Cytotoxic effect of Ad-ephrinA1-caspase-3-T on breast cancer cells in vitro
Introduction
According to the International Agency for Research on Cancer (IARC) of WHO, the number of people suffering from cancer is growing rapidly in the world, especially in developing countries. Breast cancer is the primary killer of women, and most cases are diagnosed late. Studies have suggested that the occurrence and development of breast cancer are closely related to the abnormality of signal pathways in vivo and the expression products of oncogenes. The EphA2 receptor is one of the members of the receptor tyrosine kinase family. A variety of malignant tumors and cell lines express EphA2 at high levels, and is associated with histological grade, lymph node metastasis, and prognosis of many tumors. It has become a new target for tumor therapy research (1). The blockade of apoptosis is the most common feature of malignant tumors including breast cancer. Inhibition of apoptosis will lead to tumorigenesis and abnormal immune function (2). Therefore, cytotoxic agents including induce apoptosis in cancer cells are used as an anti-cancer chemotherapeutic agent (3). Apoptosis molecule caspase-3 is located in the apoptotic pathway, the activation of caspase-3 can induce apoptosis (4). The main ligand of the EphA2 receptor is ephrinA1. Our previous studies have confirmed that in 150 cases of breast cancer tissues, the positive expression of EphA2 and ephrinA1 appeared in 123 and 129 cases, and the positive rates of them are 82% and 86%, respectively, and ephrinA1 and EphA2 overexpressed in the cell membrane and cytoplasm of breast cancer cells (5). This experiment intends to use T cell as a vector to transduce recombinant Ad-ephrinA1-caspase-3, then make Ad-ephrinA1-caspase-3-T act on breast cancer cells in vitro. Study whether Ad-ephrinA1-caspase-3-T has a targeted cytotoxic effect on breast cancer cells in vitro.
Methods
Main materials and instruments
Breast cancer tissue and normal breast tissue (Department of Pathology, Affiliated Hospital of Dali University), Ad-ephrinA1-caspase-3-T (Kunming Jiaoman Biotechnology Co., Ltd.), T lymphocytes (Xiangya School of Medicine), D-Hanks solution, collagenase, trypsin, TritonX-100, fetal bovine serum, Rabbit anti-EphA2 (Invitrogen, Catalog No. 34-7400), Rabbit anti-ephrinA1 (Invitrogen, Catalog No. 343300), FITC-labeled goat anti-rabbit Ig (G+M) antibody (ThermoFisher, Catalog No. A16097), MTT test kit, Annexin V-FITC/PI Apoptosis Detection Kit (Biyuntian Biotechnology Research Institute), anti-ephrinA1 serum (Invitrogen Technical Services), flow cytometer (Becton, Dickinson and Company).
Identification of breast cancer cells and normal breast cells
The breast cancer tissue and normal breast tissue were washed with D-Hanks solution, and such tissues as connective tissue were removed with a surgical gill. After washing again, the tissue was cut into small pieces with a scalpel and transferred into a 5 mL centrifuge tube. After adding an appropriate amount of buffer, the tissue was repeatedly cut into a paste, approximately 1 mm in size. After standing for a while, the upper liquid was absorbed with a pipette, added appropriate buffer, and then washed it over a 200-mesh sieve and collected the filtrate. The unscreened tissue block was digested with 0.25% and shaken for 1 h at 37 °C. After the digestion was completed, the digest was passed through a sieve and the cell fluid was collected. The liquid collected in the previous two times was combined and placed in a 50 mL centrifuge tube, centrifuged at 1,000 rpm for 5 min, the supernatant was removed, and the number of cells was adjusted to [2–5]×105 cells/mL with the culture solution, and the cells were installed in the culture bottle, then put the bottle in the CO2 culture box, 5% CO2, at 37 °C.The fibroblasts in the isolated cells were removed by repeated adherence gradually, and finally the purified breast cells and breast cancer cells can be obtained. The isolated human breast cells and breast cancer cells were counted by trypsinization and inoculated into a 48-well plate. After overnight culture, the expression of EphA2 receptor and ephrinA1 in cells was detected by indirect immunofluorescence (IFA), The main operations of IFA were as follows: (I) the culture medium was abandoned and cells were washed with phosphate-buffered saline (PBS) for 3 times; (II) cells were fixed with 4% polymethyl alcohol for l0min and washed with PBS for 3 times; (III) cells were permeabilized with 0.1% Triton X-100 for 10 min; (IV) cells were blocked with PBS containing 10% fetal calf serum for 1 h at 37 °C; (V) 1:1,000 dilution of Rabbit anti-EphA2 or Rabbit anti-ephrinA1 primary antibody were added, 1 h at 37 °C, then washed 3 times with PBS; (VI) FITC-labeled goat anti-rabbit Ig (G+M) antibody was added and incubated at 37 °C for 1 h, washed 3 times with PBS. Cells were observed and photographed by fluorescence microscopy.
The effect of ephrinA1-caspase-3-T on viability of breast cancer cells
The main operations were as follows: (I) 96-well plates were paved with isolated normal breast and breast cancer cells, 5×103 cells were inoculated in each well, and after overnight culture, old and non-adherent cells were removed; (II) serially diluted normal T lymphocyte culture supernatant or recombinant Ad-ephrinA1-caspase-3-T supernatant (100, 50, 25, and 0 µL) were added and filled up to 100 µL/well, and incubated at 37 °C incubator; (III) plates were taken after 24, 48 and 72 h for MTT assay; (IV) 10 µL of MTT solution (5 mg/mL) was added to each well and the culture was continued for 4–6 h in the cell incubator; (V) the culture medium in the hole was absorbed carefully and 150 µL DMSO was added to each hole. The plate was shaken at low speed for 10 min to fully dissolve the crystals; (VI) within 10 min after adding DMSO, the absorbance at 570 nm in each well was measured with a microplate reader, the cell growth inhibition rate (%) = (1− drug group OD/control group OD) ×100%.
Effect of ephrinA1-caspase-3-T on apoptosis of breast cancer cells
The effect of Ad-ephrinA1-caspase-3-T supernatant on the apoptosis of breast cancer cells was detected by flow cytometer. The main operations were as follows: (I) breast cancer cells were plated in 6-well plates and inoculated 5×105 cells in each well. After overnight culture, old culture medium and non-adherent cells were removed; (II) the supernatants of serially diluted recombinant ephrinA1-caspase-3-T were added, and the uninfected T lymphocyte supernatant was used as a control and cultured at 37 °C for 72 h; (III) culture supernatant was collected, and the cells were digested with conventional trypsin and centrifuged with the supernatant, then the cells were collected; (IV) the cells were suspended at 200 µL Binding Buffer; (V) 10 µL Annexin V-FITC and 10 µL PI were added, gently mix, react for 15 min at room temperature in the dark or 30 min at 4 °C; (VI) added 300 µL Binding Buffer and detected by flow cytometer within 1 h.
Detection of the specific cytotoxic effect of ephrinA1-caspase-3-T on target cells
In order to further detect the specificity of Ad-ephrinA1-caspase-3-T on target cells, antibody neutralization assay was used to observe whether ephrinA1 can antagonize the cytotoxic effect of Ad-ephrinA1-caspase-3-T on EphA2 positive breast cancer cells. The main operations were as follows: (I) the anti-ephrinA1 serum was inactivated at 56 °C for 30 min; (II) 50 µL of serum-free medium was previously added to each well of a 96-well plate, and the inactivated serum was successively serially diluted at a 2-fold concentration starting from the first column; (III) 50 µL Ad-ephrinA1-caspase-3-T supernatant was added, mixed and incubated in a 37 °C incubator for 60 min; (IV) 50 µL breast cancer cells or normal breast cells (density 1×104 cells/well) were added to the supernatant and serum mixture; (V) the cell culture plate was cultured at 37 °C and 5% CO2 incubator for 72 h, and then detected by MTT.
Statistical analysis
The SPSS 20.0 statistical software was used for analysis. The t-test was used for comparison between the two groups. The linear correlation analysis was used to analyze the correlation between antibody dilution and cell viability. P<0.05 was considered statistically significant.
Results
Identification of breast cancer cells and normal breast cells
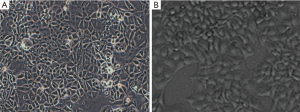
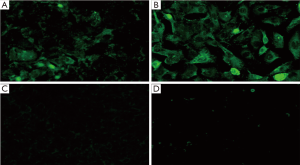
The growth of breast cancer cells was rapid, showing a variety of shapes, such as spindles and polygons, accumulating in nests and adhering to the wall; normal breast cells grown into pieces and had round nuclei in the center of the cells (Figure 1). The positive staining zones of ephrinA1 and EphA2 were located in cell membrane and cytoplasm. The results showed that there was almost no expression of EphA2 receptor and ephrinA1 in normal breast cells, while high levels expression of EphA2 receptor and ephrinA1 expression were found in breast cancer cells (Figure 2).
The effect of ephrinA1-caspase-3-T on viability of breast cancer cells
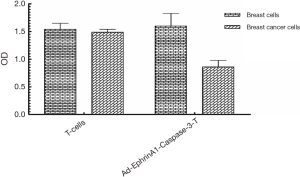
After 24, 48 and 72 h of culture, MTT was used to examine the effect of Ad-ephrinA1-caspase-3-T supernatant (100, 50, 25 and 0 µL) on the viability of breast cancer cells. According to Figure 3, T lymphocytes infected with adenovirus had little effect on the growth of normal breast cells but showed a strong growth inhibitory effect on breast cancer cells (P<0.05); the T lymphocytes uninfected with adenovirus did not affect the growth of normal cells and breast cancer cells (P>0.05). The inhibitory rate of Ad-ephrinA1-caspase-3-T on the growth of breast cancer cells was 42.3% (Figure 4).

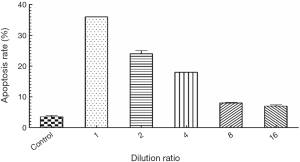
The effect of ephrinA1-caspase-3-T on the apoptosis of breast cancer cells
The apoptotic rates corresponding to dilutions ratios of 1, 2, 4, 8, and 16 were 36%, 24%, 18%, 8% and 7%, respectively, which were significantly higher than that of the control group 3.5% (P<0.05) (Figure 5).
Antibody neutralization experiment
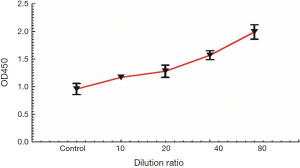
Figure 6 shows a scatter diagram of antibody dilution and OD450 values. It showed that the viability of cells increases with the increase of antibody dilution, and r=0.994, P=0.001 (<0.05).
Discussion
Apoptosis is programmed cell death controlled by genes in order to maintain the stability of the interior milieu, it was first proposed by Kerr et al. in 1972 (6). Apoptosis is a common phenomenon in organisms and has important biological significance (7). Abnormal apoptosis plays a very important role in the occurrence and development of many kinds of malignant tumors. In the treatment of cancer, radiotherapy, chemotherapy and endocrine therapy can lead to apoptosis of tumor cells, and may be the main form of cancer cell death (8). Therefore, dysfunction of apoptotic pathways can contribute to tumorigenesis and chemoresistance (9). Studies showed that down-regulation or loss of caspase expression is associated with breast cancer (10,11); caspase-3 is an important member of the cysteine aspartic acid specific protease family. It is a regulatory molecule located downstream of the cascade and is thought to be the performer of apoptosis (12,13). In our study, the Ad-ephrinA1-caspase-3-T had a relatively strong inhibitory effect on the viability of breast cancer cells. The growth inhibition rate was 42.30%, indicating that the Ad-ephrinA1-caspase-3-T can inhibit breast cancer cells viability, however, the inhibition rate is not very high, maybe it is related to concentration. In apoptosis experiment, apoptotic rates decreased with increasing of the dilution ratio. In short, Ad-ephrinA1-caspase-3 not only promotes the apoptosis of breast cancer cells, but is concentration-dependent. The authors suppose that the dose dependence upon the action of supernatant is related to the amount of key factors ephrinA1 and caspase-3 in the supernatant.
The Eph gene family is the largest member of the RTKs family, and EphA2 is the first gene in the family to be found to have tyrosine kinase activity. EphA2 is located on human chromosome 1p36.1 and encodes a polypeptide containing 976 amino acid residues and is a membrane-bound type I glycoprotein. Normal epithelial cells generally have low levels of EphA2 protein, which is itself tyrosine phosphorylated, however, EphA2 is widely expressed in many human tumors but not phosphorylated, including prostate cancer (14), breast cancer (15), malignant melanoma (16), non-small cell lung cancer (17), colon cancer (18), and ovarian cancer (19) and many other entities. Some people think the possible mechanisms of overexpression of EphA2 receptor in malignant tumors (20) were: protein stability increased and gene expression disordered. However, some people believe that such cells have unstable cell-to-cell contact. And ligand binding allows EphA2 to transmit a signal that negatively regulates tumor cell growth and survival, the lack of ligand binding also facilitates the development of these tumors (21). Noblitt (21) found that malignant cells with adenoviral vector expressing ephrinA1 is sufficient to decrease tumorigenic potential in vitro and in vivo. We also found in breast cancer cell identification experiments that there were almost no EphA2 receptor and ephrinA1 expression in normal breast cells, while high levels of EphA2 receptor and ephrinA1 expression were seen in breast cancer cells. In the test of cells identification, breast cancels express EphA2 and ephrinA1 at high levels, consistent with previous research results (22). Their positive rate in breast cancer is related to pathological type, tumor size, lymph node metastasis, clinical stage and histological grade (23). EphA2 is expected to become a new marker for the diagnosis of breast cancer and a molecular therapeutic target.
EphrinA1 is one of the main ligands of the EphA2 receptor. EphrinA1 and EphA2 are co-expressed in many malignant tumor tissues and tumor-associated vascular endothelial cells. This overlapping expression pattern allows full contact between ephrinA1 and EphA2 (24). The EphA2 ligand ephrinA1 induces EphA2 phosphorylation and intracellular internalization and degradation, thus inhibiting tumor progression (25). In the antibody neutralization experiment, cells’ viability increased with the increasing of antibody dilution ratio. it proved that neutralizing antibodies can effectively weaken the cytotoxic effect of Ad-ephrinA1-caspase-3-T on EphA2 positive breast cancer cells. Therefore, the author believes Ad-ephrinA1-caspase-3-T can specifically inhibit the growth of breast cancer cells, it is the joint effect of ephrinA1and caspase-3, caspase-3 activates apoptosis pathway and exerts cytotoxic effect directly; ephrinA1 reduce EphA2 expression and cell viability by inducing EphA2 phosphorylation in cells, consistent with experimental results of Tandon et al. (26).
In conclusion, the Ad-ephrinA1-caspase-3-T has a targeted cytotoxic effect on breast cancer cells in vitro.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Medical Ethics Committee of Dali University (No. DLDXLL2018050).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer 2010;10:165-80. [Crossref] [PubMed]
- Xue LY, Ren LQ, Luo W, et al. Expression of Fas, Fas ligand, Fas-associated death domain protein, caspase 8 and mutant P53 protein in esophageal squamous cell carcinoma. Zhonghua Yi Xue Za Zhi 2007;87:150-4. [PubMed]
- Hickman JA. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev 1992;11:121-39. [Crossref] [PubMed]
- Wang J, Sun B. Expression of caspase-3 and caspase-9 and their significance in breast cancer. J Clin Exp Pathol 2012;28:378-81.
- Zhang B, Bian S, Pan Y, et al. Expression of EphA2 and EphrinAl in the breast carcinoma tissue and its relation to clinicopathological parameters. Chinese Journal of Clinical Anatomy 2017;35:43-7.
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972;26:239-57. [Crossref] [PubMed]
- Recio-Boiles A, Ilmer M, Rhea PR, et al. JNK pathway inhibition selectively primes pancreatic cancer stem cells to TRAIL-induced apoptosis without affecting the physiology of normal tissue resident stem cells. Oncotarget 2016;7:9890-906. [Crossref] [PubMed]
- Kerr JFR, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer 1994;73:2013-26. [Crossref] [PubMed]
- Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature 2000;407:810-6. [Crossref] [PubMed]
- Devarajan E, Sahin AA, Chen JS, et al. Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene 2002;21:8843-51. [Crossref] [PubMed]
- Sun Y, Guo Y. Expression of Caspase-1 in breast cancer tissues and its effects on cell proliferation, apoptosis and invasion. Oncol Lett 2018;15:6431-5. [PubMed]
- Okinaga T, Kasai H, Tsujisawa T, et al. Role of caspases in cleavage of lamin A/C and PARP during apoptosis in macrophages infected with a periodontopathic bacterium. J Med Microbiol 2007;56:1399. [Crossref] [PubMed]
- Kleinberg L, Dong HP, Holth A, et al. Cleaved caspase-3 and nuclear factor-kappaB p65 are prognostic factors in metastatic serous ovarian carcinoma. Hum Pathol 2009;40:795-806. [Crossref] [PubMed]
- Easty DJ, Bennett DC. Protein tyrosine kinases in malignant melanoma. Melanoma Res 2000;10:401. [Crossref] [PubMed]
- Zelinski DP, Zantek ND, Stewart JC, et al. EphA2 Overexpression Causes Tumorigenesis of Mammary Epithelial Cells. Cancer Res 2001;61:2301-6. [PubMed]
- Miyazaki T, Kato H, Fukuchi M, et al. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer 2003;103:657-63. [Crossref] [PubMed]
- Rosenberg IM, Göke M, Kanai M, et al. Epithelial cell kinase-B61: an autocrine loop modulating intestinal epithelial migration and barrier function. Am J Physiol 1997;273:G824-32. [PubMed]
- Thaker PH, Deavers M, Celestino J, et al. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res 2004;10:5145-50. [Crossref] [PubMed]
- Martinet W, Abbeloos V, Van Acker N, et al. Western blot analysis of a limited number of cells: a valuable adjunct to proteome analysis of paraffin wax‐embedded, alcohol‐fixed tissue after laser capture microdissection. J Pathol 2004;202:382-8. [Crossref] [PubMed]
- Zhang B, Li Z, Bian S, et al. Progress in the study of the relationship between EphA2 receptor and malignant tumor. Chinese Journal of Anatomy 2017;40:469-72.
- Noblitt LW, Bangari DS, Shukla S, et al. Decreased tumorigenic potential of EphA2-overexpressing breast cancer cells following treatment with adenoviral vectors that express EphrinA1. Cancer Gene Ther 2004;11:757-66. [Crossref] [PubMed]
- Bian S, Zhang B, Yang G, et al. EphA2, EphrinAl expression in breast cancer and its relationship with clinical pathological study. Biomed Res 2017;28:6022-7.
- Zhang B, Li Z, Yang G, et al. Expression and clinical significance of EphA2 and EphrinA1 in the breast cancer tissue from Yunnan Dali Bai, Han and other minority women. Chinese Journal of Anatomy 2017;40:529-34.
- Valladares A, Hernández NG, Gómez FS, et al. Genetic expression profiles and chromosomal alterations in sporadic breast cancer in Mexican women. Cancer Genet Cytogenet 2006;170:147-51. [Crossref] [PubMed]
- Carles-Kinch K, Kilpatrick KE, Stewart JC, et al. Antibody targeting of the EphA2 tyrosine kinase inhibits malignant cell behavior. Cancer Res 2002;2840-7. [PubMed]
- Tandon M, Vemula SV, Sharma A, et al. EphrinA1-EphA2 interaction-mediated apoptosis and FMS-like tyrosine kinase 3 receptor ligand-induced immunotherapy inhibit tumor growth in a breast cancer mouse model. J Gene Med 2012;14:77-89. [Crossref] [PubMed]