
The future is now: beyond first line systemic therapy in hepatocellular carcinoma
Background
Hepatocellular carcinoma (HCC) is a major global health issue, being the 5th most prevalent and the 2nd most deadly cancer. Moreover, the incidence of HCC has shown a growing tendency in the recent years, the rate of newly diagnosed HCC patients increased by 75% from 1990 to 2015 (1). Projections that have been made assume that in the USA, by 2030 HCC will be the third leading cause of cancer, preceding breast, prostate and colorectal cancer (2,3). HCC is most commonly found in the elderly, with its peak incidence at about 70 years old, twice more frequent in men than women (4). Europe belongs to the lower incidence region, however, in the South the incidence is significantly higher (5).
In approximately 90% of cases, the etiology of HCC is established, chronic viral hepatitis, alcohol intake and aflatoxin exposure being the most common causes. In Western world, chronic hepatitis C virus is the main risk factor (1). Even though direct-acting antiviral (DAA) treatment has a high efficacy in the eradication of HCV, which should correlate with a lower risk of tumour development, there are some reports that suggest a higher risk of HCC occurrence and recurrence, with a more aggressive pattern in DAA treated patients. This may be due to changes in immune system because of the rapid decrease in viral load (6). Many epidemiological studies are needed regarding this concern. Mice studies, as well as epidemiological observations demonstrates that HCC may appear in the settings of non-alcoholic steatohepatitis (NASH) and obesity via liver inflammation and tumorigenesis, interleukin-6 (IL-6) and tumour necrosis factor-alpha (7).
Surveillance examinations is based on abdominal ultrasound (US). US should be performed every 6 months in high-risk groups (cirrhosis, hepatitis B, DAA treated patients) (8). Unfortunately, there is no serological test for an early diagnosis of HCC. Alpha-feto-protein (AFP) which is one of the most used biomarkers, has increased serum levels in flares of HBV, HCV infections or decompensations of underlying liver disease, which means it has low accuracy in detection (9).
There are several staging systems for the classification of HCC in order to assess the prognosis, from which the BCLC system is the most widely used because it corelates the treatment method with the tumour stage and the severity of subsequent liver cirrhosis. Although there are curative treatments for initial stages (transplantation, resection, ablation), many patients are still diagnosed when effective therapy is no longer possible; therefore advanced disease represents the major concern for the Hepatology community (10). Although sorafenib (11), a multi-kinase inhibitor, revealed improved overall survival (OS) and disease-free survival (DFS) in clinical trials and in real-life scenarios, more emerging therapies are now tested with potential benefit. Moreover, immunotherapy is showing a great promise and might stand as a revolutionary therapy.
First line treatment
In order to review what’s beyond the first line of treatment in HCC, it’s important to remember which are the current recommendations from the guidelines (12).
Sorafenib is the first drug which showed survival benefits in patients with advanced HCC and is considered a major revolution in hepatology (13). Since 2008, when the SHARP (Sorafenib HCC Assessment Randomised Protocol) trial showed an improved overall survival in Child–Pugh A (CP-A) patients with advanced HCC treated with sorafenib, this therapy is considered standard of care for advanced HCC. In addition, the benefits and safety of the administration of sorafenib were strengthen by the phase 4 large GIDEON study (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and of its treatment with sorafeNib). This prospective, observational study revealed that sorafenib has the same safety profile, drug related adverse effects and overall survival between CP-A and CP-B patients, [13.6 (12.8–14.7)] vs. [5.2 (4.6–6.3)] (14).
Sorafenib inhibits not only the RAF kinase, but some other tyrosine kinases involved in the development of HCC such as mitogen activated protein (MEK), extracellular signal regulated kinase (ERK), vascular endothelial growth factor receptor 2 (VEGFR2), platelet derived growth factor receptor (PDGFR), the receptor-type tyrosine-protein kinase FLT3, the proto-oncogenes Ret and cKIT (15). Major etiologic factors such as hepatitis B and C viruses overexpress Raf1 kinase thus leading to the activation of the Raf/MEK/ERK pathway (11). Therefore, the administration of sorafenib with consequent inhibition of MEK can further reduce cell proliferation and can induce apoptosis (15). Moreover, sorafenib demonstrates its antiangiogenic properties due to the fact that HCC is a hypervascular tumour with an overexpression of VEGFR (16).
The efficacy of sorafenib assessed by the overall survival (OS) was outlined and confirmed even from the beginning by two phase III trials (11,17) that obtained an OS of 10.7 months as compared with placebo. Moreover, recent studies, analysed the OS under sorafenib treatment according to the BCLC class. Apart from patients with BCLC-C, also the BCLC-B patients who failed to respond or had contraindication to the standard of care, transarterial chemoembolization (TACE) or other ablative therapies (18) had a benefit. The median OS ranged from 7.2 to 15.7 months in BCLC-C (18-21) and from 19.6 to 21.5 months in BCLC-B patients (18-21).
Due to the heterogeneity of the HCC population included in the BCLC-C class, where the recommended treatment option is sorafenib, there is a variable response to this systemic treatment.
In order to predict or evaluate the response to sorafenib in an early phase and therefore the impact of OS, several factors that can impact the treatment outcomes have been analysed. Regarding the tumour related factors, several histological and serological biomarkers have been identified: extra-hepatic spread (EHS), CRIPTO, AFP decrease, ANXA3, PIVKA, FGF2, BCL2, COX2/PGE2 axis (22-29).
Some patient’s characteristics and associated comorbidities and its treatment may impact the sorafenib response: type 2 diabetes mellitus (T2DM), systemic inflammatory index (SII), FT4xTSH score, neutrophil to lymphocyte ratio (NLR), platelet level and pre-sarcopenia/sarcopenia (18,19,21,30-35).
Furthermore, there are currently several clinical trials analysing weather the association of sorafenib to immunotherapy may enhance its effect, with an impact on the overall survival, which will be discussed later in the review.
Lenvatinib is the second first line drug approved in the treatment of HCC, being non-inferior to sorafenib. Lenvatinib is an orally multikinase that inhibits VEGFR 1-3, FGFR 1-2, PDGF-β, KIT and ret kinases (36).
Promising phase II trial, showing 37% response rate (by mRECIST), 7.2 months median time to progression and an acceptable safety profile led to a phase III multicentre, randomized, open-label trial (37). The REFLECT trial included CP-A patients with advanced HCC and ECOG-PS 0/1 and excluded the patients with previous systemic anticancer therapy, main portal vein invasion and tumour spread >50% of the liver volume (38). The primary end-points of the study were met, with a median OS of 13.6 months in the lenvatinib arm versus 12.3 months in the Sorafenib arm (38). Lenvatinib performed better in median progression free survival (7.4 vs. 3.7 months) and time to progression (8.9 vs. 3.7 months) (39). Therefore, for the treatment of advanced HCC there are two first line treatment options, with similar survival benefits and with a different safety profile as sorafenib is more frequently associated with HFSR as the most important AE, while lenvatinib is associated with a higher rates of hypertension, anorexia and fatigue.
Several other therapeutic agents (sunitinib, brivanib, linifanib and erlotinib) were tested as a first line treatment, showing no benefit in overall survival compared with Sorafenib. In part, the failure of these substances can be attributed according to Baxter et al. to the lack of understanding of the critical driver and flaws in the trial design, including significant toxicity due to a lack of understanding of the tyrosine kinase inhibitors (38).
Beyond first line
Inside the guidelines
Regorafenib is the first drug approved as second line treatment in HCC patients with proved survival benefits in patients that did not respond to sorafenib.
Regorafenib is an oral multikinase inhibitor that blocks the activity of several protein kinases involved in:
The benefits of regorafenib in HCC patients which progressed while on sorafenib was outlined in the phase III Resource trial, which included BCLC stage B/C patients with a good liver function (CP-A) that had radiological progression during previous sorafenib treatment. The primary endpoint of the study was met as regorafenib improved the median overall survival from 7.8 to 10.6 months, compared with placebo (42).
In an exploratory subgroup analysis (41,43) in patients treated with regorafenib, the survival benefit was similar regardless of the last sorafenib dose (800 ng/day or less). Further it was proved that Regorafenib significantly improved post-progression survival relative to placebo irrespective of the progression pattern during sorafenib therapy.
Analysing the effects of regorafenib, some studies (44) have outlined that it can influence the level of expression of PD-L1 by reducing it and also, it can prevent the engagement of PD-L1 by PD-1+ T cells. As a consequence, the T cell-receptor mediated signalling is up-regulated and the immune response to HCC is reactivated.
For the patients who do not respond to sorafenib, regorafenib seems like a good alternative with almost a 3 months survival improvement as a second line treatment. Although among the eligibility criteria there are: a previous tolerance to sorafenib and a preserved liver function (CP-A), some studies have outlined that almost 30.6% of the patients that were previously treated with sorafenib are eligible for the regorafenib treatment (45). That can be partially explained by a study that analyses the liver function during the sorafenib treatment, showing that almost 27.4% of the patients will have a CP class changed into CP-B class at 4 weeks after the beginning of treatment (35).
Therefore, regorafenib represents a good alternative for a minority of the patients that are sorafenib-resistant and further studies need to analyse if its survival benefits can be extended to a larger group of patients.
Beyond guidelines
Multikinase inhibitors
HCC tumour cells are usually characterized by heterogeneous imbalances in molecular mechanisms and signalling pathways that regulate cell proliferation, survival and death, growth factors (epidermal growth factor, EGF) and growth factor receptors (EGF receptor), angiogenetic factors (vascular endothelial growth factor, VEGF; fibroblast growth factor, FGFR; platelet-derived growth factor receptor, PDGFR; inflammatory cells, tumour stromal cells), oncogenes. Among the intracellular signalling pathways, the mitogen-activated protein kinase (MAPK) cascade, PI3K/Akt/mTOR (mammalian target of rapamycin), hepatocyte growth factor (HGF)/c-Met pathway, IGF and its receptor (IGFR), as well as Wnt/beta-catenin pathway have been studied and used for development of targeted HCC treatments (46).
Inhibition of angiogenesis is one of the therapeutic targets in HCC and several therapies targeting VEGF have entered clinical studies:
- Cabozantinib (Cabometyx®)—an oral multi-kinase inhibitor targeting MET, RET, AXL, and VEGFR1-3, has improved median OS compared with placebo in patients with advanced HCC who have previously received sorafenib in the global phase III CELESTIAL trial (NCT01908426) (47);
- Bevacizumab—a humanized monoclonal antibody that targets VEGF, which besides its antiangiogenetic effects, might also enhance chemotherapy administration by decreasing the interstitial pressure in the tumour (48);
- Sunitinib—an oral multikinase inhibitor for the receptor tyrosine kinases (RTKs), such as VEGFR-1 and -2, PDGFR-alpha/beta, c-KIT, FLT3, and RET kinases (49);
- Brivanib—a dual inhibitor of VEGFR and FGFR, undergoing evaluation in phase III studies (50);
- ABT-869—an oral inhibitor of VEGFR and PDGFR, with early evidence of efficacy and ongoing Phase III studies (51);
- AZD2171—a pan-VEGF receptor tyrosine kinase, PDGF receptors and c-Kit inhibitor (52);
- PTK787 (vatalanib)—targeting all VEGFR tyrosin kinases, with a higher activity on VEGFR-2 (53);
- Pazopanib (GW786034)—an inhibitor of VEGFR, PDGFR, and c-Kit (54).
The EGFR signaling pathways are another important target for HCC therapies and two classes of EGFR agents have proved relevant clinical activity:
- EGFR tyrosin kinase inhibitors, such as erlotinib (with modest activity), gefitinib or lapatinib (with no proven activity as single agents) (55-57);
- Monoclonal antibodies against EGFR, such as cetuximab, with demonstrated antitumor activity only in combinations (58,59).
mTOR inhibitors (sicrolimus, temsirolimus, everolimus) have demonstrated cell growth and tumour vascularity inhibition in several cancers including HCC cell lines, but studies have not shown significant therapeutic activity for the agents alone (60).
Until this year, sorafenib was the only approved systemic treatment for patients with HCC BCLC-C class. Since 2008, when it became available, many other substances were evaluated for being either superior or non-inferior to sorafenib, but without conclusive results. Meanwhile, another TKI, lenvatinib proved to be non-inferior in terms of OS but with a better progression free survival, time to progression and response rate (61).
The first substance to prove her efficacy in the second line therapy was regorafenib with an improvement in OS from 7.8 months on placebo to 10.6 months (HR: 0.63, P<0.0001) (42).
Apart from regorafenib, in the second line therapy, two other substances met their end-point of an improved survival compared to placebo: cabozantinib from 8 months on placebo to 10.2 months (HR: 0.76, P=0.005) (62) and ramucirumab, after a sub-group analysis, in patients with advanced HCC and an AFP >400 ng/mL from 4.2 months on placebo to 7.8 months (HR: 0.674; P=0.006) (63).
Several other substances have been tested alone or in combination with sorafenib in the first line (sunitinib, linifanib, brivanib, erlotinib) or in the second line treatment (everolimus, brivanib, tivantinib), but none of them met their primary end-points (64-70).
Chemotherapy
Nowadays, systemic chemotherapy is used only occasionally, in the settings of (very) advanced disease, being out shadowed by the use of multikinase inhibitors. Chemotherapy in the treatment of HCC has two main challenges, the frequent presence of cirrhosis that can perturb the drug metabolism and enhance its toxicity and the additional severity of the chemotherapy related complications in a patient already immune-compromised (71).
Doxorubicin is one of the first chemotherapeutic agents used in HCC treatment with 10% objective response rate and inconclusive survival benefits (72). Nevertheless, it is currently one of the most commonly used chemotherapeutic agents in TACE treatment (73).
Another chemotherapeutic agent with promising results is TS-1 that acts on 5FU metabolism, increasing its toxicity on neoplastic cells (74) Although the initial trial, S-CUBE, failed to fulfil its primary end-points, a subgroup analysis outlined better results in TNM stage III, IVa, IVb, CP-A patients and in those with a low level of tumour markers (74).
Regarding combo treatment schemes, some other chemotherapeutic regimens have shown negative results.
The PIAF regimen, although with higher response rates 20.9% and better OS 8.67 months, the differences in comparison to doxorubicin regimen were not statistically significant (75). Unfortunately, PIAF was also associated with a significant higher rate of myelotoxicity.
When analysed in comparison with Doxorubicin alone, FOLFOX4 showed better results in terms of progression free survival (2.7 vs. 1.7 months), better response rate but with no significant difference in OS, which was one of the primary endpoints (76).
Recently, promising results seem to come from hepatic intra-arterial chemotherapy (HIAC). It is considered a more effective method than systemic chemotherapy because it facilitates the drug to directly reach the tumour throughout the hepatic artery (76). In Japan, HIAC is indicated mainly in localized advanced HCC with evidence of vascular invasion (74).
A recent randomized multicentre, prospective study (77) has shown interesting results in the treatment of patients with HCC and portal vein thrombosis either by sorafenib alone versus sorafenib + HIAC. The OS and time to progression were significantly longer in HIAC group than in sorafenib group (14.9 vs. 7.2 months). The safety profile was good, with fewer overall side effects and fewer serious adverse events but with a higher rate of grade 3 and 4 toxicities. Although conducted on a small number of patients, this study shows promising results than need to be confirmed in further studies. As it has favourable shrinking tumour effects, lenvatinib plus HIAC could be another direction of research.
Still, up until now, there is no registered clinical trial that proves a survival benefit of systemic chemotherapy in the treatment of HCC.
Although the promising results of multi-kinase inhibitors and immune checkpoint inhibitors in the treatment of advanced HCC have taken the frontline, there seems to be a glimpse of hope for the systemic therapy that comes from some observational studies of metronomic capecitabine (MC).
The metronomic regimes which are currently becoming popular in oncology, rely in the chronic administration of chemotherapeutic agents, in a continuous manner, with the aim of optimizing the antiangiogenic properties of the drug with the reduction of the gastrointestinal and bone marrow toxicities (78,79).
Metronomic capecitabine was analysed in few observational studies, mainly in a second line setting, demonstrating in all of them a good efficacy at the cost of a low rate of adverse events in comparison with best supportive care BSC (78-81).
As second line therapy in patients unresponsive or intolerant to sorafenib, a grey area in the treatment of HCC, the treatment with MC showed superior progression free survival and median overall survival rates compared to best supportive care (79,80). Furthermore, in patients with moderate compromised liver function (CP-B) not eligible for sorafenib, there was a 42% reduction in the death risk for patients on MC compared to those receiving just BSC (78).
However, future prospective randomised clinical trials should analyse the efficacy of MC in the treatment of advanced HCC.
Immunotherapy—the revolution against cancer
Liver immunology
The liver has a specific, dual blood supply. 75% of the blood enters the liver through the portal vein bringing many microbial antigens from the gut, also known as microbial associated molecular patterns (MAMPs). One such antigen is lipopolysaccharide (LPS), an endotoxin from the Gram-negative bacteria (82), that interacts with hepatic non-parenchymal cells such as liver sinusoidal endothelial cells (LSECs), hepatic stellate cells (h-SCs), Kupffer cells, dendritic cells (DCs), and lymphocytes capable of inducing immunotolerance. Some of the most important mechanisms of inducing immunotolerance are: decrease of the costimulatory immune receptors B7-1, B7-2 versus up-regulation of programmed cell death protein 1 (PD-1) receptor and cytotoxic T-lymphocyte-associated protein 4 (CTLA4) immuno-checkpoint inhibitors of different immune cells (83,84).
Chronic inflammation of various causes (HBV, HCV, AI, NASH or alcohol) may lead to cirrhosis and liver cancer. The tumoral microenvironment promotes T cells deregulation and an increase of the immune checkpoint inhibitors expression (85,86). Probably this is one of the most studied mechanisms in the last years in cancer biology and stands as a cornerstone of the immunotherapy. Also, represents one of the hallmarks of cancer. In addition, forkhead box P3 (FOXP3)+ T-regulatory lymphocytes (Treg), a subset of CD4+ T cells found in the tumour microenvironment are specialized in the suppression of the host immune system, thus promoting tumour development (87). The main mechanisms involved in tumoral immune evasion and the main actors involved in liver cancer are illustrated in Figure 1.
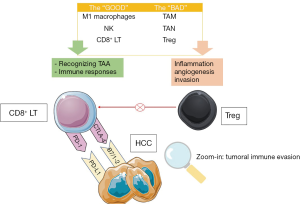
The suppressive function of FOXP3+ Tregs may be related to target cells killing, modulation of target cell signalling via cell-cell contact, and immunosuppressive cytokines secretion (IL-10, IL-35, and TGF-β) (88).
In order to combat the tumour-specific immune response oncologists revealed three possible mechanisms that could be also specifically applied to liver cancer:
- Adoptive immunotherapy—immune cells that destroy cancer cells;
- Indirect immunological therapies—immune checkpoint blockade, cancer vaccines used to increase immune system activity;
- Indirect non-immunological strategies—antigen-encoding mRNA strategy in HCC, oncolytic viruses.
Each type of approach will be described, in order to understand HCC’s complex biology and to highlight the most important discoveries in liver cancer.
Adoptive immunotherapy
The principle of adoptive immunotherapy is simple. Scientists take away from patients’ immune cells such as NK or T lymphocytes and after growing them in the laboratory gives them back to the patient in order to fight back cancer cells.
Natural killer cells destroy cancer cells or virus-infected cells, being known as key effector cells in cancer immune-surveillance and early viral immunity. Unfortunately, the cytotoxic effect of NK cells is diminished in patients with advanced HCC (89). Furthermore, it was demonstrated that radiofrequency ablation (RFA) can reactivate the NK’s (90). Following these assumptions, two clinical trials are ongoing, combining autologous NK’s reinfusion with resection and transplant. The results are expected in the near future.
The main objective of T cell engineering is to generate tumour-targeted T cells through genetic transfer of antigen specific receptors. Thus, T cells armed with chimeric (artificial) antigen receptors (CAR-T cells) are able to target and destroy cancer cells. CAR-T cell revolutionised haematology, with promising results in acute myeloid leukaemia, lymphoid leukaemia and lymphomas (91-93).
There are 7 ongoing clinical trials regarding engineered T cells in HCC. Although the experience of CAR-T in solid tumours is scarce, scientists hope to obtain promising results.
Indirect immunological therapies
Indirect immunological strategies in liver cancer are consisted of vaccines and immune check-point inhibitors.
HBV vaccination led to the decrease of HCC incidence, therefore it could be considered a prophylactic vaccine for HCC. However, a therapeutic vaccine for HCC as in prostate cancer is still awaited (94). The presence of dendritic cells (DCs) in the tumoral microenvironment of tumours has been associated with a good prognosis. In addition, it was shown that DC infiltration in HCC lesions has been associated with a better prognosis in resected patients (95). Therefore, many clinical trials bursted trying to find the best DC-immunotherapy approach. One DC vaccine pulsed with autologous tumour lysate reported that 12.9% of advanced HCC patients had partial response and 54.8% had stable disease (96). Other studies revealed tumour recurrence after combining radiotherapy or TACE with DC vaccine (97,98).
Immune check-points, found on many type of immune cells, prevent T cell overactivation against different antigens and thus limiting self tissue damage by are physiologically induced immunosuppression (99). Liver tumour cells use cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death-1 (PD-1) inhibitory pathways to silence the host’s immune cells activity in order to evade and proliferate, which are shown on Figure 1.
Both CTLA-4 and PD-1 binding share the negative effects on T-cell activity. However, the signalling mechanisms and location of the immune inhibition differ. CTLA-4 inhibits the immune priming phase by silencing the effector T cells and by recruiting more Treg cells to further support the suppression of T-cells (100).
PD-1 works on the effector phase and inhibits the function of T cells in the periphery following extended or high levels of tumoral antigen exposure (101).
All the preclinical information discussed earlier provides a valid rationale for an immunologic approach to the treatment of HCC based on the interaction with immune checkpoints. The most important checkpoint inhibitors in HCC clinical trials are outlined in the Table 1.
Table 1
| Phase | NCT number | Agents | Target |
|---|---|---|---|
| Check-point inhibitors as monotherapy | |||
| 1b/2 | 01658878 | Nivolumab | PD-1 |
| 3 | 02702414 | Pembrolizumab | PD-1 |
| 3 | 02576509 | Nivolumab vs. Sorafenib | PD-1 |
| 3 | 02702401 | Pembrolizumab vs. BSC | PD-1 |
| Combination of check-point inhibitors | |||
| 1b/2 | 01658878 | Nivolumab + Ipilimumab | PD-1 + CTLA-4 |
| 1b/2 | 03071094 | Nivolumab + PexaVac | PD-1 |
| 1b/2 | 02519348 | Tremelimumab + Durvalumab vs. Durvalumab vs. Tremelimumab | PD-L1 + CTLA-4 |
| Association of check-point inhibitors with other antineoplastic agents/therapies | |||
| 2 | 03439891 | Nivolumab + Sorafenib | PD-1 + multikinase |
| 1 | 03299946 | Nivolumab + Cabozantinib | PD-1 + multikinase |
| 1 | 03418922 | Nivolumab + Lenvatinib | PD-1 + multikinase |
| 1b/2 | 02859324 | Nivolumab + CC-122 | PD-1 & pleiotropic pathway modifier |
| 1b/2 | 02423343 | Nivolumab + Galunisertib | PD-1 & TGFb |
| 1 | 03382886 | Nivolumab + Bevacizumab | PD-1 + VEGF |
| 1b/2 | 03033446 02837029 |
Nivolumab + Y90 radioembolization | PD-1+ radiation |
| 1 | 01853618 | Tremelimumab + TACE | CTLA-4 + chemoembolization |
| Tremelimumab + RFA | CTLA-4 + ablation | ||
| 1a/b | 02572687 | Durvalumab + Ramucirumab | PD-1 + VEGFR2 |
| 1b | 02856425 | Pembrolizumab + Nintedanib | PD-1 + multikinase |
| 1 | 03006926 | Pembrolizumab + Lenvatinib | PD-1 + multikinase |
| 1b | 02988440 | PDR001 + Sorafenib | PD-1 + multikinase |
| 1b/2 | 02795429 | PDR001 vs. PDR001 + Capmatinib | PD-1 + c-met |
PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; Y90, yttrium 90; TACE, transarterial chemoembolization; RFA, radiofrequency ablation.
Clinical studies have only recently been conducted and the results are very promising. The CTLA-4 antibody (ipilimumab) became the first check-point inhibitor approved for systemic treatment of cancer patients and it was used successfully in non-resectable melanoma (102). Since then it was tested on a variety of cancers, including HCC. Regarding liver cancer, the first CTLA-4 inhibitor chosen was tremelimumab. A phase I clinical trial from Spain tested tremelimumab alone on patients with advanced HCC in a phase I trial. The treatment was well tolerated with 17.6% and 58.8% of partial response (PR) and stable disease, respectively. In addition, it decreased the viral load of HCV infected patients (103). Patients received a suboptimal dose of 15 mg/kg tremelimumab every 90 days to a maximum of 4 doses till tumour progression or toxicities occurred. Despite the suboptimal dosing, 3/17 partial responses were observed and therefore the trial was found to be positive. Stable disease was the best response in 10 patients and the median time to progression was 6.48 months (95% CI, 3.95–9.14 months), not different compared to other second line trials in HCC. Authors also attest the possibility that maybe the final results were influenced due to the high proportion of Child B patients.
In order to enhance tremelimumab’s antineoplastic effect, other scientists thought to associate it with percutaneous radiofrequency (RFA) or TACE (104). The hypothesis stands on the fact that after performing RFA or TACE on a HCC tumor, the cell death will determine a strong immunogenic response that will be further amplified by CTLA-4 blockade. Thus, the patients were treated with an optimal dose of tremelimumab at two dose levels (3.5 and 10 mg/kg IV) every 4 weeks, a total of 6 doses, followed by 3-monthly infusions until off-treatment criteria were met. 5 weeks after the first dose of the anti-immune checkpoint was performed the interventional procedure. The better OS of 12.3 months (95% CI, 9.3–15.4 months) in the combination trial could be explained by better liver function, but also by the enhanced immunologic effect of prior ablation.
Based on these findings on tremelimumab (good antitumor activity in advanced HCC and good safety profile in cirrhotic patients), other immune checkpoint inhibitors were tested. Therefore PD-1/PD-L1 pathway provides another mechanistic approach. In addition, PD1-1/PD-L1 checkpoint inhibitors revolutionised lung cancer and gave a new hope for these patients (105).
Nivolumab, a fully human molecular antibody anti-PD-1 has been tested in patients with intermediate or advanced HCC and preserved liver function (CP-A) that were candidates to systemic therapy and had progressed or were intolerant to sorafenib or had refused this drug. The trial, also known as the CheckMate 040 study (106), showed a median response duration was 17 months. Very impressive, response was ongoing beyond 24 months in 1 patient who stopped treatment with a complete response. Following the extraordinary results obtained against melanoma when combining immune checkpoint inhibitors, the next objective of the 1b phase of the Checkmate 040 trial is the dual blockade of PD-L1 and the CTLA-4 where different doses of ipilimumab and nivolumab are tested. The results will be available this year in November. Furthermore, by following the promising results of this trial, the hepatology community hopes that the administration immune checkpoints will be approved in Europe.
Pembrolizumab is another PD-1 antibody currently under investigation in HCC with promising results. It is also investigated in earlier phase combination therapy with lenvatinib or regorafenib and other immunotherapeutics as well as in combination with locoregional therapies. The results of the trials will be revealed in the recent future.
High levels of PD-1 and PD-L1 in tumor tissue is associated with negative prognostic in patients undergoing liver resection with consequent increased rate of recurrence after surgery (107). Regarding this concern, many studies are focusing on combining of immune check point inhibitors with other therapies: ablation, TACE, anti-angiogenetic therapies. From the immunological point view, vascular endothelial growth factor (VEGF) inhibits dendritic cell maturation and T-cell activation. VEGF promotes the expression of PD-1 and CTLA-4 by inducing CD8+ T cells’ exhaustion (108). Paradoxically, when given high doses of anti-VEGF therapies the induced hypoxia produces an enhancement of the check point molecules. To this respect, Huang et al. stated that a careful titration of anti-VEGF therapy with VEGF blockage but without excessive pruning of tumour vasculature may enhance immunotherapy efficacy. The clinical trials outgoing were shown in the table earlier (109).
Immune check-point inhibitors represent a hope for primary liver cancer patients.
Indirect non-immunological strategies
Mouse models of HCC revealed another antitumor strategy: vaccines with mRNA. DCs which are cultivated and electroporated with mRNA are restituted into the tumor, with important results (110). Oncolytic viruses can induce tumor cells lysis during viral replication and also being able to reveal tumor antigens (111). A randomized phase II trial tested the feasibility of two doses of JX-594 (Pexa-Vec), an oncolytic and immunotherapeutic vaccine virus in 30 HCC patients, revealing a higher OS in the high-dose arm versus the low-dose arm (14.1 and 6.7 months, respectively) (112). Currently, a phase III study investigates the administration of this virus followed by sorafenib versus sorafenib alone. In addition, another trial is associating the combination of Pexa-Vec and nivolumab.
Conclusions
Sorafenib and lenvatinib are the first line approved systemic therapies for advanced primary liver cancer, while regorafenib is the second line stated in the guidelines. Emerging therapies are on their way with important and promising results. Immune strategies such as adoptive immunotherapy and immune check-point inhibitors are the most studied in clinical trials. A possibility of obtaining efficient results stands in the combination of the immunotherapies with other antitumoral agents like multikinase inhibitors. The objectives of clinical trials will not be only to obtain an increased survival but also to determine the exact doses in order to have less toxicities. Furthermore, the future stands in to the hands of precision and translational medicine, to engineer one individual’s immune cells and tumoral antigens in order to destroy targeted cancer cells.
Acknowledgments
Funding: I Nenu and H Stefanescu were partially supported by
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giovanni Brandi; Francesco Tovoli) for the series “Primary Liver Cancer” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.11.23). The series “Primary Liver Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Akinyemiju T, Abera S, Ahmed M, et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level. JAMA Oncol 2017;3:1683-91. [Crossref] [PubMed]
- Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016;122:1757-65. [Crossref] [PubMed]
- Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019;156:477-491.e1. [Crossref] [PubMed]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-73.e1. [Crossref] [PubMed]
- Bosetti C, Levi F, Boffetta P, et al. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology 2008;48:137-45. [Crossref] [PubMed]
- Spârchez Z, Mocan T. Hepatocellular carcinoma occurrence and recurrence after antiviral treatment in HCV-related cirrhosis. Are outcomes different after direct antiviral agents? A review. J Gastrointestin Liver Dis 2017;26:403-10. [PubMed]
- Park EJ, Lee JH, Yu GY, et al. Dietary and Genetic Obesity Promote Liver Inflammation and Tumorigenesis by Enhancing IL-6 and TNF Expression. Cell 2010;140:197-208. [Crossref] [PubMed]
- Bolondi L. Screening for hepatocellular carcinoma in cirrhosis. J Hepatol 2003;39:1076-84. [Crossref] [PubMed]
- Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: Results from the HALT-C Trial. J Hepatol 2005;43:434-41. [Crossref] [PubMed]
- Llovet JM, Brú C, Bruix J. Prognosis of Hepatocellular Carcinoma: The BCLC Staging Classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J Hepatol 2016;65:1140-7. [Crossref] [PubMed]
- Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 2006;66:11851-8. [Crossref] [PubMed]
- Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 2008;7:3129-40. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Iavarone M, Cabibbo G, Piscaglia F, et al. Field-practice study of sorafenib therapy for hepatocellular carcinoma: A prospective multicenter study in Italy. Hepatology 2011;54:2055-63. [Crossref] [PubMed]
- Ganten TM, Stauber RE, Schott E, et al. Sorafenib in patients with hepatocellular carcinoma—results of the observational INSIGHT study. Clin Cancer Res 2017;23:5720-8. [Crossref] [PubMed]
- Cardoso H, Alves AM, Marques M, et al. Tratamento de Carcinoma Hepatocelular Com Sorafenib: Avaliação de Fatores de Prognóstico e um Indício Prático para a Orientação dos Doentes. GE Port J Gastroenterol 2016;23:243-8. [Crossref] [PubMed]
- Waziry R, Gomaa A, Waked I, et al. Determinants of survival following hepatocellular carcinoma in Egyptian patients with untreated chronic HCV infection in the pre-DAA era. Arab J Gastroenterol 2018;19:26-32. [Crossref] [PubMed]
- Karkampouna S, van der Helm D, Gray PC, et al. CRIPTO promotes an aggressive tumour phenotype and resistance to treatment in hepatocellular carcinoma. J Pathol 2018;245:297-310. [Crossref] [PubMed]
- Di Costanzo GG, Casadei Gardini A, Marisi G, et al. Validation of a Simple Scoring System to Predict Sorafenib Effectiveness in Patients with Hepatocellular Carcinoma. Target Oncol 2017;12:795-803. [Crossref] [PubMed]
- Tong M, Che N, Zhou L, et al. Efficacy of annexin A3 blockade in sensitizing hepatocellular carcinoma to sorafenib and regorafenib. J Hepatol 2018;69:826-39. [Crossref] [PubMed]
- Kim HY, Lee DH, Cho EJ, et al. A novel biomarker-Based model for the prediction of response to sorafenib and overall survival for advanced hepatocellular carcinoma: A prospective cohort study. Hepatology 2016;63:634A.
- Takada H, Kurosaki M, Nakanishi H, et al. Impact of pre-sarcopenia in sorafenib treatment for advanced hepatocellular carcinoma. PLoS One 2018;13:e0198812. [Crossref] [PubMed]
- Sánchez AIP, Roces LV, García IZ, et al. Value of α-fetoprotein as an early biomarker for treatment response to sorafenib therapy in advanced hepatocellular carcinoma. Oncol Lett 2018;15:8863-70. [PubMed]
- Tutusaus A, Stefanovic M, Boix L, et al. Antiapoptotic BCL-2 proteins determine sorafenib/regorafenib resistance and BH3-mimetic efficacy in hepatocellular carcinoma. Oncotarget 2018;9:16701-17. [Crossref] [PubMed]
- Dong XF, Liu QT, Zhi XT, et al. COX-2/PGE2 Axis Regulates HIF-2α Activity to Promote Hepatocellular Carcinoma Hypoxic Response and Reduce the Sensitivity of Sorafenib Treatment. Clin Cancer Res 2018;24:3204-16. [Crossref] [PubMed]
- Labeur TA, Ten Cate DWG, Bart Takkenberg R, et al. Are we SHARP enough? The importance of adequate patient selection in sorafenib treatment for hepatocellular carcinoma. Acta Oncol 2018;57:1467-74. [Crossref] [PubMed]
- Yen CJ, Kim TY, Feng YH, et al. A Phase I/Randomized Phase II Study to Evaluate the Safety, Pharmacokinetics, and Efficacy of Nintedanib versus Sorafenib in Asian Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2018;7:165-78. [Crossref] [PubMed]
- Daniele B, Croitoru A, Papandreou C, et al. Impact of sorafenib dosing on outcome from the European patient subset of the GIDEON study. Future Oncol 2015;11:2553-62. [Crossref] [PubMed]
- Ostwal V, Gupta T, Chopra S, et al. Tolerance and adverse event profile with sorafenib in Indian patients with advanced hepatocellular carcinoma. South Asian J Cancer 2017;6:144-6. [Crossref] [PubMed]
- DA Fonseca LG. Safety and efficacy of sorafenib in patients with Child-Pugh B advanced hepatocellular carcinoma. Mol Clin Oncol 2015;3:793-6. [Crossref] [PubMed]
- Terashima T, Yamashita T, Sunagozaka H, et al. Analysis of the liver functional reserve of patients with advanced hepatocellular carcinoma undergoing sorafenib treatment: prospects for regorafenib therapy. Hepatol Res 2018;48:956-66. [Crossref] [PubMed]
- de Rosamel L, Blanc JF. Emerging tyrosine kinase inhibitors for the treatment of hepatocellular carcinoma. Expert Opin Emerg Drugs 2017;22:175-90. [Crossref] [PubMed]
- Raoul JL, Kudo M, Finn RS, et al. Systemic therapy for intermediate and advanced hepatocellular carcinoma: Sorafenib and beyond. Cancer Treat Rev 2018;68:16-24. [Crossref] [PubMed]
- Baxter MA, Glen H, Evans TR. Lenvatinib and its use in the treatment of unresectable hepatocellular carcinoma. Future Oncol 2018;14:2021-9. [Crossref] [PubMed]
- Contratto M, Wu J. Targeted therapy or immunotherapy? Optimal treatment in hepatocellular carcinoma. World J Gastrointest Oncol 2018;10:108-14. [Crossref] [PubMed]
- Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245-55. [Crossref] [PubMed]
- Kissel M, Berndt S, Fiebig L, et al. Antitumor effects of regorafenib and sorafenib in preclinical models of hepatocellular carcinoma. Oncotarget 2017;8:107096-108. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol 2018;69:353-8. [Crossref] [PubMed]
- Qiu MJ, He XX, Bi NR, et al. Effects of liver-targeted drugs on expression of immune-related proteins in hepatocellular carcinoma cells. Clin Chim Acta 2018;485:103-5. [Crossref] [PubMed]
- Uchikawa S, Kawaoka T, Aikata H, et al. Clinical outcomes of sorafenib treatment failure for advanced hepatocellular carcinoma and candidates for regorafenib treatment in real-world practice. Hepatol Res 2018;48:814-20. [Crossref] [PubMed]
- Alves RCP, Alves D, Guz B, et al. Advanced hepatocellular carcinoma. Review of targeted molecular drugs. Ann Hepatol 2011;10:21-7. [PubMed]
- Abou-Alfa GK, Cheng AL, Meyer T, et al. Phase 3 randomized, double-blind, controlled study of cabozantinib (XL184) versus placebo in subjects with hepatocellular carcinoma who have received prior sorafenib (CELESTIAL; NCT01908426). J Clin Oncol 2017; [Crossref]
- Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol 2008;26:2992-8. [Crossref] [PubMed]
- Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol 2009;10:794-800. [Crossref] [PubMed]
- Raoul JL, Finn RS, Kang YK, et al. An open-label phase II study of first- and second-line treatment with brivanib in patients with hepatocellular carcinoma (HCC). J Clin Oncol 2009;27:4577.
- Toh H, Chen P, Carr BI, et al. A phase II study of ABT-869 in hepatocellular carcinoma (HCC): Interim analysis. J Clin Oncol 2009;27:4581.
- Alberts SR, Morlan BW, Kim GP, et al. NCCTG phase II trial (N044J) of AZD2171 for patients with hepatocellular carcinoma (HCC)-Interim review of toxicity. Gastrointestinal Cancers Symposium. Orlando, FL, USA; 2007.
- Koch I, Baron A, Roberts S, et al. Influence of hepatic dysfunction on safety, tolerability, and pharmacokinetics (PK) of PTK787/ZK 222584 in patients (Pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol 2005;23:4134. [Crossref]
- Yau CC, Chen PJ, Curtis CM, et al. A phase I study of pazopanib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2009;27:3561.
- Thomas MB, Chadha R, Glover K, et al. Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma. Cancer 2007;110:1059-67. [Crossref] [PubMed]
- O’Dwyer PJ, Giantonio BJ, Levy DE, et al. Gefitinib in advanced unresectable hepatocellular carcinoma: results from the Eastern Cooperative Oncology Group’s Study E1203. J Clin Oncol 2006;24:4143.
- Bekaii-Saab T, Markowitz J, Prescott N, et al. A multi-institutional phase II study of the efficacy and tolerability of lapatinib in patients with advanced hepatocellular carcinomas. Clin Cancer Res 2009;15:5895-901. [Crossref] [PubMed]
- Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): Results of a phase II study. Cancer 2007;109:1384-90. [Crossref] [PubMed]
- Sanoff HK, Bernard S, Goldberg RM, et al. Phase II Study of Capecitabine, Oxaliplatin, and Cetuximab for Advanced Hepatocellular Carcinoma. Gastrointest Cancer Res 2011;4:78-83. [PubMed]
- Chen L, Shiah HS, Chen CY, et al. Randomized, phase I, and pharmacokinetic (PK) study of RAD001, an mTOR inhibitor, in patients (pts) with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2009;27:4587.
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54-63. [Crossref] [PubMed]
- Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859-70. [Crossref] [PubMed]
- Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J Clin Oncol 2013;31:4067-75. [Crossref] [PubMed]
- Cainap C, Qin S, Huang WT, et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: Results of a randomized phase III trial. J Clin Oncol 2015;33:172-9. [Crossref] [PubMed]
- Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J Clin Oncol 2013;31:3517-24. [Crossref] [PubMed]
- Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: Results from the randomized phase III BRISK-PS study. J Clin Oncol 2013;31:3509-16. [Crossref] [PubMed]
- Zhu AX, Rosmorduc O, Evans TRJ, et al. Search: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2015;33:559-66. [Crossref] [PubMed]
- Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: The EVOLVE-1 randomized clinical trial. JAMA 2014;312:57-67. [Crossref] [PubMed]
- Kondo S, Ojima H, Tsuda H, et al. Clinical impact of c-Met expression and its gene amplification in hepatocellular carcinoma. Int J Clin Oncol 2013;18:207-13. [Crossref] [PubMed]
- Taieb J, Barbare JC, Rougier P. Medical treatments for hepatocellular carcinoma (HCC): What’s next? Ann Oncol 2006;17:x308-14. [Crossref] [PubMed]
- Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs Doxorubicin Alone in Patients With Advanced Hepatocellular Carcinoma. JAMA 2010;304:2154-60. [Crossref] [PubMed]
- Buschauer S, Koch A, Wiggermann P, et al. Hepatocellular carcinoma cells surviving doxorubicin treatment exhibit increased migratory potential and resistance to doxorubicin re-treatment in vitro. Oncol Lett 2018;15:4635-40. [PubMed]
- Ikeda M, Morizane C, Ueno M, et al. Chemotherapy for hepatocellular carcinoma: Current status and future perspectives. Jpn J Clin Oncol 2018;48:103-14. [Crossref] [PubMed]
- Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst 2005;97:1532-8. [Crossref] [PubMed]
- Le Grazie M, Biagini MR, Tarocchi M, et al. Chemotherapy for hepatocellular carcinoma: The present and the future. World J Hepatol 2017;9:907-20. [Crossref] [PubMed]
- Choi WY, Chung WJ, Bae SH, et al. A Randomized, prospective, comparative study about effects and safety of Sorafenib vs. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma patients with portal vein tumor thrombosis. Hepatology 2016;64:624A-5A.
- De Lorenzo S, Tovoli F, Barbera MA, et al. Metronomic capecitabine vs. best supportive care in Child-Pugh B hepatocellular carcinoma: A proof of concept. Sci Rep 2018;8:9997. [Crossref] [PubMed]
- Trevisani F, Brandi G, Garuti F, et al. Metronomic capecitabine as second-line treatment for hepatocellular carcinoma after sorafenib discontinuation. J Cancer Res Clin Oncol 2018;144:403-14. [Crossref] [PubMed]
- Casadei Gardini A, Foca F, Scartozzi M, et al. Metronomic capecitabine versus best supportive care as second-line treatment in hepatocellular carcinoma: A retrospective study. Sci Rep 2017;7:42499. [Crossref] [PubMed]
- Ravaioli M, Cucchetti A, Pinna AD, et al. The role of metronomic capecitabine for treatment of recurrent hepatocellular carcinoma after liver transplantation. Sci Rep 2017;7:11305. [Crossref] [PubMed]
- Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: Crosstalk between the liver and gut. J Physiol 2012;590:447-58. [Crossref] [PubMed]
- Kassel R, Cruise MW, Iezzoni JC, et al. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology 2009;50:1625-37. [Crossref] [PubMed]
- Okazaki T, Maeda A, Nishimura H, et al. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci 2001;98:13866-71. [Crossref] [PubMed]
- Miamen AG, Dong H, Roberts LR. Immunotherapeutic Approaches to Hepatocellular Carcinoma Treatment. Liver Cancer 2012;1:226-37. [Crossref] [PubMed]
- BioMed Research International. Retracted: The Immune System in Hepatocellular Carcinoma and Potential New Immunotherapeutic Strategies. Biomed Res Int 2016;2016:2514067. [PubMed]
- Pedroza-Gonzalez A, Verhoef C, Ijzermans JNM, et al. Activated tumor-infiltrating CD4+ regulatory T cells restrain antitumor immunity in patients with primary or metastatic liver cancer. Hepatology 2013;57:183-94. [Crossref] [PubMed]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057-61. [Crossref] [PubMed]
- Jinushi M, Takehara T, Tatsumi T, et al. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol 2005;43:1013-20. [Crossref] [PubMed]
- Zerbini A, Pilli M, Laccabue D, et al. Radiofrequency Thermal Ablation for Hepatocellular Carcinoma Stimulates Autologous NK-Cell Response. Gastroenterology 2010;138:1931-42. [Crossref] [PubMed]
- Grupp SA, Kalos M, Barrett D, et al. Chimeric Antigen Receptor–Modified T Cells for Acute Lymphoid Leukemia. N Engl J Med 2013;368:1509-18. [Crossref] [PubMed]
- Pizzitola I, Anjos-Afonso F, Rouault-Pierre K, et al. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia 2014;28:1596-605. [Crossref] [PubMed]
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377:2531-44. [Crossref] [PubMed]
- Wargowski E, Johnson LE, Eickhoff JC, et al. Prime-boost vaccination targeting prostatic acid phosphatase (PAP) in patients with metastatic castration-resistant prostate cancer (mCRPC) using Sipuleucel-T and a DNA vaccine. J Immunother Cancer 2018;6:21. [Crossref] [PubMed]
- Ormandy LA, Farber A, Cantz T, et al. Direct ex vivo analysis of dendritic cells in patients with hepatocellular carcinoma. World J Gastroenterol 2006;12:3275-82. [Crossref] [PubMed]
- Lee WC, Wang HC, Hung CF, et al. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother 2005;28:496-504. [Crossref] [PubMed]
- Chi KH, Liu SJ, Li CP, et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother 2005;28:129-35. [Crossref] [PubMed]
- Mizukoshi E, Nakamoto Y, Arai K, et al. Enhancement of tumor-specific T-cell responses by transcatheter arterial embolization with dendritic cell infusion for hepatocellular carcinoma. Int J Cancer 2010;126:2164-74. [PubMed]
- Brahmer JR, Pardoll DM. Immune Checkpoint Inhibitors: Making Immunotherapy a Reality for the Treatment of Lung Cancer. Cancer Immunol Res 2013;1:85-91. [Crossref] [PubMed]
- Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008;322:271-5. [Crossref] [PubMed]
- Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682-7. [Crossref] [PubMed]
- Hodi FS, Day SJO, Mcdermott DF, et al. NIH Public Access. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Sangro B, Gomez-Martin C, De La Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81-8. [Crossref] [PubMed]
- Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 2017;66:545-51. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971-9. [Crossref] [PubMed]
- Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8 + T cells in tumors. J Exp Med 2015;212:139-48. [Crossref] [PubMed]
- Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci 2012;109:17561-6. [Crossref] [PubMed]
- Van Lint S, Heirman C, Thielemans K, et al. From a chemical blueprint for protein production to an off-the-shelf therapeutic. Hum Vaccin Immunother 2013;9:265-74. [Crossref] [PubMed]
- Tenneti P, Board MJ, Babiker HM. Exploring the role of oncolytic viruses in hepatobiliary cancers. Immunotherapy 2018;10:971-86. [Crossref] [PubMed]
- Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 2013;19:329-36. [Crossref] [PubMed]


