
Circulating regulatory T cells from breast cancer patients in response to neoadjuvant chemotherapy
Introduction
Regulatory T cells (Tregs) are T cells identified in peripheral blood as CD4+CD25+ cells with regulatory properties (1). Recent studies have shown that Tregs play an essential role in sustaining self-tolerance by expressing a wide variety of pathological immune responses against self, non-self, and tumor antigens (2). Tregs are immunosuppressive lymphocytes that seem to play an important role in controlling the immune escape (3,4). Tregs represent roughly 10% of CD4 T cells in human blood (1,2). Although the exact mechanisms of Treg suppression remain unknown, this effect seems to be largely dependent on the expression of the transcription factor FOXP3 which controls some genes encoding proteins like CD25, GITR, CTLA-4, and others, capable of mediating Treg suppressive functions (5,6). In addition, FOXP3 inhibits production of effector cytokines like interleukin-2 (IL-2) after T-cell receptor (TCR) stimulation of T cells (7). Other mechanisms of immunosuppression are direct cell-to-cell contact with antigen presenting cells (APC) via transforming growth factor β (TGF-β) or CTLA-4 and secretion of immunosuppressive cytokines such as interleukin-10 (IL-10), TGF-β, and others (8,9). Specifically, in breast carcinoma, the number of Tregs and decreased ratios of CD8 T cells/Treg seem to be correlated with a poor prognosis (10). As mentioned before, immune function is generally compromised in cancer patients, which have lower absolute numbers of peripheral blood lymphocytes but increased numbers of functionally suppressive CD4+CD25+ Treg (11). In addition, higher numbers of Treg in blood from patients with breast cancer has been reported in relation to normal donors (12). Moreover, an inverse correlation has been found between Tregs and clinical stage of breast cancer (13). Besides, patients with high levels of CD4 have been found to have a lower survival, whereas a longer survival has been associated with a higher frequency of CD8 T cells (14). That is why the CD8 T cell number, together with Tregs, and the CD8/Treg ratio may be good markers to evaluate the clinical outcome of breast cancer (14).
In the present work, we aimed to analyze cellular populations in peripheral blood, including lymphocyte subpopulations in peripheral blood before, during and after neoadjuvant chemotherapy treatment.
Methods
Patients diagnosed of infiltrative breast carcinoma and suitable for neoadjuvant chemotherapy were recruited from the Breast Cancer Unit of the University Hospital Virgen Macarena (Seville, Spain) from March 2011 to May 2013. All the patients were informed about the study protocol and signed informed consent was obtained. Consecutive patients were divided by protocol in two treatment groups depending on their her2 status. Group with her2 overexpression were treated with CDH schedule (carboplatin, docetaxel and trastuzumab) plus G-CSF, 6 cycles. Patients without her2 overexpression received TAC schedule (docetaxel, doxorubicin and cyclophosphamide) plus G-CSF, 6 cycles.
Blood samples were collected in EDTA-K3 tubes before every cycle of CT (basal and cycles 1–4) to determine the immunophenotype and regulatory cell profile. Cell populations were determined by flow cytometry analysis of whole blood using the BD FACSCanto™ flow cytometry system. Six-color immunofluorescence staining was performed in one tube for lymphocytes subpopulations (CD45, CD3, CD4, CD8, CD19, CD16+CD56). Tregs were analyzed by three-color immunofluorescence (CD4, CD25, CD127). Tregs were identified as CD4+CD127−CD25high. Anti-CDs monoclonal antibodies (mAbs) were obtained from Becton Dickinson Immunocytometry Systems (BDIS, San Jose, CA, USA) and were used at the manufacturer’s recommended concentration.
Data are available upon request, through the institutional review board from Virgen Macarena University Hospital. The authors declare that there is no conflict of interest regarding the publication of this paper.
Data shown are mean ± standard error of mean (SEM). The distribution of analyzed populations was checked by the Shapiro-Wilk test. Biostatistical analysis was performed comparing circulating cell populations before and after neoadjuvant chemotherapy using the Wilcoxon matched pairs test. Circulating cell number was compared using Mann-Whitney U test (2 subgroups) or Kruskal-Wallis one-way analysis of variance test (>2 subgroups).
Results
From March 2011 to May 2013, 50 patients (21 Her2+/29 Her2−) with T2–T4 N0–N3 breast cancer treated with neoadjuvant chemotherapy in the Breast Cancer Unit of Virgen Macarena University Hospital (Seville, Spain) were included. By December 2013, 50 patients (21 Her2+/29 Her2−) were operated with curative intent. Pathological complete response (pCR), or near pathologic complete responses, were attained in 22 patients (44%). pCR was obtained in 14/21 of Her2+, representing 66.7% of tumors overexpressing Her2. On the other hand, pCR occurred only in 8/29 of Her2-negative tumors (27.6%)
Changes in peripheral blood cell leucocytes from breast cancer patients in response to neoadjuvant treatment
As shown in Figure 1, neutrophil and monocyte concentration in peripheral blood from breast cancer patients did not change after neoadjuvant treatment. On the other hand, lymphocyte concentration progressively diminished after every cycle of treatment reaching statistical significance after four cycles.
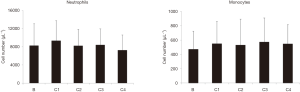
Changes in peripheral blood lymphocyte population from breast cancer patients in response to neoadjuvant treatment
As shown in Figure 2, some lymphocyte subpopulation decreased after neoadjuvant treatment. Thus, CD19 (B lymphocytes) and CD3−CD16+CD56+ (NK) cell concentration significantly decreased after four cycles of treatment. Even though total CD3+ cell concentration did not change significantly, CD3+CD4+ (helper) T lymphocytes cell concentration was significantly decreased after 4 cycles of treatment. On the other hand, CD3+CD8+ (cytotoxic) T lymphocytes did not decrease in response to the neoadjuvant treatment. CD4/CD8 ratio progressively decreased after neoadjuvant treatment but differences were not statistically significant. Finally, Treg lymphocyte concentration progressively decreased upon treatment, and changes were statistically significant after 4 cycles of neoadjuvant therapy.
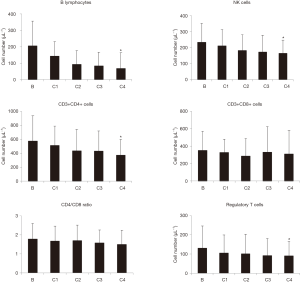
Since clinical response was better in Her2+ patients, we next compared the changes in lymphocytes populations from both groups in response to neoadjuvant treatment. As shown in Figure 3, B cells decreased in Her2− patients to significantly lower levels, compared with Her2+ patients. However, NK cells and CD4 cells decreased in a similar way in both groups, whereas CD8 cells significantly decreased only in Her2− patients. No significant changes in CD4/CD8 ratio was observed in both groups. Finally, higher decrease in Tregs was observed in Her2+ patients in response to treatment, compared with that observed in Her2− patients.
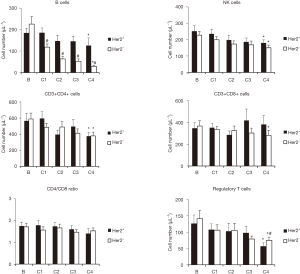
Since the patients with better clinical response seem to have lower number of Treg and higher number of CD8 cells, we have represented the CD8/Treg ratio comparing Her2+ and Her2− patients. As shown in Figure 4, Her2+ patients had an increase in CD8/Treg ratio compared with Her2− patients, that was statistically significant, after 4 cycles of treatment.
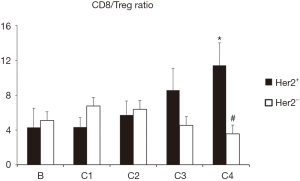
Discussion
The immune system plays an integral and complex role in breast cancer biology (15), both promoting tumor growth and mediating the eradication of disease. In this context, Tregs seem to modulate host immune response to the tumor, and may even mediate the immunopathogenesis of breast cancer (16). Thus, increased number of Tregs in the peripheral blood of cancer patients, including breast cancer has been described (12). Moreover, it has been strongly suggested that the increase in functional Treg cell levels in cancer patients might be seen as a response to malignant transformation (17). Treg cells are potent mediators of peripheral immune tolerance, suppressing a wide range of immune cells, including CD4+ and CD8+ T cells, natural killer (NK) cells, NKT cells, B cells, and APC, through inhibiting target cell activation and proliferation as well as effector functions (18). Thus, quantifying Tregs has become a valuable tool in the assessment of breast cancer progression and prognosis (12,19).
Previous works have studied the effects of conventional therapeutic intervention on the number and function of Tregs in different carcinoma patients, including breast cancer (20). Thus, tamoxifen plus leuprolide has minimal effect on Tregs, whereas docetaxel appears to significantly increase the ratio between effector T cells and Tregs (19). On the other hand, other groups have previously shown that taxanes do not change NK cell number but increase the activity (21). We have found that conventional neoadjuvant treatment with carboplatin, docetaxel and trastuzumab, or with docetaxel, doxorubicin and cyclophosphamide significantly decrease the number of Tregs in peripheral blood. Moreover, Her2+ patients had a greater decrease in Treg number. This result along with the lower decrease in B cell number, and the maintenance of CD8 T cells in Her2+ patients may be relevant, considering the better clinical response of these subjects compared with Her2− patients. In this sense, a greater decrease in Treg number, and a better CD8/Treg ratio, seems to be associated with a better response, that especially in the Her2+ population is strongly related with best overall survival (22).
The results reported in the present work may represent only a part of the complex immune responses of the host to the neoadjuvant treatment of breast cancer. But, in any case, data released in this article strengthen the hypothesis that conventional neoadjuvant treatment induces a positive impact in the immune system of these patients, at least those related with the inhibition of the Treg mediated immunosuppressive effects. Moreover, even though the number of B cells and NK cells were also decreased in peripheral blood in response to the treatment, the decrease in B cells was less pronounced in Her2+ patients, and the number of effector cytotoxic T lymphocytes (CD8+ cells) was maintained, especially in Her2+ patients, suggesting that the neoadjuvant chemotherapy does not negatively affect the possible specific immune response of the host to the breast cancer, and may even improve their immune profile. Consequently, effects of these kinds of therapies could be combined with modern immunotherapeutics, in order to achieve a better clinical response (23-25). Therefore, changes observed in peripheral blood during neoadjuvant treatment indicate that Tregs may represent an interesting therapeutic target in breast carcinoma and provides the rationale for the combination of active immunotherapy with standard therapies to improve clinical outcomes in breast cancer.
Acknowledgments
We are especially grateful to the Nursery Team of the “Hospital de Día” of Virgen Macarena University Hospital by their support and aid along the study. Finally, we are in debt and thank warm and respectfully to all the patients and families that gave consent to participate in this study.
Funding: We want to thank the
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.12.30). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the local IRB (Ethics Committee of the institution Virgen Macarena University Hospital) (LCM-RCL-2013-01). The study conforms to the provisions of in accordance with the Helsinki Declaration as revised in 2013. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jonuleit H, Schitt E, Stassen M, et al. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med 2001;193:1285-94. [Crossref] [PubMed]
- Dieckmann D, Plottner S, Berchtold S, et al. Ex vivo isolation and characterization of CD4(+)CD(25) T cells with regulatory properties from human blood. J Exp Med 2001;193:1303-10. [Crossref] [PubMed]
- Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Curr Opin Immunol 2004;16:157-62. [Crossref] [PubMed]
- Beyer M, Schuktze JL. Regulatory T cells in cancer. Blood 2006;108:804-11. [Crossref] [PubMed]
- Gavin MA, Rasmussen JP, Fontenot JD, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature 2007;445:771-5. [Crossref] [PubMed]
- Zheng Y, Josefowicz SZ, Kas A, et al. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature 2007;445:936-40. [Crossref] [PubMed]
- Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nature Reviews Immunology 2008;8:523-32. [Crossref] [PubMed]
- Collison LW, Pillai MR, Chaturvedi V, et al. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol 2009;182:6121-8. [Crossref] [PubMed]
- de la Cruz-Merino L, Henao-Carrasco F, García-Manrique T, et al. Role of transforming growth factor beta in cancer microenvironment. Clin Transl Oncol 2009;11:715-20. [Crossref] [PubMed]
- Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 2006;24:5373-80. [Crossref] [PubMed]
- Wolf AM, Wolf D, Steurer M, et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 2003;9:606-12. [PubMed]
- Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002;169:2756-61. [Crossref] [PubMed]
- Zhu S, Lin J, Qiao G, et al. Differential regulation and function of tumor-infiltrating T cells in different stages of breast cancer patients. Tumour Biol 2015;36:7907-13. [Crossref] [PubMed]
- Huang Y, Ma C, Zhang Q, et al. CD4+ and CD8+ T cells have opposing roles in breast cancer progression and outcome. Oncotarget 2015;6:17462-78. [PubMed]
- de la Cruz-Merino L, Chiesa M, Caballero R, et al. Breast Cancer Immunology and Immunotherapy: Current Status and Future Perspectives. Int Rev Cell Mol Biol 2017;331:1-53. [Crossref] [PubMed]
- Beyer M, Kochanek M, Giese T, et al. In vivo peripheral expansion of naïve CD4+CD25highFoxP3+ regulatory T cells in patients with multiple myeloma. Blood 2006;107:3940-9. [Crossref] [PubMed]
- Watanabe MA, Oda JM, Amarante MK, et al. Regulatory T cells and breast cancer implications for immunopahogenesis. Cancer Metastasis Rev 2010;29:569-79. [Crossref] [PubMed]
- Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010;10:490-500. [Crossref] [PubMed]
- Ohara M, Yamaguchi Y, Matsuura K, et al. Possible involvement of regulatory T cells in tumor onset and progression in primary breast cancer. Cancer Immunol. Immunother 2009;58:441-7. [Crossref] [PubMed]
- Roselli M, Cereda V, di Bari MG, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology 2013;2:e27025. [Crossref] [PubMed]
- Carson WE, Shapiro CL, Crespin TR, et al. Cellular immunity in breast cancer patients completing taxane treatment. Clin Cancer Res 2004;10:3401-9. [Crossref] [PubMed]
- Cortazar P, Geyer CE Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol 2015;22:1441-6. [Crossref] [PubMed]
- Page DB, Postow MA, Callahan MK, et al. Immune modulation in cancer with antibodies. Annu Rev Med 2014;65:185-202. [Crossref] [PubMed]
- Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol 2015;27:39-46. [Crossref] [PubMed]
- Solinas C, Gombos A, Latifyan S, et al. Targeting immune checkpoints in breast cancer: an update of early results. ESMO Open 2017;2:e000255. [Crossref] [PubMed]


