mutant; 66% in 1st-line; phase III | 13.0 (erlotinib) vs. 10.4 (gefitinib); ns | 22.9 (erlotinib) vs. 20.1 (gefitinib); ns | 56.3 (erlotinib) vs. 52.3 (gefitinib); ns | (54) |
| LUX-Lung 7 | Afatinib [160] vs. gefitinib [159]; EGFR-mutant; 1st-line; phase IIb | 11.0 (afatinib) vs. 10.9 (gefitinib); P=0.017 | 27.9 (afatinib) vs. 24.5 (gefitinib); ns | 72.5 (afatinib) vs. 56.0 (gefitinib); P=0.0018 | (55,56) |
| ARCHER 1050 | Dacomitinib [227] vs. gefitinib [225]; EGFR-mutant; 1st-line; phase III | 14.7 (dacomitinib) vs. 9.2 (gefitinib); P<0.0001 | 34.1 (dacomitinib) vs. 26.8 (gefitinib); P=0.0438 | 75.0 (dacomitinib) vs. 72.0 (gefitinib); ns | (57,58) |
ns, not significant; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; PFS, progression-free survival; OS, overall survival; ORR, objective response rate.
Second-generation EGFR TKIs
The second-generation inhibitors, including afatinib and dacomitinib, are irreversible inhibitors, which covalently bind to EGFR. Just as gefitinib and erlotinib, afatinib is globally approved for the first-line therapy of EGFR-mutant patients, while dacomitinib is also under consideration for regulatory approval.
Afatinib
Afatinib (Gilotrif/Giotrif; Boehringer Ingelheim, Germany) is an ATP-competitive anilinoquinazoline derivative harboring a reactive acrylamide group. It covalently binds and irreversibly blocks enzymatically active ErbB receptor family members (60). Afatinib contains an electrophilic group capable of Michael addition to conserved cysteine residues within the catalytic domains of EGFR (Cys797), HER2 (Cys805), and ErbB-4 (Cys803) (60). The efficacy of afatinib in NSCLC has been evaluated in the LUX-lung program (Figure 3). Many of the trials in the program initially evaluated afatinib in unselected for EGFR populations, until we reached the pivotal 1st line trials of afatinib in EGFR-mutant NSCLC, LUX-Lung 3 and 6 (Figure 3).
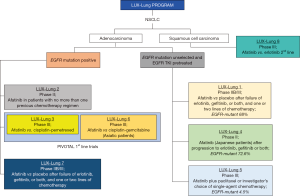
Afatinib is approved in U.S (July 2013) and Europe (September 2013) for the first-line therapy of EGFR-mutant NSCLC patients (Figure 2). The LUX-Lung 3 and 6 trials compared the efficacy of afatinib versus pemetrexed plus cisplatin (LUX-Lung 3) (32) or gemcitabine plus cisplatin (LUX-Lung 6) (34) and demonstrated its superiority regarding PFS and responses compared to chemotherapy (Table 1). The pooled analysis of LUX-Lung 3 and LUX-Lung 6 (33) was performed in order to assess the effect of afatinib on OS of patients with EGFR-mutant lung adenocarcinoma. In the whole population, there was no survival benefit with afatinib compared to platinum-based chemotherapy, but patients with EGFR deletion 19 derived a significant OS benefit in comparison with those carrying exon 21 L858R mutations (Table 1).
The LUX-Lung 8 study compared afatinib versus erlotinib as second-line therapy in patients with metastatic squamous-cell lung cancer (53). Afatinib was superior to erlotinib concerning PFS and OS (Table 2). The absolute improvement in PFS with afatinib over erlotinib was only half a month and was also associated with increased toxicities. Afatinib is approved in Europe and U.S. since April 2016, for the treatment of locally advanced or metastatic squamous-cell NSCLC after progression to platinum-based chemotherapy (Figure 2). At the time that the LUX-Lung 8 study was conceived, there were limited therapies for squamous-cell lung cancer after progression to platinum-based chemotherapy. Still, under these conditions, erlotinib was not the appropriate comparator in the LUX-Lung 8 study. As mentioned above in the EMPHASIS study, erlotinib was compared with docetaxel in the same setting and was not able to show a superiority of the EGFR TKI (48). The LUX-Lung 8 is far from being clinically relevant for this subset of patients, and, despite the regulatory approvals, afatinib should not be considered a standard second-line treatment in squamous-cell NSCLC.
In January 2018, FDA approved a supplemental New Drug Application for afatinib for the first-line treatment of patients with metastatic NSCLC whose tumors have non-resistant EGFR mutations including three additional EGFR mutations: L861Q, G719X, and S768I (Figures 1,2). The approval is based on a pooled analysis of the phase II LUX-Lung 2 and the phase III LUX-Lung 3 and LUX-Lung 6 studies. All three studies included patients with uncommon EGFR mutations, including L861Q, G719X or S768I (Table 3). Afatinib was active in 32 patients with rare nonresistant EGFR mutations based on ORR, duration of response, disease control, PFS and, OS (61,62).
Table 3
| Afatinib treated patients (N=32) | Confirmed responses (N=21) |
|---|---|
| L861Q (N=12) | 7 out of 12 (58%) |
| G719X (N=8) | 6 out of 8 (75%) |
| S768I + G719X (N=5) | 4 out of 5 (80%) |
| G719X + L861Q (N=3) | 2 out of 3 (67%) |
| S768I + L858R (N=2) | 1 out of 2 (50%) |
| S768I (N=1) | 1 out of 1 |
| L861Q + deletion 19 (N=1) | 0 out of 1 |
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer.
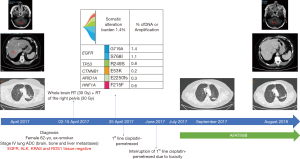
We report the case of a 62-year-old woman, ex-smoker, who was diagnosed in April 2017, with a stage IV (brain, bone and liver metastases) lung cancer. A biopsy of a bone metastasis with significant soft-tissue component revealed that it was a pan-negative lung adenocarcinoma. The patient started platinum-based chemotherapy after completing whole brain radiotherapy for the symptomatic brain metastases, and palliative pelvic radiotherapy for the right iliac and sacral symptomatic bone metastasis (Figure 4). She was included in the observational Spanish Lung Liquid versus Invasive biopsy Program (SLLIP, NCT03248089). Blood was collected before chemotherapy initiation, and her plasma DNA genome profile was performed with a clinically validated cell-free DNA (cfDNA) assay (Guardant360, Guardant Health Inc., CA, U.S.) (63,64). The results of the study revealed that the patient was carrying a double exon 18 (G719X) and exon 20 (S768I) EGFR mutation (Figure 4). After less than two months of treatment, we had to interrupt platinum-based chemotherapy due to grade IV hematologic toxicity and grade III asthenia. Based on the results of the SLIPP, we decided to switch therapy to afatinib 40 mg per day. After two weeks of therapy, she had to reduce afatinib to 30 mg per day due to grade III diarrhea and rash. The patient is still in therapy with no toxicity and almost complete response of her disease after 16 months from the diagnosis and 13 months from afatinib initiation (Figure 4).
Dacomitinib
Dacomitinib was developed by Pfizer as a second-generation irreversible EGFR TKI. It is a pan-HER inhibitor that was initially thought to inhibit the acquired resistance EGFR mutation T790M (65). However, the inhibitory concentration needed to inhibit 50% of the purified kinase activity of T790M is in the range of hundreds of nanomolar (65). The development of dacomitinib in NSCLC has been performed through the Advanced Research for Cancer targeted pan-HER therapy (ARCHER) program. As it is shown in Table 4, dacomitinib has been tested in several clinical studies and overall has failed to show better clinical efficacy over first-generation EGFR TKIs and with more toxicities in unselected NSCLC patients. Maybe the failure of ARCHER 1009 and NCIC BR-26 to achieve their primary endpoints delayed the approval and potential use of dacomitinib in NSCLC (72,73). For example, in the ARCHER 1009, dacomitinib was found to have similar efficacy to erlotinib as second-line therapy of NSCLC patients unselected for EGFR mutations. However, dacomitinib was more toxic than erlotinib, and the trial was not powered to be a non-inferiority trial (72). Dacomitinib failed to improve OS compared to placebo in the NCIC BR-26 trial in patients who had progressed to first-generation EGFR TKIs (73). These results remind us of those of the LUX-Lung-1 trial, where afatinib improved PFS over placebo, but there was no OS difference (74). Still, in the LUX-Lung-1 trial, there was OS benefit in the subgroup of patients whose best response to first-generation EGFR TKIs was not disease progression (74). Therefore, we consider that the negative results from the ARCHER 1009 and NCIC-BR-26 trials have delayed, and may have diminished, the clinical relevance of dacomitinib approval. In addition, the era of enrolling molecularly unselected NSCLC patients into clinical trials involving EGFR TKIs (or other targeted agents) has come to an end. A lot of time was lost during the development of dacomitinib, until the ARCHER 1050 was finally conducted and the results were recently presented (57,58), now that the third-generation EGFR TKIs have taken the lead in the first line setting.
Table 4
| ARCHER | Design title | Population | Endpoints | Ref. |
|---|---|---|---|---|
| 1001 | Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors | 121 (U.S) | RP2D =45 mg orally once daily | (66) |
| 1003 | Safety and efficacy of dacomitinib in Korean patients with KRAS wild-type advanced NSCLC refractory to chemotherapy and erlotinib or gefitinib: a phase I/II trial | 12 (South Korea) | RP2D =45 mg orally once daily; PFS at 4 mo =47.2%; median PFS 15.4 weeks, median OS =46.3 weeks; ORR 17.1% | (67) |
| 1005 | Phase I and pharmacokinetic study of dacomitinib (PF-00299804), an oral irreversible, pan-HER inhibitor in Japanese patients with advanced solid tumors | 13 (Japan) | RP2D = 45 mg orally once daily | (68) |
| 1002 | A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER inhibitor, in patients with advanced NSCLC after failure of prior chemotherapy and erlotinib | 66. ADC: 4.8%; non-ADC: 6.3%; EGFR-mutant: 8% | ADC: ORR=5%; non-ADC: ORR=6%; median PFS =12 weeks; median PFS for EGFR-mutant =18 weeks | (69) |
| 1017 | Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced NSCLC: a multicenter, open-label, phase 2 trial | 89. EGFR-mutant: 60% (51% with del 19 or L858R) | Median PFS=11.5 mo; median PFS for EGFR-mutant =18.2 mo; median OS= 29.5 mo; median OS for EGFR-mutant =40.2 mo; ORR =53.9%; ORR for EGFR-mutant =75.6% | (70) |
| 1028 | Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-HER inhibitor, vs. erlotinib in patients with advanced NSCLC | 188. EGFR-mutant: 15.9% | Median PFS=2.86 mo (dacomitinib) vs. 1.91 mo (erlotinib); P= 0.012; KRAS wild-type/EGFR any type: median PFS =3.71 mo (dacomitinib) vs. 1.91 mo (erlotinib); P= 0.006; KRAS wild-type/EGFR wild-type: median PFS =2.21 mo (dacomitinib) vs. 1.84 mo (erlotinib); P=0.043; median OS =9.53 mo (dacomitinib) vs. 7.44 mo (erlotinib); ns | (71) |
| 1009 | Dacomitinib vs. erlotinib in patients with advanced-stage, previously treated NSCLC (ARCHER 1009): a randomized, double-blind, phase 3 trial | 878. EGFR-mutant: 10% | Median PFS =2.6 mo for both dacomitinib and erlotinib; KRAS wild-type: median PFS =2.6 mo for both dacomitinib and erlotinib; median OS =7.9 mo (dacomitinib) vs. 8.4 mo (erlotinib); ns; KRAS wild-type: median OS =8.1 mo (dacomitinib) vs. 8.5 mo (erlotinib); ns | (72) |
| BR-26 | Dacomitinib compared with placebo in pretreated patients with advanced or metastatic NSCLC (NCIC CTG BR.26): a double-blind, randomized, phase 3 trial | 720. EGFR-mutant: 25.3% | Median OS =2.38 mo (dacomitinib) vs. 6.31 mo (placebo); ns. No differences in the subgroups | (73) |
| 1050 | Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive NSCLC (ARCHER 1050): a randomized, open-label, phase 3 trial | 452 EGFR-mutant | Median PFS =14.7 mo (dacomitinib) vs. 9.2 mo (gefitinib); P<0.0001; median PFS =34.1 mo (dacomitinib) vs. 26.8 mo (gefitinib); P=0.0438 | (57,58) |
RP2D, recommended phase II dose; mo, months; ADC, adenocarcinoma; ns, not significant; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PFS, progression-free survival; OS, overall survival; ORR, objective response rate.
Comparison of first and second-generation EGFR TKIs
Studies have head-to-head compared first and second-generation EGFR TKIs, but until now have failed to become clinically relevant. The phase III CTONG 0901 study compared gefitinib with erlotinib in a Chinese population, but did not identify a superior drug regarding PFS, OS or toxicity (54). Randomized clinical trials have compared the efficacy of gefitinib or erlotinib with the second-generation EGFR TKIs. Gefitinib has been compared with afatinib and dacomitinib in the LUX-Lung-7 (55,56) and the ARCHER-1050 (57,58) studies, respectively. In both studies, the second-generation EGFR TKIs, afatinib and dacomitinib were found to be superior to gefitinib, but with some caveats. For instance, in the LUX-Lung-7, although the difference in PFS between the two compounds was statistically significant, from the clinical point of view a PFS of 11 months with afatinib versus 10.9 months with erlotinib is not clinically relevant (55). There were no differences in OS, while ORR was significantly higher with afatinib (56) (Table 2). In the most recent ARCHER-1050 study, PFS was significantly longer with dacomitinib compared to gefitinib (14.7 versus 9.2 months, P<0.0001). Patients with brain metastases were excluded from the study, and the benefit of dacomitinib in comparison with gefitinib was more evident for Asian patients with a hazard ratio (HR) of 0.51 (0.39–0.66), compared to non-Asians HR of 0.89 (0.57–1.39) (57). In addition, the patients in the dacomitinib arm suffered more toxicity in comparison with gefitinib. Besides these caveats, dacomitinib is the first EGFR TKI (among the first- and second-generation EGFR TKIs) that shows a significant improvement in OS, with an estimated HR for OS of 0.760 (95% CI, 0.582–0.993; two-sided P=0.044) and a median OS of 34.1 months with dacomitinib versus 26.8 months with gefitinib (58) (Table 2). On the 4th of April 2018, FDA granted priority review for dacomitinib for the first-line treatment of EGFR-mutant NSCLC patients (Figure 2). The European Medicines Agency has also accepted the Marketing Authorization Application for dacomitinib for the same indication.
Although any comments on third-generation EGFR TKIs is out of the scope of the present review, it is worth mentioning that both erlotinib and gefitinib were compared with the third-generation inhibitor, osimertinib in the phase III FLAURA clinical trial (38). Based on a significant improvement in PFS with osimertinib compared to standard EGFR TKIs (18.9 versus 10.2 months) (38), osimertinib has received FDA (April 2018) and EMA (June 2018) approval for the first-line therapy of EGFR-mutant NSCLC patients. We are running the AZENT clinical trial (NCT02841579), which is testing the efficacy of osimertinib as first-line therapy in EGFR-mutant NSCLC patients that carry concomitant pre-treatment T790M mutation.
First- and second-generation EGFR TKIs and brain metastases
Lung cancer patients with EGFR mutations have high prevalence of brain metastases at the time of diagnosis (1) which increases more during the disease (75), making the role EGFR TKIs on brain metastases of great relevance (76). Gefitinib has a limited brain penetration rate of only 1% (77,78), while studies have shown that erlotinib penetrates the cerebrospinal fluid (CSF) (79-81). EGFR mutations were discovered in 2004 (4,6,7), and 1 year later we were shown the case of a Spanish female patient with an EGFR-mutant (deletion 19) lung adenocarcinoma and severe neurologic symptoms with impairment of walking, eating, and speaking that required a gastric feeding tube (82). The brain computed tomography showed multiple brain metastases. Gefitinib was given through a gastric feeding tube, and rapid recovery of neurologic functions was observed, accompanied by a regression of the brain metastases (82). We and others have found an activity of erlotinib against brain metastases (80,83).
Most of the information about the activity of gefitinib and erlotinib in brain metastases comes from retrospective or prospective studies not focused on EGFR-mutant patients [see also Table 1 in reference (76)]. It was in 2004, before understanding the role of EGFR mutations and targeted therapies, that gefitinib showed some activity in two reports of NSCLC patients with brain metastases, (84,85). Later, in a cohort of Korean patients with NSCLC and asymptomatic brain metastases, not previously irradiated, gefitinib or erlotinib therapy resulted in an intracranial response rate of 74% (86). An intracranial response rate of 57% was observed with gefitinib or erlotinib in a cohort of 43 Chinese NSCLC patients with brain metastases (87). Prospective studies have reported a response rate with gefitinib ranging from 10–33% in European (88) and Taiwanese (89) to 31–81% in Chinese (90) NSCLC patients with brain metastases.
It was only in 2013 that a Japanese phase II study of gefitinib concentrated on NSCLC patients with EGFR mutations and brain metastases (91). In this study, among 41 patients, an ORR of almost 90% with 13 complete responses was found with gefitinib. Twenty of the patients required brain radiotherapy (91). A phase II open-label single-institution clinical trial evaluated erlotinib or gefitinib for NSCLC patients harboring EGFR mutations with measurable metastatic brain disease (92). Among 28 patients enrolled, 83% achieved a partial response, and 11% had stable disease (92). No differences on efficacy (PFS and OS) were observed between the two EGFR TKIs used in the study (92). Dose escalation of erlotinib has been studied for increasing their CNS permeability. In a retrospective analysis, nine EGFR-mutant NSCLC patients with brain or leptomeningeal metastases occurring under regular doses of EGFR TKIs, were treated with 1,500 mg of erlotinib weekly (93). Six of them (67%) obtained a partial response. However median time to CNS progression was less than three months (93). Subsequently, a phase I study found that 1,200 mg of erlotinib given for two days of the week (D1 + D2) and then 50 mg of erlotinib for the rest of the week (D3 + D7) is the maximum tolerated dose. Among the patients who had brain metastases at study entry, none of them progressed in an untreated brain metastasis or had a new brain metastasis while on the study (94). In a retrospective analysis including patients with leptomeningeal carcinomatosis, erlotinib was superior to gefitinib with a cytologic conversion rate of 64.3% compared to 9.1% with gefitinib (95). Also, erlotinib offered clinical benefit in a series of NSCLC patients with leptomeningeal metastasis who had failed gefitinib treatment (96). The BRAIN phase III clinical trial comparing icotinib versus whole brain radiotherapy for EGFR-mutant patients with brain metastases demonstrated that icotinib was superior to radiotherapy regarding intracranial and overall PFS, response, and disease control rate (97). Still, these results have not established the role of icotinib in this setting (98).
As far as the second-generation EGFR TKIs concerns, subgroup analyses of patients with asymptomatic brain metastases from the LUX-Lung 3 and 6 trials, have shown that afatinib is more efficacious than compared to chemotherapy (99). However, the rates of CNS progression were similar among patients treated with afatinib or chemotherapy. Furthermore, in both studies, those patients who had received whole brain radiation therapy appeared to have better PFS benefit from afatinib than those who did not receive radiation (32,34). In the compassionate use program of afatinib, the activity of the drug in CNS has also been reported (100). In the LUX-Lung 7 trial, no statistically significant differences were found between afatinib and gefitinib in the subgroup of patients with asymptomatic brain metastasis (55). Finally, in most of the dacomitinib studies, patients with brain metastases are excluded.
In conclusion, data about the potential efficacy of first- and second-generation EGFR TKIs in patients with brain metastases need to be interpreted with caution, because they are derived from mainly retrospective reports and from small numbers of patients. We are still not confident to defer radiotherapy in EGFR-mutant patients with brain metastases that are about to start therapy with a first- or second-generation EGFR TKI. Furthermore, a recent pooled analysis has shown that the upfront use of an EGFR TKI (mainly erlotinib), in EGFR-mutant NSCLC patients with brain metastases and deferred radiotherapy is associated with shorter OS compared with radiotherapy followed by EGFR TKI (101). Of course, we are in the era of the CNS-active EGFR TKI osimertinib that has become the EGFR TKI of choice in newly diagnosed EGFR-mutant NSCLC patients considering that it triples median PFS, but also has high CNS response rate compared to gefitinib or erlotinib (38).
Mechanisms of resistance to first- and second-generation EGFR TKIs
It is very well known that after a median PFS of 11 months in the 50–80% of initially responding patients to first-and second-generation EGFR TKIs, different mechanisms of resistance occur (10,11,102,103). Acquirement of the T790M mutation in exon 20 is one such example and was detected in 50–60% of patients who were resistant to EGFR-TKIs (102). This secondary mutation causes impaired binding of the TKI, by increasing the binding pocket affinity for ATP. The result is a blockage of reversible first-generation TKI binding, and thus steric hindrance (104).
We have shown that besides being induced by EGFR-TKIs, the T790M mutation is detected in treatment-naive patients. According to our experience, pre-treatment T790M can be present in more than 30% of the patients and diminishes the magnitude of response to erlotinib (105-107). We have also demonstrated that redundant signaling pathways (108,109) can sustain and even hyper-activate pro-survival signaling, despite EGFR inhibition (110-116). Recently, we convincingly demonstrated that no EGFR TKI can abrogate STAT3 and Src-YAP1 (Yes-associated protein 1) (117-119). EGFR is co-expressed with other receptor and non-RTK, like AXL, MET, CUB domain-containing protein 1 (CDCP1) or Src homology-2 domain containing phosphatase (SHP2) which undermines responses to single EGFR TKIs, forbids complete responses and ultimately deprives patients of cure (117-119). Rational combinatorial therapies that target STAT3 and Src-YAP1 overcome this problem at least in culture and in vivo (117-119).
Combinations of first- and second-generation EGFR TKIs with chemotherapy, antiangiogenic agents, and immunotherapy
Considering the abundance of mechanisms of resistance that indeed can similarly occur with the third-generation EGFR TKIs, many efforts are ongoing, combining EGFR TKIs with other drugs, including anti-angiogenic, chemotherapy, immunotherapy or other targeted agents.
With anti-angiogenic agents
The addition of the vascular endothelial growth factor (VEGF) inhibitor, bevacizumab, to erlotinib was started to be tested in unselected populations, in several phase II and III clinical trials, before the clinical relevance of EGFR mutations was established (120-123). Retrospective analyses of EGFR-mutant patients demonstrated that the combination of erlotinib plus bevacizumab was better than the control arms. The phase II JO25567 clinical trial was conducted in Japan showing a significant benefit in PFS with the addition of the anti-angiogenic agent to erlotinib [HR, 0.54; 95% CI: 0.36–0.79; P=0.0015; median PFS 16.0 months for erlotinib plus bevacizumab and 9.7 months for erlotinib alone] (124,125). The combination has manageable toxicity, and it is well tolerated (125). Our BELIEF phase II single-arm clinical trial evaluated the combination of erlotinib plus bevacizumab in the first-line setting of EGFR-mutant patients, all of whom have been evaluated for the presence of the pre-treatment exon 20 missense mutation T790M (126). The study was based on a biological rational from preclinical studies. As previously mentioned, we and others have explored the role of pre-treatment T790M (105,107,127,128), and we have seen a worse outcome to erlotinib for patients with the double mutation (105,107). Preclinical studies had shown that the combination of gefitinib with bevacizumab inhibits tumor growth in H1975 xenograft tumors (129,130) carrying the 21 missense mutation (L858R) and T790M (4). The BELIEF study found that pre-treatment T790M was present in 37 out of the 109 patients included in the study. Median PFS was 16 months in the T790M-positive versus 10.5 months in the T790M-negative group (unadjusted HR, 0.52, 95% CI: 0.30–0.88; P=0.016) (126). From the results of the JO25567 and the BELIEF trials, erlotinib plus bevacizumab has received EMA (June 2016) approval for the first-line therapy of EGFR-mutant NSCLC patients (Figure 1). The NEJ026 and the BEVERLY studies are the first phase III clinical trials that compare erlotinib plus bevacizumab versus erlotinib alone as first-line therapy in Japanese and Caucasian EGFR-mutant patients, respectively (Table 5). A preplanned interim analysis of the NEJ026 study was presented at the 2018 ASCO Annual Meeting showing a median PFS of 16.9 months with erlotinib plus bevacizumab compared to 13.3 months with erlotinib alone (131). Finally, a meta-analysis of 2,802 unselected for EGFR-mutation patients showed that the combination of erlotinib plus bevacizumab did not improve OS, responses or PFS overall, but enhanced OS in EGFR mutant patients (132). The premise of a combination of an EGFR TKI plus bevacizumab in EGFR-mutant NSCLC is strong, given the signals from the already performed and the ongoing (Table 5) clinical trials. However, considering that both FDA an EMA have approved the third-generation EGFR TKI osimertinib for first-line treatment of metastatic NSCLC with most common EGFR mutations, the erlotinib plus bevacizumab combination may be obsolete and will not change the standard of care.
Table 5
| ClinicalTrials.gov identifier or other identifier | Phase; population | Treatment | Status |
|---|---|---|---|
| UMIN000017069 | Phase III; 1st-line; NEJ026 | Erlotinib + bevacizumab vs. erlotinib | Recruiting |
| NCT02633189 | Phase III; 1st-line; BEVERLY | Erlotinib + bevacizumab vs. erlotinib | Recruiting |
| NCT00436332 | Phase II; EGFR TKI-naive; BAC1 and ADENOBAC2; S0635 | Erlotinib + bevacizumab | Ongoing, not recruiting |
| NCT02655536 | Phase II; with brain metastases; EGFR TKI-naive; BRILLIANT | Erlotinib + bevacizumab vs. erlotinib | Recruiting |
| NCT02411448 | Phase III; EGFR TKI-naive; RELAY | Erlotinib + ramucirumab vs. erlotinib (part A and B); gefitinib + ramucirumab vs. gefitinib (part C) | Recruiting |
| NCT03461185 | Phase II; EGFR TKI-pretreated (stable disease for at least 2 months | Erlotinib or gefitinib + anti-angiogenic drugs (endostatin3 or apatinib4 or anlotinib5) vs. erlotinib or gefitinib | Not yet recruiting |
| NCT03050411 | Phase I; EGFR TKI-pretreated | Erlotinib + apatinib4 | Recruiting |
| NCT03628521 | Phase I; EGFR TKI-naive patients | Erlotinib + apatinib4 (arm A) | Recruiting |
| NCT03602027 | Phase I; EGFR TKI-naive patients | Gefitinib + apatinib4 | Not yet recruiting |
1BAC, bronchioloalveolar carcinoma; 2ADENOBAC, adenocarcinoma with BAC features; 3endostatin, naturally occurring, 20-kDa C-terminal fragment derived from type XVIII collagen with anti-angiogenic activity; 4anlotinib, receptor tyrosine kinase (RTK) inhibitor (including vascular endothelial growth factor receptor type 2 (VEGFR2) and type 3 (VEGFR3); 5apatinib (also known as YN968D1), TKI against VEGFR2. EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; NSCLC, non-small cell lung cancer.
With other targeted agents
The group of Dr. Rosell was among the first to be in favor of upfront combinatorial therapies for EGFR-mutant patients. Besides the abovementioned BELIEF study, we have carried out the GOAL phase I/II trial that compares gefitinib plus the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib versus gefitinib alone as first-line therapy in EGFR-mutant NSCLC patients (133). The rationale of the GOAL study comes from previous findings that low levels of breast cancer type 1 susceptibility (BRCA1) gene expression can be related with a better outcome to erlotinib and furthermore they can neutralize the nefarious effect of pre-treatment T790M (105). Although the results of the phase I part of the study were promising (134), in the phase II part of the study in which 186 EGFR-mutant patients were included, we were not able to find statistically significant differences in PFS between the two treatment arms. Specifically, the median PFS was 12.8 months for gefitinib plus olaparib versus 10.4 months for gefitinib alone (P=0.329) (135). We are now actively working to see whether the prespecified assessment of BRCA1, p53-binding protein 1 (53BP1), and other biomarkers including C-terminal binding protein interacting protein (CtIP) could determine whether a subgroup of patients may derive benefit from the combination.
There are multiple clinical trials ongoing, combining first- or second-generation EGFR TKIs with other targeted agents, like MET, AXL, PI3K, MEK or Src inhibitors (Table 6). There are also trials combining gefitinib or erlotinib with third-generation inhibitors as well as others that combine EGFR TKIs with monoclonal antibodies against EGFR (Table 6). We are carrying out in Spain and Latin America (Colombia) the phase Ib EPICAL clinical trial, which evaluates the safety and efficacy of afatinib with EGF pathway targeting immunization (EGF-PTI) in treatment-naive patients with EGFR-mutant NSCLC (136). The study was designed based on preclinical findings, generated in our laboratory, showing that the addition of EGF-PTI (that is already in phase III clinical trials) to EGFR TKIs, delays the emergence of resistance and more efficiently than EGFR TKI alone abrogates the EGFR downstream signaling pathways among them STAT3 and NOTCH (137). The study is currently accruing patients (NCT03623750).
Table 6
| ClinicalTrials.gov identifier | Phase; population | Treatment | Status |
|---|---|---|---|
| NCT01487265 | Phase II; erlotinib-pretreated (sensitive) | Erlotinib + buparlisib (BKM120; selective PI3K inhibitor) | Ongoing, not recruiting |
| NCT01570296 | Phase Ib; EGFR TKI-pretreated; molecular alterations of PI3K pathway; EGFR overexpression | Gefitinib + buparlisib | Ongoing, not recruiting |
| NCT02424617 | Phase I/II; erlotinib-pretreated | Erlotinib + BGB324 (bemcentinib, R428; AXL inhibitor) | Recruiting |
| NCT03599518 | Phase I; EGFR TKI-pretreated | Gefitinib + DS-1205c (AXL inhibitor) | Not yet recruiting |
| NCT03333343 | Phase Ib; EGFR TKI-naive or not | Gefitinib + nazartinib (EGF816)1 | Recruiting |
| NCT03292133 | Phase II; EGFR TKI-naive | Gefitinib + nazartinib | Recruiting |
| NCT00600496 | Phase I; EGFR TKI-pretreated | Erlotinib + selumetinib (arm 3) | Ongoing, not recruiting |
| NCT03076164 | Phase I/II; erlotinib-pretreated | Erlotinib + trametinib | Recruiting |
| NCT01859026 | Phase I/Ib; EGFR TKI-naive or not | Erlotinib + MEK162 (binimetinib; selective MEK inhibitor) | Recruiting |
| NCT02468661 | Phase I/II; EGFR TKI-pretreated; c-MET amplified | Erlotinib + INC280 (capmatinib; c-MET inhibitor) vs. chemotherapy (phase II) | Recruiting |
| NCT01610336 | Phase IB/II; EGFR TKI-pretreated; c-MET amplified | Gefitinib + INC280 | Ongoing, not recruiting |
| NCT02374645 | Phase Ib; EGFR TKI-pretreated; c-MET amplified | Gefitinib + volitinib (c-MET inhibitor) | Ongoing, not recruiting |
| NCT01982955 | Phase Ib/II; EGFR TKI-pretreated; c-MET amplified and T790M negative | Gefitinib + tepotinib (c-MET inhibitor) | Ongoing, not recruiting |
| NCT03122717 | Phase I; EGFR TKI-naive | Gefitinib + osimertinib (combined or alternative) | Recruiting |
| NCT02535338 | Phase I/II; EGFR TKI-pretreated | Erlotinib + onalespib lactate2 | Ongoing, not recruiting |
| NCT02716311 | Phase II; EGFR TKI-naive | Afatinib + cetuximab | Recruiting |
| NCT03623750 | Phase I/II; EGFR TKI-naive; EPICAL study | Afatinib + EGF-PTI3 | Recruiting |
| NCT03054038 | Phase I; EGFR TKI-pretreated | Afatinib + necitumumab | Recruiting |
| NCT02438722 | Phase II/III; EGFR TKI-naive; S1403 | Afatinib + cetuximab vs. afatinib | Recruiting |
| NCT01999985 | Phase I; EGFR TKI-pretreated; objective response, or stable disease for at least 6 months | Afatinib + dasatinib | Ongoing, not recruiting |
1EGF816, irreversible, third-generation, mutant-selective EGFR, with activity against T790M; 2onalespib lactate, the lactate form of onalespib, a synthetic, orally bioavailable, small-molecule inhibitor of heat shock protein 90 (Hsp90); 3EGF-PTI, EGF pathway targeting immunisation. EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; NSCLC, non-small cell lung cancer.
With immunotherapy
The effect of immunotherapy in EGFR-mutant NSCLC is debatable. In most of the studies with immune checkpoint inhibitors, EGFR-mutant patients derive no benefit (138). Whether this can be because patients with EGFR mutations are light or never smokers, with therefore low tumor mutational burden, or because EGFR-mutant tumors have low PD-L1 expression and infiltrating CD8+ T cells, is still not very clear (139). The only exception until now comes from the IMpower 150 trial, in which there was a PFS benefit with the combination of atezolizumab plus bevacizumab plus chemotherapy for EGFR-mutant patients compared to those receiving bevacizumab and chemotherapy (median PFS 9.7 months vs. 6.1 months; unstratified HR, 0.59; 95% CI: 0.37–0.94) (140). However, the comparison of the triple combination (atezolizumab plus bevacizumab plus chemotherapy) versus atezolizumab plus chemotherapy is not reported in the study. Indeed, the main biological question behind the IMpower 150 trial was whether VEGF blockade enhanced the efficacy of immunotherapy. Considering that this important information is omitted, we cannot rely on the findings of the IMpower 150 trial (140).
There are several trials ongoing exploring the combination of first and second-generation EGFR TKIs with immunotherapy (Table 7). Preliminary results from the CheckMate 012 study suggest that nivolumab plus erlotinib may provide some benefit with acceptable safety profile EGFR TKI-resistant (141). In an ongoing phase Ib study 10 EGFR TKI-naive patients were treated with concurrent durvalumab and gefitinib (arm 1) or with gefitinib monotherapy for 4 weeks followed by concurrent durvalumab plus gefitinib (arm 2) (142). No clinically relevant differences in the ORR (approximately 79%) compared to what is already known for gefitinib monotherapy (73.7%) (25) were observed, and 55% of the patients suffered grade 3 adverse events (142). In the GEFTREM study that combined the anti-cytotoxic T-lymphocyte associated protein 4 (anti-CTLA4) antibody, tremelimumab, with gefitinib in previously treated EGFR-mutant patients, the best overall response was stable disease in 67% of patients, and all patients discontinued treatment after median duration of 8 weeks mainly due to disease progression (143).
Table 7
| ClinicalTrials.gov identifier | Phase; population | Treatment | Status |
|---|---|---|---|
| NCT01998126 | Phase I; EGFR TKI-naive or not | Nivolumab/ipilimumab + erlotinib | Ongoing, not recruiting |
| NCT01454102 | Phase I; EGFR TKI-naive or not; CheckMate 012 | Nivolumab + erlotinib (arm E) | Ongoing, not recruiting |
| NCT02574078 | Phase I/II; EGFR TKI-naive or not (maintenance) | Nivolumab + erlotinib | Ongoing, not recruiting |
| NCT02039674 | A Phase I/II; EGFR TKI-naive | Pembrolizumab + erlotinib (cohort E); pembrolizumab + gefitinib (cohort F) | Ongoing, not recruiting |
| NCT02364609 | Phase I; erlotinib-resistant | Pembrolizumab + afatinib | Recruiting |
| NCT03157089 | Phase II; squamous cell lung cancer; 3rd-line and more; (LUX-Lung IO) | Pembrolizumab + afatinib | Recruiting |
| NCT02013219 | Phase I; EGFR TKI-naive | Atezolizumab + erlotinib | Ongoing, not recruiting |
| NCT02088112 | Phase I; EGFR TKI-naive or not | Durvalumab + gefitinib | Ongoing, not recruiting |
| NCT01998126 | Phase I; EGFR TKI-naive or not | Ipilimumab + erlotinib | Ongoing, not recruiting |
| NCT02040064 | Phase I; EGFR TKI-pretreated; GEFTREM | Tremelimumab + gefitinib | Ongoing, not recruiting |
| NCT02906163 | Phase I/II; EGFR TKI-naive | Afatinib, gefitinib, erlotinib + thymosin alpha 1 (a peptide immune modulator) (phase II) vs. afatinib, gefitinib, erlotinib | Ongoing, not recruiting |
EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Currently, there are several clinical studies in progress, and data will soon be published about the combination of EGFR TKIs and immunotherapy (Table 7). Until then, we should deprioritize immunotherapy (alone or in combinations) for the first- or second-line therapy of EGFR-mutant patients and relegate it to a later treatment, if at all.
First and second-generation EGFR TKIs in early-stage disease
It is still not clear whether EGFR TKIs have similar efficacy in metastatic disease as in patients with early-stage disease. Caution should be taken, since single therapy with EGFR TKIs abrogates EGFR downstream signaling, but also activates parallel signaling pathways and factors involved in cell migration, invasion, and metastasis (117-119,144,145). When, for instance, gefitinib was compared with placebo (the NCIC CTG BR19 study) for the post-operative treatment of operated stage IB–IIIA NSCLC patients, no benefit, or even worse outcome with gefitinib was found, even for the subpopulation carrying EGFR mutations (146). The trial was prematurely closed (146). Similar results were generated from the RADIANT phase III clinical trial, in which erlotinib was not able to prolong DFS in resected IB-IIIA EGFR overexpressing NSCLC patients (147).
However, there are also studies with promising results. Gefitinib has been tested as six-month maintenance after platinum-based chemotherapy in a phase II study which included surgically resected stage IIIA (N2 positive) EGFR-mutant NSCLC patients (148). The combination was related to a significant improvement in disease-free survival (DFS) in comparison with adjuvant chemotherapy alone (148) (Table 8). The phase III ADJUVANT study followed in China. The ADJUVANT study is the only phase III clinical trial in which gefitinib was associated with longer DFS compared with platinum-based chemotherapy for the adjuvant therapy of surgically resected stage II–IIIA EGFR-mutant NSCLC patients (149). Still, the study has been strongly criticized (154-156), due to its several caveats, including the non-proportionality of the Kaplan-Meier curves. The ADJUVANT study has not changed the clinical practice for postoperative therapy of EGFR-mutant NSCLC patients. The EVAN phase II study, similar to the ADJUVANT trial, was conducted in Asia in stage IIIA EGFR-mutant, surgically resected patients. Patients were allocated to cisplatin plus vinorelbine for four cycles or erlotinib for 2 years (151). The study demonstrated a longer DFS with erlotinib compared to chemotherapy (Table 8). In the phase II, non-randomized SELECT trial, adjuvant erlotinib after standard chemotherapy conferred a 90% 2-year DFS (150). The phase II RECEL study compared erlotinib versus etoposide plus cisplatin both with concurrent radiotherapy in unresectable stage III EGFR-mutant NSCLC. The results were in favor of erlotinib (152) (Table 8).
Table 8
| EGFR TKI | Study | Number of patients, population | Design | Primary endpoint | Ref. |
|---|---|---|---|---|---|
| Gefitinib | ADJUVANT, phase III | 222, II–IIIA EGFR-mutant | Gefitinib vs. platinum-based chemotherapy | HR for DFS =0.60; P=0.0054 | (149) |
| NCIC CTG BR19, phase III | 503 stage IB–IIIA, unselected | Gefitinib vs. placebo for 2 years | HR for OS =1.24; P=0.14 | (146) | |
| Phase II, randomized | 60, IIIA (N2), EGFR-mutant | Platinum-based chemotherapy followed by gefitinib for 6 months vs. platinum-based chemotherapy | HR for DFS =0.37; P=0.014 | (148) | |
| Erlotinib | SELECT, phase II, non-randomised | 100, IA–IIIA, EGFR-mutant | Platinum-based chemotherapy followed by erlotinib for 2 years | 2-year DFS rate =90% | (150) |
| EVAN, phase II | 100 IIIA EGFR-mutant | Erlotinib (2 years) vs. platinum-based chemotherapy | HR for DFS =0.26; P<0.001 | (151) | |
| RADIANT, phase III | 973, IB–IIIA, EGFR overexpression (IHC or FISH) | Erlotinib vs. placebo for 2 years | HR for DFS =0.90; P=0.324 | (147) | |
| RECEL, phase II | 41, unresectable stage III, EGFR-mutant | Erlotinib + RT (erlotinib for 2 years) vs. cisplatin-etoposide + RT | HR for PFS =0.053; P<0.001 | (152) | |
| Icotinib | Phase II, randomized | 41, IB–IIIA, EGFR-mutant | Platinum-based chemotherapy followed by icotinib for 4–8 months vs. platinum-based chemotherapy | 24 months DFS 90.5% vs. 66.7%; P=0. 066 | (153) |
EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; NSCLC, non-small cell lung cancer; DFS, disease-free survival; HR, hazard ratio; PFS, progression-free survival.
Icotinib was combined with chemotherapy in a phase II clinical trial for the adjuvant therapy of surgically resected stage IB–IIIA EGFR-mutant Chinese NSCLC. The study demonstrated a non-statistically significant trend for better 2-year DFS when icotinib was combined with adjuvant chemotherapy (153) (Table 8). There are several phase II clinical trials ongoing that explore the effect of adjuvant EGFR TKIs (including the third-generation EGFR TKI, osimertinib) for EGFR-mutant NSCLC patients [see for details Table 2 in ref (157)].
Conclusions
With the availability of multiple compounds for EGFR-mutant NSCLC, treatment needs to be considered with a long-term plan to maximize survival. There is evidence that the second-generation EGFR TKIs, and even more so, the third-generation EGFR TKI, osimertinib, are more potent than gefitinib or erlotinib. However, several factors should be considered when deciding on the first-line therapy of an EGFR-mutant patient, including the presence of brain metastases, the type of EGFR-mutation and the tolerability profile. It is unavoidable that research in the next few years will focus on outcomes and tolerability of different sequences so that we finally turn EGFR-mutant NSCLC into a chronic disease.
Acknowledgments
Funding: Work in Dr. Rosell’s laboratory is partially supported by a grant from
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Umberto Malapelle, Christian Rolfo) for the series “Targeted Therapy and Non-Small Cell Lung Cancer: A New Era?” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.10.06). The series “Targeted Therapy and Non-Small Cell Lung Cancer: A New Era?” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [Crossref] [PubMed]
- Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci 2008;65:1566-84. [Crossref] [PubMed]
- Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res 2006;12:5268-72. [Crossref] [PubMed]
- Sordella R, Bell DW, Haber DA, et al. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 2004;305:1163-7. [Crossref] [PubMed]
- Lazzara MJ, Lane K, Chan R, et al. Impaired SHP2-mediated extracellular signal-regulated kinase activation contributes to gefitinib sensitivity of lung cancer cells with epidermal growth factor receptor-activating mutations. Cancer Res 2010;70:3843-50. [Crossref] [PubMed]
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Kosaka T, Yatabe Y, Endoh H, et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res 2004;64:8919-23. [Crossref] [PubMed]
- Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer 2007;7:169-81. [Crossref] [PubMed]
- Rosell R, Moran T, Carcereny E, et al. Non-small-cell lung cancer harbouring mutations in the EGFR kinase domain. Clin Transl Oncol 2010;12:75-80. [Crossref] [PubMed]
- Bartholomew C, Eastlake L, Dunn P, et al. EGFR targeted therapy in lung cancer; an evolving story. Respir Med Case Rep 2017;20:137-40. [Crossref] [PubMed]
- Kazandjian D, Blumenthal GM, Yuan W, et al. FDA Approval of Gefitinib for the Treatment of Patients with Metastatic EGFR Mutation-Positive Non-Small Cell Lung Cancer. Clin Cancer Res 2016;22:1307-12. [Crossref] [PubMed]
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) J Clin Oncol 2003;21:2237-46. [corrected]. [Crossref] [PubMed]
- Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 2003;290:2149-58. [Crossref] [PubMed]
- Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527-37. [Crossref] [PubMed]
- Kim ES, Hirsh V, Mok T, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 2008;372:1809-18. [Crossref] [PubMed]
- Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol 2004;22:777-84. [Crossref] [PubMed]
- Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol 2004;22:785-94. [Crossref] [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [Crossref] [PubMed]
- Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med 2005;353:133-44. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Yoshioka H, Mitsudomi T, Morita S, et al. Final overall survival results of WJTOG 3405, a randomized phase 3 trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer (NSCLC) harboring mutations of the epidermal growth factor receptor (EGFR). J Clin Oncol 2014;32:8117. [Crossref]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Nakamura A, Inoue A, Morita S, et al. Phase III study comparing gefitinib monotherapy (G) to combination therapy with gefitinib, carboplatin, and pemetrexed (GCP) for untreated patients (pts) with advanced non-small cell lung cancer (NSCLC) with EGFR mutations (NEJ009). J Clin Oncol 2018;36:9005. [Crossref]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877-83. [Crossref] [PubMed]
- Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015;26:1883-9. [Crossref] [PubMed]
- Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol 2017;28:2443-50. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9. [Crossref] [PubMed]
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8. [Crossref] [PubMed]
- Douillard JY, Ostoros G, Cobo M, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. Br J Cancer 2014;110:55-62. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Moyer JD, Barbacci EG, Iwata KK, et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res 1997;57:4838-48. [PubMed]
- Johnson JR, Cohen M, Sridhara R, et al. Approval summary for erlotinib for treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. Clin Cancer Res 2005;11:6414-21. [Crossref] [PubMed]
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [Crossref] [PubMed]
- Cohen MH, Johnson JR, Chattopadhyay S, et al. Approval summary: erlotinib maintenance therapy of advanced/metastatic non-small cell lung cancer (NSCLC). Oncologist 2010;15:1344-51. [Crossref] [PubMed]
- Benlloch S, Botero ML, Beltran-Alamillo J, et al. Clinical validation of a PCR assay for the detection of EGFR mutations in non-small-cell lung cancer: retrospective testing of specimens from the EURTAC trial. PLoS One 2014;9:e89518. [Crossref] [PubMed]
- Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2005;23:5892-9. [Crossref] [PubMed]
- Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol 2007;25:1545-52. [Crossref] [PubMed]
- Lee SM, Khan I, Upadhyay S, et al. First-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy (TOPICAL): a double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2012;13:1161-70. [Crossref] [PubMed]
- Karachaliou N, Wei J, Marques AD, et al. Front-line erlotinib in unselected patients with advanced NSCLC and poor performance status - the TOPICAL study. Transl Lung Cancer Res 2013;2:58-61. [PubMed]
- Peters S, Stahel RA, Dafni U, et al. Randomized Phase III Trial of Erlotinib versus Docetaxel in Patients with Advanced Squamous Cell Non-Small Cell Lung Cancer Failing First-Line Platinum-Based Doublet Chemotherapy Stratified by VeriStrat Good versus VeriStrat Poor. The European Thoracic Oncology Platform (ETOP) EMPHASIS-lung Trial. J Thorac Oncol 2017;12:752-62. [Crossref] [PubMed]
- Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981-8. [Crossref] [PubMed]
- Kawaguchi T, Ando M, Asami K, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol 2014;32:1902-8. [Crossref] [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [Crossref] [PubMed]
- Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol 2013;14:953-61. [Crossref] [PubMed]
- Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 2015;16:897-907. [Crossref] [PubMed]
- Yang JJ, Zhou Q, Yan HH, et al. A phase III randomised controlled trial of erlotinib vs. gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer 2017;116:568-74. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Mok TS, Cheng Y, Zhou X, et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non–Small-Cell Lung Cancer and EGFR-Activating Mutations. J Clin Oncol 2018;36:2244-50. [Crossref] [PubMed]
- Shi Y, Sun Y, Ding C, et al. China experts consensus on icotinib for non-small cell lung cancer treatment (2015 version). Ann Transl Med 2015;3:260. [PubMed]
- Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 2012;343:342-50. [Crossref] [PubMed]
- D'Arcangelo M, Hirsch FR. Clinical and comparative utility of afatinib in non-small cell lung cancer. Biologics 2014;8:183-92. [PubMed]
- Karachaliou N, Molina-Vila MA, Rosell R. The impact of rare EGFR mutations on the treatment response of patients with non-small cell lung cancer. Expert Rev Respir Med 2015;9:241-4. [Crossref] [PubMed]
- Blakely CM, Watkins TBK, Wu W, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet 2017;49:1693-704. [Crossref] [PubMed]
- Odegaard JI, Vincent JJ, Mortimer S, et al. Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue- and plasma-based methodologies. Clin Cancer Res 2018;24:3539-49. [Crossref] [PubMed]
- Engelman JA, Zejnullahu K, Gale CM, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res 2007;67:11924-32. [Crossref] [PubMed]
- Janne PA, Boss DS, Camidge DR, et al. Phase I dose-escalation study of the pan-HER inhibitor, PF299804, in patients with advanced malignant solid tumors. Clin Cancer Res 2011;17:1131-9. [Crossref] [PubMed]
- Park K, Cho BC, Kim DW, et al. Safety and efficacy of dacomitinib in korean patients with KRAS wild-type advanced non-small-cell lung cancer refractory to chemotherapy and erlotinib or gefitinib: a phase I/II trial. J Thorac Oncol 2014;9:1523-31. [Crossref] [PubMed]
- Takahashi T, Boku N, Murakami H, et al. Phase I and pharmacokinetic study of dacomitinib (PF-00299804), an oral irreversible, small molecule inhibitor of human epidermal growth factor receptor-1, -2, and -4 tyrosine kinases, in Japanese patients with advanced solid tumors. Invest New Drugs 2012;30:2352-63. [Crossref] [PubMed]
- Reckamp KL, Giaccone G, Camidge DR, et al. A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer 2014;120:1145-54. [Crossref] [PubMed]
- Janne PA, Ou SH, Kim DW, et al. Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: a multicentre, open-label, phase 2 trial. Lancet Oncol 2014;15:1433-41. [Crossref] [PubMed]
- Ramalingam SS, Blackhall F, Krzakowski M, et al. Randomized phase II study of dacomitinib (PF-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2012;30:3337-44. [Crossref] [PubMed]
- Ramalingam SS, Janne PA, Mok T, et al. Dacomitinib versus erlotinib in patients with advanced-stage, previously treated non-small-cell lung cancer (ARCHER 1009): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:1369-78. [Crossref] [PubMed]
- Ellis PM, Shepherd FA, Millward M, et al. Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR.26): a double-blind, randomised, phase 3 trial. Lancet Oncol 2014;15:1379-88. [Crossref] [PubMed]
- Miller VA, Hirsh V, Cadranel J, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 2012;13:528-38. [Crossref] [PubMed]
- Li L, Luo S, Lin H, et al. Correlation between EGFR mutation status and the incidence of brain metastases in patients with non-small cell lung cancer. J Thorac Dis 2017;9:2510-20. [Crossref] [PubMed]
- Karachaliou N, Rosell R. Treatment of brain metastases in non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations: the role of EGFR tyrosine kinase inhibitors. Ann Palliat Med 2013;2:114-7. [PubMed]
- McGranahan T, Nagpal S. A Neuro-oncologist's Perspective on Management of Brain Metastases in Patients with EGFR Mutant Non-small Cell Lung Cancer. Curr Treat Options Oncol 2017;18:22. [Crossref] [PubMed]
- Chen Y, Wang M, Zhong W, et al. Pharmacokinetic and pharmacodynamic study of Gefitinib in a mouse model of non-small-cell lung carcinoma with brain metastasis. Lung Cancer 2013;82:313-8. [Crossref] [PubMed]
- Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399-405. [Crossref] [PubMed]
- Deng Y, Feng W, Wu J, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol Clin Oncol 2014;2:116-20. [Crossref] [PubMed]
- Weber B, Winterdahl M, Memon A, et al. Erlotinib accumulation in brain metastases from non-small cell lung cancer: visualization by positron emission tomography in a patient harboring a mutation in the epidermal growth factor receptor. J Thorac Oncol 2011;6:1287-9. [Crossref] [PubMed]
- Taron M, Ichinose Y, Rosell R, et al. Activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor are associated with improved survival in gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer Res 2005;11:5878-85. [Crossref] [PubMed]
- Porta R, Sanchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J 2011;37:624-31. [Crossref] [PubMed]
- Hotta K, Kiura K, Ueoka H, et al. Effect of gefitinib ('Iressa', ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer 2004;46:255-61. [Crossref] [PubMed]
- Namba Y, Kijima T, Yokota S, et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: review of 15 clinical cases. Clin Lung Cancer 2004;6:123-8. [Crossref] [PubMed]
- Kim JE, Lee DH, Choi Y, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer 2009;65:351-4. [Crossref] [PubMed]
- Zhang Q, Zhang X, Yan H, et al. Effects of epidermal growth factor receptor-tyrosine kinase inhibitors alone on EGFR-mutant non-small cell lung cancer with brain metastasis. Thorac Cancer 2016;7:648-54. [Crossref] [PubMed]
- Ceresoli GL, Cappuzzo F, Gregorc V, et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol 2004;15:1042-7. [Crossref] [PubMed]
- Chiu CH, Tsai CM, Chen YM, et al. Gefitinib is active in patients with brain metastases from non-small cell lung cancer and response is related to skin toxicity. Lung Cancer 2005;47:129-38. [Crossref] [PubMed]
- Wu C, Li YL, Wang ZM, et al. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer 2007;57:359-64. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282-7. [Crossref] [PubMed]
- Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012;77:556-60. [Crossref] [PubMed]
- Grommes C, Oxnard GR, Kris MG, et al. "Pulsatile" high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol 2011;13:1364-9. [Crossref] [PubMed]
- Yu HA, Sima C, Feldman D, et al. Phase 1 study of twice weekly pulse dose and daily low-dose erlotinib as initial treatment for patients with EGFR-mutant lung cancers. Ann Oncol 2017;28:278-84. [PubMed]
- Lee E, Keam B, Kim DW, et al. Erlotinib versus gefitinib for control of leptomeningeal carcinomatosis in non-small-cell lung cancer. J Thorac Oncol 2013;8:1069-74. [Crossref] [PubMed]
- Katayama T, Shimizu J, Suda K, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol 2009;4:1415-9. [Crossref] [PubMed]
- Yang JJ, Zhou C, Huang Y, et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med 2017;5:707-16. [Crossref] [PubMed]
- Rosell R, Karachaliou N. Brain metastases in patients with EGFR-mutant non-small-cell lung cancer. Lancet Respir Med 2017;5:669-71. [Crossref] [PubMed]
- Schuler M, Wu YL, Hirsh V, et al. First-Line Afatinib versus Chemotherapy in Patients with Non-Small Cell Lung Cancer and Common Epidermal Growth Factor Receptor Gene Mutations and Brain Metastases. J Thorac Oncol 2016;11:380-90. [Crossref] [PubMed]
- Hoffknecht P, Tufman A, Wehler T, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol 2015;10:156-63. [Crossref] [PubMed]
- Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naive Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol 2017;35:1070-7. [Crossref] [PubMed]
- Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014;11:473-81. [Crossref] [PubMed]
- Karachaliou N, Rosell R, Morales-Espinosa D, et al. Systemic treatment in EGFR-ALK NSCLC patients: second line therapy and beyond. Expert Rev Anticancer Ther 2014;14:807-15. [Crossref] [PubMed]
- Heydt C, Michels S, Thress KS, et al. Novel approaches against epidermal growth factor receptor tyrosine kinase inhibitor resistance. Oncotarget 2018;9:15418-34. [Crossref] [PubMed]
- Rosell R, Molina MA, Costa C, et al. Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutations. Clin Cancer Res 2011;17:1160-8. [Crossref] [PubMed]
- Rosell R, Karachaliou N. Implications of Blood-Based T790M Genotyping and Beyond in Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3361-2. [Crossref] [PubMed]
- Costa C, Molina MA, Drozdowskyj A, et al. The impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trial. Clin Cancer Res 2014;20:2001-10. [Crossref] [PubMed]
- Rosell R, Karachaliou N, Morales-Espinosa D, et al. Adaptive resistance to targeted therapies in cancer. Transl Lung Cancer Res 2013;2:152-9. [PubMed]
- Rosell R, Bivona TG, Karachaliou N. Genetics and biomarkers in personalisation of lung cancer treatment. Lancet 2013;382:720-31. [Crossref] [PubMed]
- Karachaliou N, Costa C, Gimenez-Capitan A, et al. BRCA1, LMO4, and CtIP mRNA expression in erlotinib-treated non-small-cell lung cancer patients with EGFR mutations. J Thorac Oncol 2013;8:295-300. [Crossref] [PubMed]
- Karachaliou N, Codony-Servat J, Teixido C, et al. BIM and mTOR expression levels predict outcome to erlotinib in EGFR-mutant non-small-cell lung cancer. Sci Rep 2015;5:17499. [Crossref] [PubMed]
- Jacobsen K, Bertran-Alamillo J, Molina MA, et al. Convergent Akt activation drives acquired EGFR inhibitor resistance in lung cancer. Nat Commun 2017;8:410. [Crossref] [PubMed]
- Karachaliou N, Rosell R, Molina MA, et al. Predicting resistance by selection of signaling pathways. Transl Lung Cancer Res 2014;3:107-15. [PubMed]
- Karachaliou N, Gimenez-Capitan A, Drozdowskyj A, et al. ROR1 as a novel therapeutic target for EGFR-mutant non-small-cell lung cancer patients with the EGFR T790M mutation. Transl Lung Cancer Res 2014;3:122-30. [PubMed]
- Zhang Z, Lee JC, Lin L, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet 2012;44:852-60. [Crossref] [PubMed]
- Bivona TG, Hieronymus H, Parker J, et al. FAS and NF-kappaB signalling modulate dependence of lung cancers on mutant EGFR. Nature 2011;471:523-6. [Crossref] [PubMed]
- Karachaliou N, Chaib I, Cardona AF, et al. Common Co-activation of AXL and CDCP1 in EGFR-mutation-positive Non-Small Cell Lung Cancer Associated With Poor Prognosis. EBioMedicine 2018;29:112-27. [Crossref] [PubMed]
- Chaib I, Karachaliou N, Pilotto S, et al. Co-activation of STAT3 and YES-Associated Protein 1 (YAP1) Pathway in EGFR-Mutant NSCLC. J Natl Cancer Inst 2017;109: [Crossref] [PubMed]
- Codony-Servat C, Codony-Servat J, Karachaliou N, et al. Activation of signal transducer and activator of transcription 3 (STAT3) signaling in EGFR mutant non-small-cell lung cancer (NSCLC). Oncotarget 2017;8:47305-16. [Crossref] [PubMed]
- Herbst RS, O'Neill VJ, Fehrenbacher L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol 2007;25:4743-50. [Crossref] [PubMed]
- Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 2011;377:1846-54. [Crossref] [PubMed]
- Ciuleanu T, Tsai CM, Tsao CJ, et al. A phase II study of erlotinib in combination with bevacizumab versus chemotherapy plus bevacizumab in the first-line treatment of advanced non-squamous non-small cell lung cancer. Lung Cancer 2013;82:276-81. [Crossref] [PubMed]
- Johnson BE, Kabbinavar F, Fehrenbacher L, et al. ATLAS: randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2013;31:3926-34. [Crossref] [PubMed]
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236-44. [Crossref] [PubMed]
- Kato T, Seto T, Nishio M, et al. Erlotinib Plus Bevacizumab Phase ll Study in Patients with Advanced Non-small-Cell Lung Cancer (JO25567): Updated Safety Results. Drug Saf 2018;41:229-37. [Crossref] [PubMed]
- Rosell R, Dafni U, Felip E, et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): an international, multicentre, single-arm, phase 2 trial. Lancet Respir Med 2017;5:435-44. [Crossref] [PubMed]
- Watanabe M, Kawaguchi T, Isa S, et al. Ultra-Sensitive Detection of the Pretreatment EGFR T790M Mutation in Non-Small Cell Lung Cancer Patients with an EGFR-Activating Mutation Using Droplet Digital PCR. Clin Cancer Res 2015;21:3552-60. [Crossref] [PubMed]
- Chen LY, Molina-Vila MA, Ruan SY, et al. Coexistence of EGFR T790M mutation and common activating mutations in pretreatment non-small cell lung cancer: A systematic review and meta-analysis. Lung Cancer 2016;94:46-53. [Crossref] [PubMed]
- Naumov GN, Nilsson MB, Cascone T, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res 2009;15:3484-94. [Crossref] [PubMed]
- Ichihara E, Ohashi K, Takigawa N, et al. Effects of vandetanib on lung adenocarcinoma cells harboring epidermal growth factor receptor T790M mutation in vivo. Cancer Res 2009;69:5091-8. [Crossref] [PubMed]
- Furuya N, Fukuhara T, Saito H, et al. Phase III study comparing bevacizumab plus erlotinib to erlotinib in patients with untreated NSCLC harboring activating EGFR mutations: NEJ026. J Clin Oncol 2018;36:9006. [Crossref]
- Zhao B, Zhang W, Yu D, et al. Erlotinib in combination with bevacizumab has potential benefit in non-small cell lung cancer: A systematic review and meta-analysis of randomized clinical trials. Lung Cancer 2018;122:10-21. [Crossref] [PubMed]
- Campelo RG, Felip E, Massuti B, et al. Phase IB study of olaparib (AZD2281) plus gefitinib in EGFR-mutant patients (p) with advanced non-small-cell lung cancer (NSCLC) (NCT01513174/GECP-GOAL). J Clin Oncol 2013;31:2581.
- Campelo RG, Felip E, Massuti B, et al. Phase IB study to evaluate efficacy and tolerability of olaparib (AZD2281) plus gefitinib in patients (P) with epidermal growth factor receptor (EGFR) mutation positive advanced non-small cell lung cancer (NSCLC) (NCT=1513174/GECP-GOAL). J Clin Oncol 2014;32:8079. [Crossref]
- Campelo RG, Rodriguez OGA, Massuti B, et al. Combination of gefitinib and olaparib versus gefitinib alone in EGFR mutant non-small-cell lung cancer (NSCLC): A randomized phase 2 study (GOAL, Spanish Lung Cancer Group). J Clin Oncol 2018;36:9012. [Crossref]
- Karachaliou N, Zorrilla AC, Rodríguez-Abreu D, et al. 198TiP Afatinib plus EGF pathway targeting immunization (EGF-PTI) as first line therapy for EGFR mutant NSCLC: The EPICAL study. J Thorac Oncol 2018;13:S118-9. [Crossref]
- Codony-Servat J, García-Roman S, Molina-Vila MÁ, et al. Anti-Epidermal Growth Factor Vaccine Antibodies Enhance the Efficacy of Tyrosine Kinase Inhibitors and Delay the Emergence of Resistance in EGFR Mutant Lung Cancer Cells. J Thorac Oncol 2018;13:1324-37. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 2017;12:403-7. [Crossref] [PubMed]
- Karachaliou N, Gonzalez-Cao M, Sosa A, et al. The combination of checkpoint immunotherapy and targeted therapy in cancer. Ann Transl Med 2017;5:388. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Sundar R, Cho BC, Brahmer JR, et al. Nivolumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol 2015;7:85-96. [Crossref] [PubMed]
- Gibbons DL, Chow LQ, Kim DW, et al. 57O Efficacy, safety and tolerability of MEDI4736 (durvalumab [D]), a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib (G): A phase I expansion in TKI-naïve patients (pts) with EGFR mutant NSCLC. J Thorac Oncol 2016;11:S79. [Crossref]
- Planchard D, Barlesi F, Gomez-Roca C, et al. Phase I, safety, tolerability and preliminary efficacy study of tremelimumab (Trem) in combination with gefitinib (Gef) in EGFR-mutant (EGFR-mut) NSCLC (GEFTREM). Ann Oncol 2016;27:1245. [Crossref]
- Arasada RR, Amann JM, Rahman MA, et al. EGFR blockade enriches for lung cancer stem-like cells through Notch3-dependent signaling. Cancer Res 2014;74:5572-84. [Crossref] [PubMed]
- Thiagarajan PS, Wu X, Zhang W, et al. Transcriptomic-metabolomic reprogramming in EGFR-mutant NSCLC early adaptive drug escape linking TGFbeta2-bioenergetics-mitochondrial priming. Oncotarget 2016;7:82013-27. [Crossref] [PubMed]
- Goss GD, O'Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [Crossref] [PubMed]
- Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant Erlotinib Versus Placebo in Patients With Stage IB-IIIA Non-Small-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2015;33:4007-14. [Crossref] [PubMed]
- Li N, Ou W, Ye X, et al. Pemetrexed-carboplatin adjuvant chemotherapy with or without gefitinib in resected stage IIIA-N2 non-small cell lung cancer harbouring EGFR mutations: a randomized, phase II study. Ann Surg Oncol 2014;21:2091-6. [Crossref] [PubMed]
- Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:139-48. [Crossref] [PubMed]
- Pennell NA, Neal JW, Chaft JE, et al. SELECT: A multicenter phase II trial of adjuvant erlotinib in resected early-stage EGFR mutation-positive NSCLC. J Clin Oncol 2014;32:7514. [Crossref]
- Yue D, Xu S, Wang Q, et al. OA 16.04 Efficacy and Safety of Erlotinib vs. Vinorelbine/Cisplatin as Adjuvant Therapy for Stage IIIA EGFR Mutant NSCLC Patients. J Thorac Oncol 2017;12:S1789. [Crossref]
- Xing L, Wu G, Wang L, et al. A multicenter, randomized, open-label, phase II trial of erlotinib versus etoposide plus cisplatin with concurrent radiotherapy in unresectable stage III non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) activating mutation. J Clin Oncol 2017;35:8531. [Crossref]
- Feng S, Wang Y, Cai K, et al. Randomized Adjuvant Chemotherapy of EGFR-Mutated Non-Small Cell Lung Cancer Patients with or without Icotinib Consolidation Therapy. PLoS One 2015;10:e0140794. [Crossref] [PubMed]
- Wu F, Liu X, Ma JA, et al. Adjuvant therapy for resected EGFR-mutant non-small-cell lung cancer. Lancet Oncol 2018;19:e124. [Crossref] [PubMed]
- Rosell R, Karachaliou N. Adjuvant therapy for resected EGFR-mutant non-small-cell lung cancer. Lancet Oncol 2018;19:e126. [Crossref] [PubMed]
- Ma JA, Jiang S, Hu C, et al. Adjuvant therapy for resected EGFR-mutant non-small-cell lung cancer. Lancet Oncol 2018;19:e125. [Crossref] [PubMed]
- Tazza M, Metro G. Adjuvant treatment of non-small cell lung cancer: focus on targeted therapy. J Thorac Dis 2017;9:4064-9. [Crossref] [PubMed]


