
MicroRNA-1275 targets PKCα to depress proliferation and the invasion of pancreatic cancer cells
Introduction
Worldwide, pancreatic cancer (PC) is the eighth highest cause of cancer mortality (1). Although great progress in surgical operation has been made, such as the development of novel perioperative administration, perioperative chemotherapy and radiotherapy techniques, the prognosis of PC patients is still very poor. On account of PC developing no symptoms, local invasiveness, and metastases to distant organs in the early stage of clinical process (2), the median survival time of PC patients is 5–8 months and their 5-year-survival rate is less than 10% (3). PC carcinogenesis is a multistep course, involving dysfunction of oncogenes and suppressor genes (4). Therefore, there is a pressing need to understand what potential molecular mechanisms take place for the progression of PC, and to detect suitable strategies for a targeted therapy.
MicroRNAs (miRNAs) play roles in the malignant progression of different types of tumors, belonging to endogenous single-stranded non-coding RNA (5). It could function as either oncogenes or tumor suppressors (6). Via these mechanisms, miRNAs accommodate as much as 30% of human genes, and play an important role in many kinds of biological processes, including cell metastasis, apoptosis, differentiation, proliferation, and others (7). MiRNAs have been proven to exert a significant amount of influence on the etiology and pathogenesis of cancer (8). Many reports have shown that a lot of cancers are regulated by these miRNAs like prostatic cancer, breast cancer, and hepatocellular cancer (9-11). Recent reports have shown that miRNAs are also associated with PC, providing new methods for PC treatment (12). Fawzy et al. have reported that miR-1275 repressed the growth of hepatocellular carcinoma cells, through targeting the three IGF2-mRNA-binding proteins (5). However, the function of miR-1275 in PC remains unknown. In this paper, we show that up-regulated miR-1275 expression can depress growth and invasion of pancreatic cancer cells. Our results also demonstrate that miR-1275 restrained growth and invasion of PC cells, through directly targeting PKCα.
Methods
Ethics statement
The informed consent was signed by every patient. Our research was approved by the Renmin Hospital of Wuhan University ethical organization and abided by the rules set by the Declaration of Helsinki.
Patients and tumor samples and cell lines
We collected 20 primary PC samples and their homologous adjacent non-cancerous pancreatic tissues specimens from Renmin Hospital of Wuhan University from 2012 to 2015. All patients were enrolled without undergoing blood transfusion, radiotherapy, or chemotherapy in preoperative course. Tissue specimens were grouped into 2 parts randomly. The first group that underwent a pathological diagnosis used a 10% formalin to fix. The second group for RNA extraction was stored in liquid nitrogen. The PC cell lines PANC-1, SW1990, PC-2, MIA PaCa-2 and immortalized human pancreatic ductal epithelial cells line HPDE6-C7 were cultured in DMEM medium (GIBCO; San Diego, CA, USA) containing a 10% solution of fetal bovine serum (GIBCO) at 37 °C with 5% CO2.
Plasmids and cell transfection
We purchased the miR-1275 mimic (HMI0136) and its inhibitor (HLTUD0136) from Sigma (San Francisco, CA, USA), controls from Ribo (Guangzhou, China). PANC-1 cell lines were grown in 6-well plates. The confluence condition of cells was transfected at 30% for 24 hours. According to the instructions of manufacturer, cells were either transfected with a miR-1275 mimic/inhibitor or the controls were transfected by Lipofectamine 2000 reagent (Invitrogen Carlsbad, CA, USA). 10 nmol/L was the optimal concentration of miR-1275.
RNA isolation and quantitative real-time PCR
Trizol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract the total RNA from PC cells or tissue specimens. A reverse transcription kit (Tiangen) was used in reverse transcription. MiR-1275 expression was quantified by the qRT-PCR kit (Applied Biosystems). PKCα expression was detected by qRT-PCR by SYBRGreen assays (Takara, Da-liang, China). GAPDH was selected as a control.
SiRNA of PKCα (sense 5'-CCGAGUGAAACUCACGGACUUCAAU-3');
SiRNA of miR-1275 (5'-GUGGGGGAGAGGCUGUC-3').
Cell growth
Cell growth was tested using the Cell Counting Kit-8 (CCK-8) (Dojindo, Shanghai, China). Cells were grown in the medium with a 10% diluted concentration of CCK-8 at 37 °C. After staining, 0, 24, 48, and 72 h were selected as determining times for transfected cell growth rates. A 450 nm wavelength was used to analyze values of optical density (OD).
Assay of cell migration and invasion
Invasion assay was done using the Transwell insert (Corning, NY, USA). Cells (1×105) were resuspended into the membrane of the upper chamber with Matrigel (BD Bioscience, San Jose, CA) on the 24-well plate. In the assays of cell migration, each group of cells (5×104) was resuspended in the upper chamber of the insert with 8-well plate of BD Bioscience. In both detections, PC cells were grown in medium without serum, and medium of the lower chamber contained 10% FBS. After several hours of incubation, a cotton swab was used to clean the cells without migration or invasion in every well. Then, the inserted cells were retained in methyl alcohol and colored with hematoxylin stained. The stained cells were counted with the microscope (Olympus).
Luciferase assays
HEK293T cells (8×103/per well) were added into 96-well plates for transfection. Each well of HEK293T cells was transfected with a compound of 100 ng of PKC-3'UTR, 200 ng of PKC or PKC-miR-1275, and 20 ng of Renilla plasmid (without 3'UTR) using Lipofectamine 2000. After 48 hours, a dual-luciferase reporter gene system was implemented to monitor the activities of firefly and Renilla luciferase. Transfection efficiency was evaluated by the activities of Renilla luciferase, that also acted as a control.
Rescue assays of PKCα gene expression
We amplified the integrate PKCα cDNA (consisting of the ORF and 3'-UTR) by PCR and inserted it into the pcDNA3.1 vector. Then, the (Object) it produced the pcDNA-PKC constructs. Cells were seeded in 6-well plates. Then, the cells were transfected by 20 nM miR-1275 or a 20 nM scrambled dsRNA. After 24 hours, we used pcDNA-PKCα plasmid DNA or the pcDNA plasmid (2 µg) to co-transfect the treated cells. Finally, the cells were detected.
Western blot
Protein of the cell and tissue specimens were collected as previously described (10). The concentration of protein was determined by BCA assay (Thermo Fisher, Rockford, IL, USA). The same quantities of cells lysate were resolved by SDS–PAGE. Then, the protein was shifted to the PVDF membranes (Takara, Da-lian, China). Membranes were blocked with the following antibodies: the primary antibody was rabbit anti-PKCα (1:1,000; Abcam, Cambridge, USA), and the secondary was rabbit anti-GAPDH (1:5,000, Abcam, Cambridge, USA). Secondary HRP-conjugated antibodies (Santa Cruz) were used to detect the bound antibodies while the proteins were imaged by Pierce enhanced chemiluminescent substrate (Thermo Fisher).
Results
MiR-1275 expression decreased in PC cell lines and tissue specimens
H&E staining of the normal pancreatic tissues and the PC tissue is shown in Figure 1A. The miR-1275 mRNA expression levels in the PC cell lines were significantly lower than those observed in the normal pancreatic epithelial cell lines (Figure 1B). Figure 1C shows the miR-1275 mRNA expression of 20 PC specimens. The average miR-1275 mRNA expression of PC tissues was significantly lower than the normal counterparts (Figure 1D) (P<0.01).

MiR-1275 suppressed PC cell growth, migration on and invasion
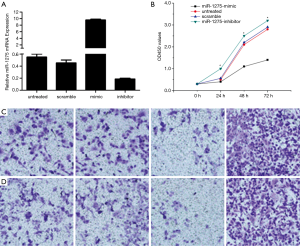
CCK-8 assays, migration assays and transwell assays were used to detect miR-1275 function on the proliferation, migration and invasion of PANC-1. PC cells were transfected with scrambled, control oligo or miR-1275 mimics and inhibitors. Efficiencies of the transfection are exhibited in Figure 2A. The proliferation of the PANC-1 cells transfected with the miR-1275 inhibitor was increased (Figure 2B); in contrast, the proliferation of PANC-1 cells transfected with the miR-1275 mimic was restrained (Figure 2B). Furthermore, the percentage of the migrated cells in cells which had been transfected with the miR-1275 mimic was lower than that of the cells transfected with the miR-1275 inhibitor (Figure 2C). Moreover, the percentage of invasive cells was similar to results of migrated cells. A higher amount of the invasive cell percentage was shown in cells which had been transfected with the miR-1275 inhibitor, and a lower invasive cells percentage was shown in the cells which had been transfected with the miR-1275 mimic (Figure 2D). Therefore, the data indicated that miR-1275 depressed cell proliferation, migration and invasion of PC cells.
PKCα 3’ UTR was a goal for miR-1275 and was negatively regulated by miR-1275
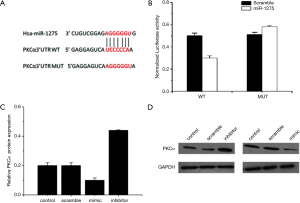
To research the goal of miR-1275 in PC cells, we used a target-scan soft website to forecast the latent goals of miR-1275. Figure 3A indicated there was complementarity relationship between has-miR-1275 and the PKCα 3' UTR. Using luciferase reporter assay, we verified the effect between PKCα and miR-1275 in the PC cell lines. Up-regulated miR-1275 expression in PANC-1 cells led to a reduced luciferase expression (Figure 3B). Furthermore, miR-1275 mimics reduced PKCα expression, and miR-1275 inhibitor played an opposite role in PC cell lines (Figure 3C and Figure3D). These data sets have revealed that PKCα expression was negatively regulated by miR-1275 through reducing miR-1275 mRNA in PC cells.
Up-regulated PKCα expression destroyed the miR-1275- influenced inhibition of proliferation and invasion
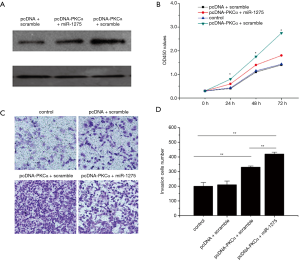
Rescue experiments were executed to confirm that PKCα was associated to the miR-1275 effect on PC cells. The expression of PKCα was restored by vector pcDNA3.1- PKCα. Less PKCα expression was shown in PC cells transfected with the pcDNA- PKCα and miR-1275 mimic after 1 day (Figure 4A). The proliferation and migration of cells was increased by the exogenous expression of PKCα (Figure 4B,C). Furthermore, the number of cell proliferations and cell invasions (Figure 4B) in the PC cells transfected with pcDNA-PKCα and miR-1275 was less than that of the PC cells which had been transfected with pcDNA-PKCα, while remaining more than that of the PC cells which had been transfected with scramble (Figure 4B,C,D). It was shown that mir-1275 could negatively regulate PKCα. Therefore, it can be conjectured from the data that miR-1275 could repress cell growth and invasion results from the decreased PKCα expression in the PC cells.
PKCα expression was negative correlation with miR-1275 in PC and non-cancerous tissues
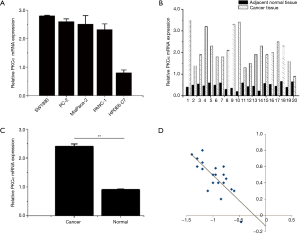
In comparison to the immortalized human pancreatic ductal epithelial cells line HPDE6-C7 (Figure 5A), PKCα mRNA was significantly increased in the PC cell lines (PANC-1, PANC-1, MIA PaCa-2, SW1990, PC-2). The level of PKCα mRNA was higher in tumor tissues than that in non-cancerous tissues (Figure 5B). Furthermore, the level of PKCα protein in PC was also higher than that in matched non-cancerous tissues (Figure 5C). Figure 5D illustrates that there is frequent inverse correlation, when the level of PKCα mRNA is plotted against miR-1275 expression (r = −0.79; P<0.01).
Discussion
Recently, numerous studies have shown that many gene expressions are regulated by miRNAs in various cancers, including pancreatic cancer (8,13). Through this regulation, miRNAs may make a great impact on cellular stress, cell differentiation, proliferation, invasion etc. (14,15). Due to the low survival rate in pancreatic cancer patients, there are numerous attempts being made to target the different molecular pathways in pancreatic cancer. Our data revealed that miR-1275 expression was higher in normal pancreatic tissues compared with PC tissues. Furthermore, an up-regulated miR-1275 expression depressed cell proliferation, migration and invasion in PC cells. Furthermore, we also revealed miR-1275 to be a suppressor by directly interacting with PKCα.
Therefore, our data discovered that miR-1275 could be acting as a suppressor gene via depressing cell proliferation and invasion in PC carcinogenesis. Fawzy et al. reported that miR-1275 was correlated with hepatocellular carcinoma targeting with the three IGF2-mRNA-binding compounds to prevent carcinoma proliferation (5). However, some reports have shown that miR-1275 could promote cell migration, invasion and proliferation through IGF-1R and CCR7in the head and neck squamous cells for carcinoma patients (5,16). The function of miR-1275 in PC remains to be determined. As per our results, miR-1275 expression was lower in PC when it was compared with the matching normal pancreatic tissues. To determine the functions of miR-1275 in PC carcinogenesis, the effects of miR-1275 were observed on a PC malignant specimen, which included testing for cell growth, migration and invasion. Up-regulated miR-1275 expression decreased PC cell growth, migration and invasion.
Our study found the potential target of miR-1275 through four miRNAs target prediction algorithms. The 3'-UTR of PKCα mRNA was confirmed for complementary sequences of miR-1275. The results of the luciferase assays displayed an active decline in 3'-UTR of PKCα luciferase activity when con-transfected with miR-1275. Compared with the control and the scrambled group, the effects of up-regulated miR-1275 expression decreased the expression of PKCα protein and mRNA. The data strongly support the hypothesis that PKCα can be directly targeted to miR-1275 in PC. Protein kinase C (PKC), which belongs to the serine-threonine kinases family, has been observed regulating cell adhesion, secretion, proliferation, differentiation and apoptosis (17-19). Furthermore, many studies have shown that PKCα can play a role in diagnosing cancers (20). In pancreatic cancer, PKCα expression was thought to be directly correlated with tumorigenicity and survival rate (21). PKCα was also observed advancing PC cell growth and invasion (22). Taken together, this evidence strongly indicates that decreasing PKCα expression can also be a significant method for regulating and treating PC. For PC, our data was first to reveal that miR-1275 could be a new suppressor and a negative regulator of PKCα expression, furnishing a possible new method for PC therapeutics.
Acknowledgments
Funding: Our experiment was funded by the Hubei Province Natural Science Foundation of China (No. 2018CFB136), the National Natural Science Foundation of China (Grant No. 61401263 and No. 61672333), and the Natural Science Basic Research Plan in Shanxi Province of China (No. 215JQ6228).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.11.32). The authors have no conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The informed consent was signed by every patient. The study was approved by the Renmin Hospital of Wuhan University ethical organization (No. 2018CFB136).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ansari D, Ansari D, Andersson R, et al. Pancreatic cancer and thromboembolic disease, 150 years after Trousseau. Hepatobiliary Surg Nutr 2015;4:325-35. [PubMed]
- Zheng Z, Zheng R, He Y, et al. Risk Factors for Pancreatic Cancer in China: A Multicenter Case-Control Study. J Epidemiol 2016;26:64-70. [Crossref] [PubMed]
- Dickson I. Pancreatic cancer: PanNETs elude elimination. Nat Rev Gastroenterol Hepatol 2016;13:314-5. [Crossref] [PubMed]
- Denham DW, Franz MG, Denham W, et al. Directed antisense therapy confirms the role of protein kinase C-alpha in the tumorigenicity of pancreatic cancer. Surgery 1998;124:218-23; discussion 223-4. [Crossref] [PubMed]
- Fawzy IO, Hamza MT, Hosny KA, et al. miR-1275: A single microRNA that targets the three IGF2-mRNA-binding proteins hindering tumor growth in hepatocellular carcinoma. FEBS Lett 2015;589:2257-65. [Crossref] [PubMed]
- Chen JT, Yao KH, Hua L, et al. MiR-338-3p inhibits the proliferation and migration of gastric cancer cells by targeting ADAM17. Int J Clin Exp Pathol 2015;8:10922-8. [PubMed]
- Wu X, Deng L, Tang D, et al. miR-615-5p prevents proliferation and migration through negatively regulating serine hydromethyltransferase 2 (SHMT2) in hepatocellular carcinoma. Tumour Biol 2016;37:6813-21. [Crossref] [PubMed]
- Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature 2005;435:834-8. [Crossref] [PubMed]
- Liu Y, Wang Y, Sun X, et al. miR-449a promotes liver cancer cell apoptosis by downregulation of Calpain 6 and POU2F1. Oncotarget 2016;7:13491-501. [PubMed]
- Wallis CJ, Gordanpour A, Bendavid JS, et al. MiR-182 Is Associated with Growth, Migration and Invasion in Prostate Cancer via Suppression of FOXO1. J Cancer 2015;6:1295-305. [Crossref] [PubMed]
- Yoo B, Kavishwar A, Ross A, et al. Combining miR-10b-Targeted Nanotherapy with Low-Dose Doxorubicin Elicits Durable Regressions of Metastatic Breast Cancer. Cancer Res 2015;75:4407-15. [Crossref] [PubMed]
- Zhou W, Li Y, Gou S, et al. MiR-744 increases tumorigenicity of pancreatic cancer by activating Wnt/beta-catenin pathway. Oncotarget 2015;6:37557-69. [Crossref] [PubMed]
- Tsukasa K, Ding Q, Miyazaki Y, et al. miR-30 family promotes migratory and invasive abilities in CD133(+) pancreatic cancer stem-like cells. Hum Cell 2016;29:130-7. [Crossref] [PubMed]
- Luo ZL, Luo HJ, Fang C, et al. Negative correlation of ITCH E3 ubiquitin ligase and miRNA-106b dictates metastatic progression in pancreatic cancer. Oncotarget 2016;7:1477-85. [PubMed]
- Chen Z, Chen LY, Dai HY, et al. miR-301a promotes pancreatic cancer cell proliferation by directly inhibiting Bim expression. J Cell Biochem 2012;113:3229-35. [Crossref] [PubMed]
- Liu MD, Wu H, Wang S, et al. MiR-1275 promotes cell migration, invasion and proliferation in squamous cell carcinoma of head and neck via up-regulating IGF-1R and CCR7. Gene 2018;646:1-7. [Crossref] [PubMed]
- Storz P. Targeting protein kinase C subtypes in pancreatic cancer. Expert Rev Anticancer Ther 2015;15:433-8. [Crossref] [PubMed]
- Zafar A, Hardy K, Wu F, et al. The role of protein kinase-C theta in control of epithelial to mesenchymal transition and cancer stem cell formation. Genom Data 2014;3:28-32. [Crossref] [PubMed]
- Zhang LL, Cao FF, Wang Y, et al. The protein kinase C (PKC) inhibitors combined with chemotherapy in the treatment of advanced non-small cell lung cancer: meta-analysis of randomized controlled trials. Clin Transl Oncol 2015;17:371-7. [Crossref] [PubMed]
- Pinto Brod LM, Fronza MG, Vargas JP, et al. Modulation of PKA, PKC, CAMKII, ERK 1/2 pathways is involved in the acute antidepressant-like effect of (octylseleno)-xylofuranoside (OSX) in mice. Psychopharmacology (Berl) 2017;234:717-25. [Crossref] [PubMed]
- El-Rayes BF, Ali S, Philip PA, et al. Protein kinase C: a target for therapy in pancreatic cancer. Pancreas 2008;36:346-52. [Crossref] [PubMed]
- Liu J, Sun HH, Ying SH, et al. Characterization of three mitogen-activated protein kinase kinase-like proteins in Beauveria bassiana. Fungal Genet Biol 2018;113:24-31. [Crossref] [PubMed]


