
Exosomal miR-196b-5p is a potential diagnostic marker for colorectal cancer with metachronous liver metastasis
Introduction
Colorectal cancer (CRC) has become the second most common cancer in females and the third most common cancer in males (1). In China, mortality caused by CRC was estimated to reach 191,000 in 2015 (2). Most CRCs arise from preexisting adenomatous or serrated polyps, and the liver was the most common site of CRC metastasis (3). According to liver metastasis (LM) time, CRC LM can be divided into synchronous liver metastases (SLM) (simultaneously occurring liver metastases) and metachronous liver metastases (MLM) (liver metastases occurring 6 months after radical resection). Reports show that approximately 20–25% of CRC patients are initially diagnosed with SLM, and approximately 50–60% of the patients developed MLM after primary tumor resection (4,5). Unfortunately, approximately 75% of patients will experience recurrence within 2 years, after liver metastases resection (6). Patients with SLM are thought to have a less favorable prognosis and easy to relapse than those with CRC alone (4-6). Although it is not clear which liver metastases are more prone to recurrence, CRC patients with SLM are considered as a less biologically favorable group. Therefore, it is extremely important to find effective markers to identify SLM and MLM.
Exosomes, containing microRNAs (miRNAs), DNA, and/or proteins, are small homogenous membrane vesicles that released by cells in both physiological and pathological situations, and involve in tumor growth, metastasis, and therapy resistance (7,8). Therefore, exosomes have the potential to be utilized as cancer biomarkers. MiRNAs are short, noncoding RNAs that play important roles in many biological processes, including cell apoptosis, proliferation and differentiation (9). Dysregulation of miRNAs contribute to the tumorigenesis, progression and metastasis in a variety of cancers and also can be use as biomarkers for early diagnosis, prognosis and prediction the sensitivity of tumor to clinical treatment (10). Our previous research found that miR-196b-5p was upregulated in CRC tissues and upregulation of miR-196b-5p was associated with poor survival in CRC patients (11). Especially, miR-196b-5p was detected at significantly higher levels in the serum exosomes of CRC patients compared to the healthy control subjects (11). Thus, we want to further explore whether miR-196b-5p is related to LM.
Methods
Patients and serum samples
Serum samples from CRC patients were obtained at Clinical laboratory in Jiangmen Central Hospital (Guangdong, China) between April 2011 and September 2012. All cases were diagnosed as CRC by preoperative colonoscopy and postoperative pathological diagnosis. No adjuvant therapy, such as neoadjuvant chemotherapy and radiotherapy, was used before operation. Serum samples were collected after diagnosis and before operation, and stored at –80 °C. All cases were followed up for 5 years to determine whether or not liver metastases occurred after the operation. According to the follow-up results, 49 cases of CRC patients with NLM, 51 cases of CRC patients with SLM and 50 cases of CRC patients with MLM were included in this study. The pathological characteristics were detailed in Table 1. In addition, 100 cases of serum from healthy volunteers (HV) were collected as controls during the same period (Table 1).
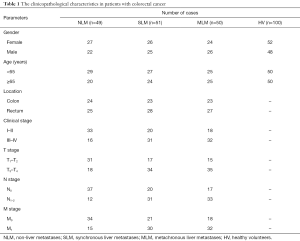
Full table
RNA extraction, reverse transcription, and real-time polymerase chain reaction (RT-PCR)
The exoEasy centrifuge column filtration method was used to separate the serum exosome, and the specific operation was carried out according to the instructions. The exosome RNA of serum without hemolysis was extracted according to the operation instructions of the exoRNeasy Serum/Plasma Midi kit (QIAGEN China, Shanghai, China). miRNA was reversely transcribed from total mRNA using the Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc., Waltham, USA) according to the manufacturer’s protocol. Complementary DNA (cDNA) was amplified and quantified on CFX96 system (Bio-Rad, Redmond, USA) using iQ SYBR Green (Bio-Rad, Redmond, USA). Primers for U6 and miR-196b-5p (Cat#: miRQ0001080) were synthesized and purified by RiboBio (Guangzhou, China). U6 was used as endogenous controls. Relative fold expression was calculated with the comparative threshold cycle (2−ddCt) method.
Carcinoembryonic antigen (CEA) and carbohydrate antigen 199 (CA199) detection
CEA, CA199 detection kit and COBAS e 602 instruments were provided by Roche (Basel, Switzerland). The detection method was electrochemiluminescence, and the specific operation was carried out according to the instructions (12).
Statistical analysis
SPSS 20 statistical software was used to analyze the data. The data of non-normal distribution was represented by median (four quantile interval). Mann-Whitney U test and t-test were used between the two groups. Kruskal-Wallis H test was used in comparison among multiple groups, Spearman was used in correlation analysis. Survival curves were plotted using the Kaplan Meier method and compared by log-rank test. P<0.05 was considered significant.
Results
Serum exosomal miR-196b-5p was elevated in CRC patients and associated with liver metastases
In comparison with HV, higher levels of exosomal miR-196b-5p were observed in CRC patients including patients with LM and non-liver metastases (NLM) (Figure 1A). Interestingly, more significantly elevated levels of serum exosomal miR-196b-5p were found in CRC patients with MLM, compared to CRC patients with NLM or CRC patients with SLM (Figure 1B). Besides, CRC patients with MLM also showed higher levels of CEA and CA199 than patients with NLM (Figure 1C,D). However, unlike miR-196b-5p, CEA levels were not significantly different between NLM and SLM patients, and CA199 levels were not significantly different between SLM and MLM patients (Figure 1B,C,D). These results suggested that the serum exosomal miR-196b-5p was increased in patients with CRC, particularly in patient with MLM.

Higher levels of serum exosomal miR-196b-5p was related to unfavorable clinicopathological features in CRC patients with NLM, SLM or MLM
Consistent with the results we reported previously in CRC tissues (11), we also found that the relative expression of serum exosomal miR-196b-5p in III–IV stage, T3–T4 stage, and N1–2 stage CRC patients were correspondingly higher than I–II stage, T1–T2 stage, and N0 stage CRC patients, respectively, whether the patient has liver metastases or NLM (Table 2).
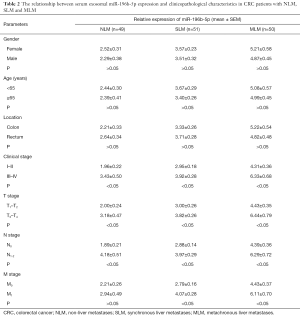
Full table
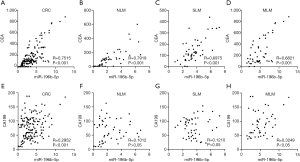
Serum exosomal miR-196b-5p was positively correlated with CEA and CA199 in CRC patients with MLM
In order to explore the relationship of serum exosomal miR-196b-5p with CEA or CA199 respectively, the correlation of miR-196b-5p with CEA or CA199 levels was analyzed. Results showed that miR-196b-5p had a positive correlation with CEA levels in CRC patients in this study (Figure 2A). Moreover, miR-196b-5p also had a strong positive correlation with CEA in CRC patients with NLM, SLM or MLM (Figure 2B,C,D). Additionally, miR-196b-5p had a positive correlation with CA199 levels in CRC patients (Figure 2E), especially. However, miR-196b-5p only had a positive correlation with CA199 in patients with MLM, but not in patients with NLM or SLM (Figure 2F,G,H).
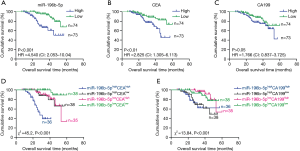
CRC patients with higher levels of both serum exosomal miR-196b-5p and CEA, or both serum exosomal miR-196b-5p and CA199 had a shorter overall survival (OS)
Kaplan-Meier analysis showed the evidence that the OS was shorter in CRC patients with higher levels of serum exosomal miR-196b-5p than those patients with lower levels of exosomal miR-196b-5p, regardless of whether the patient had liver metastases (Figure 3A). In addition, the OS was shorter in CRC patients with higher CEA but not CA199 levels than those patients with lower levels (Figure 3B,C), respectively. Additionally, according to the miR-196b-5p, CEA and CA199 levels, patients were divided into miR-196b-5phighCEAhigh, miR-196b-5phighCEAlow, miR-196b-5plowCEAhigh, miR-196b-5plowCEAlow, miR-196b-5phighCA199high, miR-196b-5phighCA199low, miR-196b-5plowCA199high, miR-196b-5plowCA199low groups. Results showed that miR-196b-5phighCEAhigh patients had a better prognosis than miR-196b-5phighCEAlow, miR-196b-5plowCEAhigh, miR-196b-5plowCEAlow patients, respectively (Figure 3D). miR-196b-5phighCA199high patients had a better prognosis than miR-196b-5plowCA199high, miR-196b-5plow CA199low patients (Figure 3E), respectively.
Serum exosomal miR-196b-5p was a good biomarker for the diagnosis of CRC patient with MLM
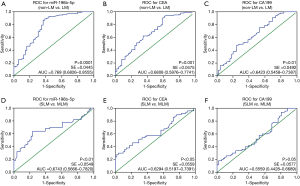
Receiver operating characteristic (ROC) analysis of serum exosomal miR-196b-5p, CEA and CA199 in CRC patients with LM and NLM, SLM and MLM were shown in Figure 4. The area under curve (AUC) of exosomal miR-196b-5p was 0.769 (95% CI: 0.6826–0.8555) (non-LM vs. LM, Figure 4A) and 0.6743 (95% CI: 0.5666–0.7820) (SLM vs. MLM, Figure 4D). Both of them were higher than AUC of CEA [non-LM vs. LM: 0.6808 (95% CI: 0.5876–0.7741), SLM vs. MLM: 0.6294 (95% CI: 0.5197–0.7391)] and AUC of CA199 [non-LM vs. LM: 0.6423 (95% CI: 0.5458–0.7387), SLM vs. MLM: 0.5559 (95% CI: 0.4428–0.6689)], respectively. These results suggested that serum exosomal miR-196b-5p could be a good diagnostic marker for CRC patient with MLM.
Discussion
miRNAs of different cell types can be secreted outside the cell and then enter peripheral circulating body fluids such as blood. According to reports, 10% of known human miRNAs can be detected in plasma (13-15). These detectable miRNAs in plasma or serum have a very stable form that can be encapsulated into the extracellular vesicles (apoptosis bodies, macrovesicles), or bound with special lipid proteins and are resistant to RNase activity (13,16). At present, miRNAs as biomarkers are mainly derived from tissues and circulating fluids, including plasma (or serum) and feces. Abnormal expression of miRNA is closely related to LM of CRC patients (17-20). Perilli et al. found that miR-182 was significantly overexpressed in both primary and LM CRCs, compared to normal tissues (17). They also found miR-182 alone or in combination with its target genes (ENTPD5, TSP-1, and PDCD4) may serve as prognostic markers to monitor the relapse in CRC patients (17). By comparing serum miRNA expression in CRC patients with LM and NLM, Wang et al. found that miR-29a expression significantly increased in patients with LM (18), and Yin et al. confirmed that serum miR-126, miR-141, and miR-21 levels were closely related to the early onset of CRC patients with LM (19). Additionally, elevated plasma miR-141 levels also predict poor prognosis in CRC patients. Meanwhile, miR-141 combined with CEA improved the diagnostic accuracy of CRC (19,20).
Exosomes can be taken up by neighboring or distant cells and thereby modulate the functional of recipient cells and play a key role in disease progression or facilitate metastasis in cancers (14). Increasing evidence shows that some exosomal miRNAs (e.g., miR-192, let-7a, miR-150, miR-21, miR-223, and miR-23a) have prognostic and diagnostic value on CRC. Recently, Monzo et al. found that plasma exosomal miR-328 of CRC patients with liver metastases was much greater in the mesenteric vein than in the peripheral vein, indicating a possible role of miR-328 in the development of liver metastases (21). miR-196b-5p are endogenous single-stranded non-coding small RNA molecules that can be secreted into the circulation and exist stably. Several research groups and our previous study found that overexpression of miR-196b-5p was significantly associated with the progression of myelodysplastic syndrome (22), the regulation of CRC cell migration and metastases (23), and the maintenance of stem cell property and chemotherapeutic resistance in CRC (11). Herein, we found serum exosomal miR-196b-5p was elevated in CRC patients and associated with liver metastases, and positively correlated with CEA or CA199 in CRC patients with MLM. Furthermore, CRC patients with higher levels of both serum exosomal miR-196b-5p and CEA, or both serum exosomal miR-196b-5p and CA199 had a shorter OS. ROC analysis of serum exosomal miR-196b-5p on SLM and MLM showed that AUC of exosomal miR-196b-5p were 0.6743 (95% CI: 0.5666–0.7820), which was higher than AUC of CEA [0.6294 (95% CI: 0.5197–0.7391)] and AUC of CA199 [0.5559 (95% CI: 0.4428–0.6689)], respectively.
Conclusions
Taken together, our results indicate that serum exosomal miR-196b-5p could be a good diagnostic marker for CRC patients with MLM. Although miRNAs have reliable stability and have good prospects in detecting liver metastases, the high cost and long latency of serum miRNAs are the limitations of miRNAs as blood-based diagnostic markers for screening purposes. Therefore, we still have a long way to go in the research of miRNA, but the prospects for the future are bright.
Acknowledgments
Funding: This study was supported by grants from the National Natural Science Foundation of China (81500007), the Medical Science Foundation of Guangdong Province (A2018205, A2018123 and 2016431), the Science and Technology Project of Dongguan (2018507150251294, 2016108101030 and 201750715005451), the National and Guangdong Provincial College Students’ innovative Entrepreneurial Training Program (201710571009, 201710571033).
Footnote
Conflicts of Interest: TAll authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.11.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived. The present study was approved by the Animal Ethics Committee of the Dongguan Key Laboratory of Medical Bioactive Molecular Developmental and Translational Research, Guangdong Medical University (Approval no. 20150032) and Jiangmen Central Hospital, Affiliated Jiangmen Hospital of Sun Yat-sen University (Approval no. JCH2015000834).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Gourevitch RA, Rose S, Crockett SD, et al. Variation in Pathologist Classification of Colorectal Adenomas and Serrated Polyps. Am J Gastroenterol 2018;113:431-9. [Crossref] [PubMed]
- Lykoudis PM, O’Reilly D, Nastos K, et al. Systematic review of surgical management of synchronous colorectal liver metastases. Br J Surg 2014;101:605-12. [Crossref] [PubMed]
- Adam R. Colorectal cancer with synchronous liver metastases. Br J Surg 2007;94:129-31. [Crossref] [PubMed]
- Jones RP, Jackson R, Dunne DF, et al. Systematic review and meta-analysis of follow-up after hepatectomy for colorectal liver metastases. Br J Surg 2012;99:477-86. [Crossref] [PubMed]
- Carretero-González A, Otero I, Carril-Ajuria L, et al. Exosomes: Definition, Role in Tumor Development and Clinical Implications. Cancer Microenviron 2018;11:13-21. [Crossref] [PubMed]
- Osier N, Motamedi V, Edwards K, et al. Exosomes in Acquired Neurological Disorders: New Insights into Pathophysiology and Treatment. Mol Neurobiol 2018;55:9280-93. [Crossref] [PubMed]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281-97. [Crossref] [PubMed]
- Wang H, Peng R, Wang J, et al. Circulating microRNAs as potential cancer biomarkers: the advantage and disadvantage. Clin Epigenetics 2018;10:59. [Crossref] [PubMed]
- Ren D, Lin B, Zhang X, et al. Maintenance of cancer stemness by miR-196b-5p contributes to chemoresistance of colorectal cancer cells via activating STAT3 signaling pathway. Oncotarget 2017;8:49807-23. [Crossref] [PubMed]
- Chen G, Liang Y, Guan X, et al. Circulating low IL-23: IL-35 cytokine ratio promotes progression associated with poor prognosisin breast cancer. Am J Transl Res 2016;8:2255-64. [PubMed]
- Lindner K, Haier J, Wang Z, et al. Circulating microRNAs: emerging biomarkers for diagnosis and prognosis in patients with gastrointestinal cancers. Clin Sci 2015;128:1-15. [Crossref] [PubMed]
- Hosseini M, Khatamianfar S, Hassanian SM, et al. Exosome-Encapsulated microRNAs as Potential Circulating Biomarkers in Colon Cancer. Curr Pharm Des 2017;23:1705-9. [Crossref] [PubMed]
- Beg F, Wang R, Saeed Z, et al. Inflammation-associated microRNA changes in circulating exosomes of heart failure patients. BMC Res Notes 2017;10:751. [Crossref] [PubMed]
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-9. [Crossref] [PubMed]
- Perilli L, Vicentini C, Agostini M, et al. Circulating miR-182 is a biomarker of colorectal adenocarcinoma progression. Oncotarget 2014;5:6611-9. [Crossref] [PubMed]
- Wang LG, Gu J. Serum microRNA-29a is a promising novel marker for early detection of colorectal liver metastasis. Cancer Epidemiol 2012;36:e61-7. [Crossref] [PubMed]
- Yin J, Bai Z, Song J, et al. Differential expression of serum miR-126, miR-141 and miR-21 as novel biomarkers for early detection of liver metastasis in colorectal cancer. Chin. J. Cancer Res 2014;26:95-103. [PubMed]
- Cheng H, Zhang L, Cogdell DE, et al. Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS ONE 2011;6:e17745 [Crossref] [PubMed]
- Monzo M, Santasusagna S, Moreno I, et al. Exosomal microRNAs isolated from plasma of mesenteric veins linked to liver metastases in resected patients with colon cancer. Oncotarget 2017;8:30859-69. [Crossref] [PubMed]
- Wen J, Huang Y, Li H, et al. Over-expression of miR-196b-5p is significantly associated with the progression of myelodysplastic syndrome. Int J Hematol 2017;105:777-83. [Crossref] [PubMed]
- Stiegelbauer V, Vychytilova-Faltejskova P, Karbiener M, et al. miR-196b-5p Regulates Colorectal Cancer Cell Migration and Metastases through Interaction with HOXB7 and GALNT5. Clin Cancer Res 2017;23:5255-66. [Crossref] [PubMed]


