
Characterization of PD-L1 expression and its prognostic value in patients with ovarian cancer
Introduction
Ovarian cancer is the second most common cancer and the leading type of lethal gynecological cancer (1). Among ovarian cancer cases, about 90% of the cases are epithelial ovarian cancer (EOC). While the majority of patients are diagnosed at advanced stages, the 5-year survival rate for a diagnosis during the early-stage of the disease is approaching 90%. With the development of chemotherapy and cytoreductive surgery, over 50% of these patients have gone into remission. Nonetheless, the majority of these cases would inevitably have chemotherapeutic drugs resistance, resulting in a recurrence (2). Currently, there is still a lack of reliable biomarkers for predicting the prognosis in the patients with ovarian cancer. Therefore, identifying the predictive biomarkers for the prognosis or a novel therapeutic strategy, is urgently needed in the clinical setting.
There is more and more evidence supporting the fact that ovarian cancer could also be regarded as an immunogenic ailment, just like other solid malignant cancers (3). The escape of a host immune surveillance system is critical for tumor progression (4). Among them, a programmed cell death ligand 1 (PD-L1) plays a major role in keeping the homeostasis in an immune response (5-10). Meanwhile, immunosuppressive cytokines which are released by tumor cells, such as the IL-10, TNFα and IFN-γ cells, could up-regulate the expression of PD-L1 to avoid cytolysis by using the activated T cells (8-10). The binding of PD-L1 with its receptor, and the programmed death 1 (PD-1) on the T cells, leads to their apoptosis, and thereafter helps the tumor cells avoid an immune clearance (10). Preliminary data has shown that there is a promising response rate to the PD-1/PD-L1 blockade drugs for the patients with advanced ovarian cancers and PD-L1 expression (11-14). Thus, a further understanding of the demographic features and their association with PD-L1 in the prognosis of patients with ovarian cancer is needed.
Several studies (15,16) have looked into the feasibility of using PD-L1 to serve as a prognosis-related specific biomarker for ovarian cancer. However, its prognostic role in ovarian cancer is still controversial. Therefore, this study performed an up-to-date meta-analysis to reveal the association between the PD-L1 expression and the demographic characteristics for the prognosis in patients with ovarian cancer.
Methods
Search strategy
A comprehensive publication searching method was conducted online via the Web of Science, EMBASE, Medline/PubMed, and the Cochrane Library databases from all dates until November 8th, 2017. The search terms used for finding the literature were, “programmed cell death ligand 1” or “PD-L1” and “ovarian cancer” and “outcome” or “prognosis” or “survival”. We also searched reference lists and conference abstracts to avoid missing data points during the retrieval process. Reference lists in identified articles were searched by hand. The current study was carried out under the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement.
Selection criteria
The study inclusion criteria entailed the following: (I) ovarian cancer related studies; (II) pathologically confirmed; (III) PD-L1 expression was tested using tumor tissue, not by any other kinds of specimens or cell lines; and (IV) association of clinicopathological features, as well as the prognosis and PD-L1 being reported. The study exclusion criteria entailed the following: (I) papers published in non-English; (II) letters, case reports or review articles; (III) repeated publication; (IV) non-human experiments; and (V) an unavailable survival curve, or insufficient data to generate a risk ratio.
Data extraction
Data extraction was performed independently by 2 different reviewers (BH Cheng, T Jiang). We used the Newcastle-Ottawa Scale (NOS) to assess the quality of the identified articles (17). Data tables were generated to extract all relevant data, such as texts, tables and figures, from the studies. These figures included the number of patients, publication year, authors, country, ethnicity, analysis procedure, testing assay, the cut-off, duration of follow-up, risk ratio, together with the rates of PD-L1 positive expression. In the articles which provided only a Kaplan-Meier curve instead of the survival data, we used software which had been developed by Sydes & Tierney to digitize and extract the risk ratio (RR) data together with its 95% CI (18). Any disagreement regarding a conflicting study was solved through a comprehensive discussion arrive at by consensus. If no consensus was achieved, the article was not included into the final analysis.
Statistical analysis
STATA Version 12.0 (Stata Corporation LP, College Station, TX, USA) was used to perform the statistical analyses. I2 tests and Chi-squared tests were adopted to evaluate the heterogeneity of the included studies. As for survival data, we directly extracted or used the previously published methods to calculate the hazard ratios (HRs) and their 95% CIs from the included articles (18). Egger’s tests and a Begg’s funnel plot chart were used to evaluate the bias of publication. Progression-free survival (PFS) and overall survival (OS) were calculated using effect variables.
Results
Search results
A total of 457 publications were initially identified from using the above searching strategy. Among them, 450 articles were excluded after we reviewed the titles together with their abstracts. This was due to duplicate records, non-ovarian cancer-related studies, incomplete data and non-original data (e.g., commentary, case report, review). Finally, 7 studies (15,16,19-23) were included into this meta-analysis. All 7 studies comprehensively evaluated PD-L1 expression and provided survival information (Figure 1).
Study characteristics and PD-L1 expression testing methods
The characteristics of the 7 included studies are shown in Table 1 (15,16,19-23). The publication years were from 2007 to 2017. Among them, 3 studies were conducted in China, and the other 4 were conducted in Canada, Germany, Japan and Austria. The included number of patients ranged from 19 to 195. The quality assessment results showed a mean of 7 with a variation from 5 to 8, suggesting that these included studies were good quality . The enrolled patients had diseases from stages I to IV. The clinicopathological features including FIGO stage were reported in 7 studies, while the median age was reported in 5 studies (Table 1). All of the included patients did not receive previous neo-adjuvant chemotherapy or radiotherapy before surgery. PD-L1 expression was evaluated on tumor tissue using the tissue immunochemistry staining (IHC) method. Six different kinds of assays were utilized for PD-L1 staining and among them were 7 studies which included 27A2, ab205921, EPR1161[2], SP142, E1L3N and SP263. The cut-off values to determine a positive relationship were not the same in the used assays (Table 1).
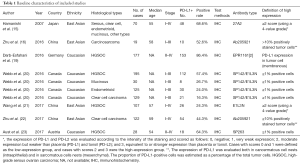
Full table
PD-L1 expression in ovarian cancer
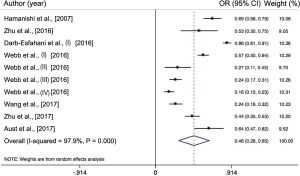
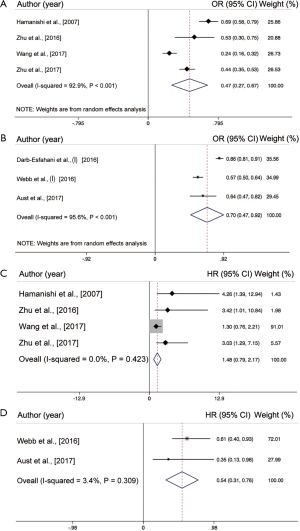
The incidence rate of the PD-L1 positive expressions ranged from 16% to 86% (Figure 2). This meta-analysis showed a 46% (increase/decrease) of PD-L1 positive expression (95% CI, 0.28–0.65) by using a random-effects model with a high heterogeneity between the studies extracted (I2=97.9%; P<0.001). The meta-analysis of the HR rate for PFS/DFS and the OS/disease specific survival (DSS) rate for the ovarian cancer patients which depend on PD-L1 expression status, is shown in Figure 3. We further conducted a subgroup analysis according to ethnicity and histological type. We found the incidence rate of PD-L1 positive expression in the East Asian group was similar when compared with the Caucasian group (47% vs. 70%; P>0.05) (Figure 4). However, the incidence rate of PD-L1 positive expression in a high-grade serous ovarian carcinoma (HGSOC) was significantly higher than the less common ovarian histopathology (LOCH) (58% vs. 38%, P<0.05) (Figure 5A,B).
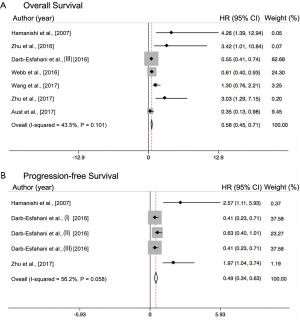
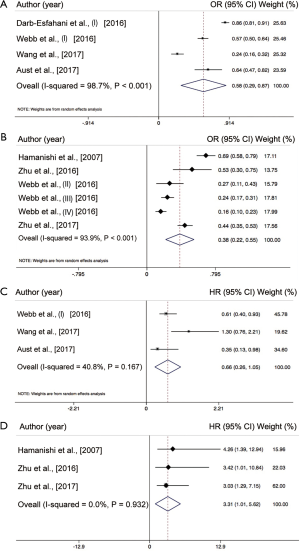
Association between PD-L1 expression and survival
All of the included studies had investigated the predictive role of PD-L1 expression and the overall survival rate of the patients with ovarian cancer. As shown in Figure 3, the pooled analysis suggests that a positive PD-L1 expression is significantly associated with a reduced mortality risk in the patients with ovarian cancer by using the random-effects model (HR =0.63; 95% CI, 0.41–0.84, P<0.001). The heterogeneity among studies is intermediate (I2=43.5%, P=0.101) (Figure 3A). As for PFS, 3 of the studies have provided the details needed, and were included into this analysis. The result revealed that a higher PD-L1 expression was not significantly associated with longer PFS (HR =1.36; 95% CI, −0.06 to 2.78, P>0.05) in patients of ovarian cancer. However, the heterogeneity among studies is high (I2=56.2%, P=0.058) (Figure 3B). We also performed a sub-analysis in regards to the distinct ethnicity and histological types. As we previously mentioned, 4 of the studies had enrolled primarily East-Asian patients and the 3 other studies enrolled primarily Caucasian patients. The pooled results found that a positive PD-L1 expression was significantly associated with OS in Caucasians (HR =0.54; 95% CI, 0.31–0.76, P<0.001) (Figure 4C), while this was not the case in the East-Asian group (HR =1.48; 95% CI, 0.79–2.17, P>0.05) (Figure 4D). As for the distinct histopathology, a positive PD-L1 expression was associated with a better OS rate (HR =0.66; 95% CI, 0.26–1.05, P=0.059) (Figure 5C) in patients with HGSOC, whereas patients with a LOCH PD-L1 expression had a significantly worse OS rate (HR =3.31; 95% CI, 1.01–5.62, P<0.001) (Figure 5D).
Publication bias
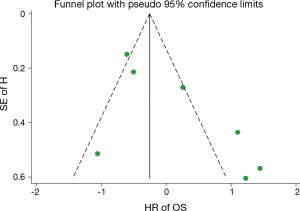
Publication bias was assessed by using an Egger’s tests and a Begg’s funnel plot test, and was only looked at during the analysis stages of the HR of the high PD-L1 expression on OS rate (P<0.05 for Egger’s test). Further analyses showed that the Begg’s funnel plot was symmetric and Egger’s tests suggested that there was no obvious publication bias in the current study (Figure 6).
Discussion
Immunotherapy that targets the PD-1/PD-L1 checkpoint has changed the medical landscape for several different kinds of solid tumors like non-small cell lung cancer, lymphoma, urothelial cancer, renal cell cancer, and melanoma (24-29). Biomarker analysis further indicated that the efficacy was superior in patients with a positive PD-L1 expression (29); therefore, PD-L1 expression was regarded as an accompanying diagnostic biomarker, and it was recommended that patients undergo a detection process before immunotherapy treatment. As for ovarian cancer, recent phase I studies also found that anti-PD-1/PD-L1 based immunotherapy showed promising results in patients with PD-L1 expressed ovarian cancer (12-14). Thus, identifying the role that PD-L1 expression plays is important for the patients with ovarian cancer in the near future.
As far as we know, this meta-analysis is the first one to comprehensively analyze the clinicopathological features, together with the prognostic roles of PD-L1 in the patients with ovarian cancer. We included 7 studies with a total of 1,002 patients with ovarian cancer. We found a PD-L1 positively expressed incidence rate of 46%, which is similar to the other kinds of malignant cancers (24,29) and this shows again, that PD-L1 might play an important role in the immune escape in ovarian cancer (5-10). Unlike the discrepancy of the EGFR mutation in different ethnicities (30,31), we found the incidence of PD-L1 expression had comparable results between the East Asian group and the Caucasian group. Importantly, we found that the rate of PD-L1 expression in HGSOC was significantly higher than in LOCH, which suggests that ovarian cancer should be divided into different subgroups in the era of different types of immunotherapy options (32). Among the included 7 studies, 5 of the PD-L1 testing assays were utilized, and the cut-off was not consistent (15,16,19-23), which might result in some bias to our findings. However, we know that in current practice different pharmaceutical companies use their unique assays for the accompanying type of PD-L1 expression detection method. Meanwhile, several studies have compared these different assays and found the results from most of the different assays to be consistent (33-36), which suggests it might be reasonable and feasible to perform this meta-analysis by using different PD-L1 testing assays.
The interaction of the PD-1 and PD-L1 pathways is the main mechanism by which cancer cells escape immunological surveillance (5-8). Several studies have investigated the prognostic role of PD-L1 expression and found it could predict a worse OS rate in renal cancer, non-small cell lung cancer, and pancreatic ductal adenocarcinoma (37-40), while it showed a reversed effect in the patients with triple-negative breast cancer (41). Similarly, in patients with ovarian cancer, several studies have suggested that PD-L1 expression was correlated to a worse OS rate and PFS (15,16,22), while some others did not (19-21,23). In the current study, we found that PD-L1 positive expression was associated with a better OS rate together with a longer PFS in the patients with ovarian cancer. Meanwhile, we observed significant heterogeneity which could not be eliminated even after using the random-effects model. Therefore, we further performed a subgroup analysis in regards to the ethnicity and histological types, and have found the diagnostic role of PD-L1 expression to have been consistent between the Asian population and the Caucasian population. More importantly, we found that the prognosis-predictive role of the PD-L1 expression was contrary between HGSOC and LOCH (42-44), which might be due to the different formation or genomic alteration in these histological subtypes. Our findings may strengthen the role of PD-L1 in predicting the survival chance for the patients with ovarian cancer.
Recently, several phase I–II clinical trials have investigated the efficacy of the PD-1/PD-L1 blockade and have shown promising results for the patients with ovarian cancer (12-14). Hamanishi et al. explored the activity of nivolumab in the patients of a platinum-resistant EOC, and the study revealed a partial response of 20% and a stable disease rate of 26% in the 15 enrolled patients (12). Similarly, in a phase IB clinical trial, the Keynote 028 study found there to be a 23.1% (6/26) reduction in tumor size, which suggested that pembrolizumab had an antitumor effect for the patients with PD-L1 expression positive ovarian cancer (13). Moreover, avelumab achieved an objective response rate of 9.7% together with a disease control rate of 54.0% in the 124 patients of a refractory or a recurrent EOC, and further analysis found that the response rate was significantly higher in the PD-L1 expressed positive patients than those of the negative group (14). Besides this research, several other clinical trials, including a combinational strategy, need to be undertaken to further validate the efficacy of anti-PD-1/PD-L1 monotherapy in ovarian cancer (45). Thus, our findings might be helpful to guide the PD-1/PD-L1 blockade immunotherapy process in ovarian cancer patients in the near future.
There are several other limitations which need to be mentioned in this study. First, the studies meeting the criteria which were included into this analysis were relatively small, and several of them were retrospective. There was also publication bias, which was inevitable. Several abstracts were identified but without detailed further information. We tried our best to obtain the primary data by contacting the authors but received no response. Therefore, we did not include the abstract publications when we performed this analysis. Second, the quality of 7 included studies had a heterogeneous feature, since several pieces of the important clinic-pathological information were not consistently reported. Third, 6 different staining assays (27A2, EPR1161, SP142, E1L3N, Ab205921, and SP263) and different cut-off values were used in the 7 selected studies to test PD-L1 expression. In addition to this, there were different pathologist interpretations, and the different patient populations might result in a heterogeneity in the PD-L1 expression rate here. However, previous studies have shown that there is a high degree of consistency among the 3 assays (SP142, E1L3N, and SP263) used in this meta-analysis (33,35). Thus, our analysis still needs to be further validated by a large scale data test with prospective evidence.
In conclusion, this meta-analysis indicated that the PD-L1 expression had a positive correlation in the group of patients with ovarian cancer, with PD-L1 expression being a predictive biomarker to both PFS and OS, which suggests that PD-1/PD-L1 might be a relatively encouraging targeted marker for ovarian cancer in the near future.
Acknowledgments
We thank Tienan Feng, an assistant professor from Shanghai Jiaotong University Medical college for his supervisor in the whole statistical procedure.
Funding: This study was sponsored by scientific research projects from the Health and Family Planning Commission of Hubei Province (No. WJ2017M026).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.10.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet 2009;374:1371-82. [Crossref] [PubMed]
- Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203-13. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [Crossref] [PubMed]
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [Crossref] [PubMed]
- Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol 2007;19:309-14. [Crossref] [PubMed]
- Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009;206:3015-29. [Crossref] [PubMed]
- Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev 2008;224:166-82. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti-PD- 1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 2015;33:4015-22. [Crossref] [PubMed]
- Varga A, Piha-Paul SA, Ott PA, et al. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: Interim results from a phase Ib study. J Clin Oncol 2015;33:abstr 5510.
- Disis ML, Patel MR, Pant S, et al. Avelumab (MSB0010718C; anti-PD- L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: Safety and clinical activity. J Clin Oncol 2016;34:abstr 5533.
- Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007;104:3360-5. [Crossref] [PubMed]
- Zhu J, Wen H, Ju X, et al. Clinical Significance of Programmed Death Ligand-1 and Intra-Tumoral CD8+ T Lymphocytes in Ovarian Carcinosarcoma. PLoS One 2017;12:e0170879 [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Darb-Esfahani S, Kunze CA, Kulbe H, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget 2016;7:1486-99. [Crossref] [PubMed]
- Webb JR, Milne K, Kroeger DR, et al. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol 2016;141:293-302. [Crossref] [PubMed]
- Wang Q, Lou W, Di W, et al. Prognostic value of tumor PD-L1 expression combined with CD8+ tumor infiltrating lymphocytes in high grade serous ovarian cancer. Int Immunopharmacol 2017;52:7-14. [Crossref] [PubMed]
- Zhu J, Wen H, Bi R, et al. Prognostic value of programmed death-ligand 1 (PD-L1) expression in ovarian clear cell carcinoma. J Gynecol Oncol 2017;28:e77 [Crossref] [PubMed]
- Aust S, Felix S, Auer K, et al. Absence of PD-L1 on tumor cells is associated with reduced MHC I expression and PD-L1 expression increases in recurrent serous ovarian cancer. Sci Rep 2017;7:42929. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376:1015-26. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Sholl LM, Aisner DL, Varella-Garcia M, et al. Multi-institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: The Lung Cancer Mutation Consortium Experience. J Thorac Oncol 2015;10:768-77. [Crossref] [PubMed]
- Ren S, Chen X, Kuang P, et al. Association of EGFR mutation or ALK rearrangement with expression of DNA repair and synthesis genes in never-smoker women with pulmonary adenocarcinoma. Cancer 2012;118:5588-94. [Crossref] [PubMed]
- Morgan RJ Jr, Armstrong DK, Alvarez RD, et al. Ovarian Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1134-63. [Crossref] [PubMed]
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol 2017;12:208-22. [Crossref] [PubMed]
- Büttner R, Gosney JR, Skov BG, et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:3867-76. [Crossref] [PubMed]
- Rimm DL, Han G, Taube JM, et al. A Prospective, Multi-institutional, Pathologist-Based Assessment of 4 Immunohistochemistry Assays for PD-L1 Expression in Non-Small Cell Lung Cancer. JAMA Oncol 2017;3:1051-8. [Crossref] [PubMed]
- Ilie M, Khambata-Ford S, Copie-Bergman C, et al. Use of the 22C3 anti-PD-L1 antibody to determine PD-L1 expression in multiple automated immunohistochemistry platforms. PLoS One 2017;12:e0183023 [Crossref] [PubMed]
- Xu F, Xu L, Wang Q, et al. Clinicopathological and prognostic value of programmed death ligand-1 (PD-L1) in renal cell carcinoma: a meta-analysis. Int J Clin Exp Med 2015;8:14595-603. [PubMed]
- Zhou C, Tang J, Sun H, et al. PD-L1 expression as poor prognostic factor in patients with non-squamous non-small cell lung cancer. Oncotarget 2017;8:58457-68. [PubMed]
- Boffa DJ, Graf RP, Salazar MC, et al. Cellular Expression of PD-L1 in the Peripheral Blood of Lung Cancer Patients is Associated with Worse Survival. Cancer Epidemiol Biomarkers Prev 2017;26:1139-45. [Crossref] [PubMed]
- Tessier-Cloutier B, Kalloger SE, Al-Kandari M, et al. Programmed cell death ligand 1 cut-point is associated with reduced disease specific survival in resected pancreatic ductal adenocarcinoma. BMC Cancer 2017;17:618. [Crossref] [PubMed]
- Beckers RK, Selinger CI, Vilain R, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology 2016;69:25-34. [Crossref] [PubMed]
- Rechsteiner M, Zimmermann AK, Wild PJ, et al. TP53 mutations are common in all subtypes of epithelial ovarian cancer and occur concomitantly with KRAS mutations in the mucinous type. Exp Mol Pathol 2013;95:235-41. [Crossref] [PubMed]
- Vereczkey I, Serester O, Dobos J, et al. Molecular characterization of 103 ovarian serous and mucinous tumors. Pathol Oncol Res 2011;17:551-9. [Crossref] [PubMed]
- Reade CJ, McVey RM, Tone AA, et al. The fallopian tube as the origin of high grade serous ovarian cancer: review of a paradigm shift. J Obstet Gynaecol Can 2014;36:133-40. [Crossref] [PubMed]
- Ventriglia J, Paciolla I, Pisano C, et al. Immunotherapy in ovarian, endometrial and cervical cancer: State of the art and future perspectives. Cancer Treat Rev 2017;59:109-16. [Crossref] [PubMed]



