
Akt/mTOR and AMPK signaling pathways are responsible for liver X receptor agonist GW3965-enhanced gefitinib sensitivity in non-small cell lung cancer cell lines
Introduction
Non-small cell lung cancer (NSCLC) is a prominent cause of cancer-related deaths. The introduction of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (EGFR-TKIs), including erlotinib and gefitinib, is the most significant advance in the treatment of NSCLCs. Single-agent treatments with EGFR-TKIs have been shown to significantly improve outcomes of patients with NSCLC harboring EGFR specific mutations compared with chemotherapy (1-3). Unfortunately, almost all patients who initially responded to EGFR-TKIs finally developed resistance (4), and their prognosis remains poor. Acquired resistance to gefitinib might involve T790M mutations, hepatocyte growth factor (HGF) over-expression, compensatory MET overactivity and loss of PTEN (5-8).
Autophagy is a highly conserved self-digesting process in cells, which is considered to be closely linked to both tumourigenesis and chemoresistance. There are increasing evidences showing that autophagy plays important role in resistance to EGFR-TKIs. EGFR inhibitors can induce autophagy, but the specific role of the induction of autophagy remains controversial (9). Recent work has demonstrated that upregulated autophagy is involved in resistance to EGFR-TKIs, including gefitinib (10,11) and erlotinib (12,13). Moreover, blocking autophagy can increase sensitivity of resistant cancer cells to EGFR-TKIs. On the contrary, there are also some data suggesting that a low level of autophagy is strongly linked to EGFR-TKIs resistance, and the combination of EGFR-TKIs with autophagy inducer may sensitize EGFR-TKI-resistant cancer cells to EGFR-TKIs (14-17). Thus, autophagy regulation may be a viable strategy to conquer the treatment of lung cancers with resistance to EGFR-TKIs.
Liver X receptors (LXRs) play crucial roles in glucose metabolism and the modulation of inflammatory responses. There are increasing evidence showing the association and potential efficacy LXRs and their ligands, including synthetic ligands T0901317 and GW3965, with malignancies. As reported, LXR ligands were shown to have antiproliferative effects on a variety of cancer cell lines (18-22). Several previous studies had proven that LXR agonists promoted cancer cell death through reduction of PI3K or AKT activity via EGFR downstream pathways (23,24). What’s more, it had been reported that treatment with LXR agonists could inhibit Akt activation and reserve gefitinib-resistance in NSCLC cell lines (25,26). As we all known, there is a close relationship between the changes of autophagy and the expression of PI3K/AKT signaling. Previous studies had shown that gefitinib-induced cell autophagy and apoptosis was mediated by PI3K/Akt inactivation, and GW3965 had synergistic effect on gefitinib in gefitinib-resistant in cancer cells (25,27). However, there was no systemic analysis showing the association of LXR ligand GW3965-induced autophagy and apoptosis with EGFR-TKI in EGFR-TKI-resistant NSCLC cell lines.
To clarify the association of GW3965-induced autophagy and apoptosis with EGFR-TKI in EGFR-TKI-resistant NSCLC cell line, we designed in vitro experiments to investigate the effects of GW3965-induced autophagy on the development of gefitinib resistance in NSCLC cell line PC9. We firstly established the gefitinib-resistant PC9 cell lines, and treated cells with single and combined treatments with gefitinib and GW3965. Cell autophagy and apoptosis were detected to evaluate the effect of both single and combined treatments. We are confident that with these experiments we will be able to provide new insights into overcoming the chemotherapy resistance to EGFR-TKIs in NSCLC cells.
Methods
Cells, antibodies, and reagents
The human NSCLC PC9 cell line (EGFR exon 19 deletion, Del E746-A750) was obtained from the central laboratory of Shanghai Pulmonary Hospital, and was maintained in RPMI-1640 (Hyclone, Logan, UT, USA) supplemented with 10% FBS (Hyclone, Logan, UT, USA) at 37 °C, with 5% CO2. Gefitinib (ZD1839) was purchased from Selleck (Selleck Chemicals, TX, USA), GW3965 was obtained from Sigma-Aldrich (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). RIPA buffer, 3-(4,5-Dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) solution and β-actin antibody was obtained from Sigma-Aldrich (Sigma-Aldrich; Merck KGaA, USA). Propidium iodide (PI) was obtained from Beijing Beyotime Company (Beijing, China) and acridine orange (AO) was purchased from Solarbio (Beijing, China). The Annexin V-FITC Apoptosis Detection Kit was obtained from BD Biosciences (San Jose, CA, USA). Primary antibodies against total (t)-Akt (#4691, 1:1,000), phosphor(p)-Akt (#9611, 1:1,000), t-AMPK (#5831, 1:1,000), p-AMPK (#2535, 1:1,000), t-mTOR (#2972, 1:1,000), p-mTOR (#2971,1:1,000), t-p70s6k1, p-p70s6k1, Beclin-1 (#3738, 1:1,000) and LC3 (#2775, 1:1,000) were purchased from Cell Signaling Technology (Beverly, MA, USA). Immunofluorescence antibody LC3 II was obtained from Abcam (Cambridge, UK, USA). Trypsinization (Biosharp Co., China), SDS-PAGE, (Sigma-Aldrich, St Louis, MO, USA), PVDF membranes were purchased from Bio-Rad Laboratories, USA. Secondary antibodies including HRP-conjugate goat anti-rabbit IgG and goat anti-mouse IgG were obtained from Abcam. N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Cells, culture conditions, and establishment of gefitinib-resistant PC9 cell line
Gefitinib-resistant PC9 cell line was generated by exposing to RPMI-1640 medium containing 2.5 µg/mL MNNG for 24 h, and culturing in RPMI-1640 medium supplementing with 0.2 µM gefitinib for 7 days according to previously described methods (26). Cells were then maintained in gefitinib-free medium until 90% confluence, followed by incubation with 0.3−0.5 µM gefitinib for 21 days for subcloning. Single colonies were obtained by trypsinization. The cell line was maintained in medium consisting of 0.05 µM gefitinib at 37 °C, with 5% CO2, and verification of integrin β1 expression was conducted by Western blot and PCR analyses, while no T790M mutation occurred (28).
Analysis of cell viability
The inhibitory effect of gefitinib on cell proliferation was detected using the MTT assay. PC9 cells were seeded into 96-well plates for 24 h prior to exposing to gefitinib and GW3965. Cells were incubated by drugs for 48 h, followed by incubation with 5 mg/mL MTT solutions at 37 °C for 4 h and 50 µL of DMSO. Finally, plates were detected using a microplate reader (Multiskan MK3; Thermo Labsystems, Helsinki, Finland) at 570 nm, and the inhibition ratio was calculated using the following formula: Inhibition ratio (%) = [OD570nm (test) − OD570nm (control, 0 µM)]/OD570nm (control) ×100%. All experiments were performed at least in three replications.
Western blot analysis
PC9 cells were incubated with single or combined GW3965 and Gefitinib for 48 hour. Cellular proteins were isolated with RIPA buffer. For immunoblotting, cell lysates were separated with 10% SDS-PAGE and then were transferred onto PVDF membranes, which were then blocked with 5% non-fat milk at room temperature for 1 h. Incubation with primary antibodies against LC3 II/I, Beclin-1, total Akt, p-Akt, total AMPK, p-AMPK, mTOR, p-mTOR, p70S6K1, and p-p70S6K1 were performed at 4 °C overnight, followed by secondary incubation in HRP-conjugated secondary antibodies [goat anti-rabbit (1:20,000); anti-mouse (1:4,000) IgG] at room temperature for enhanced chemiluminescence (ECL). NIH ImageJ software (http://rsb.info.nih.gov/nih-image/) was used for immunoreactive bands analysis.
AO staining
Cells were treated with different drugs for 48 h and then AO staining was performed. For AO staining, prepared cells were stained with 0.01% AO in dark at 37 °C for 15 min and then cells were washed with serum-free media. A Leica fluorescence microscope (Leica, Wetzlar, Germany) was employed for the visualization of fluorescent staining cells. With using 492 nm blue excitation light, the green (510–530 nm) and red (>650 nm) fluorescence emission from cells was visually examined under a fluorescence microscope. The autophagy cell rate (%) was calculated as the percentage of positively stained cells for three fields (containing at least 50 cells per field) for each experimental condition.
Apoptosis analysis
Cell apoptosis was determined using Annexin V-FITC Apoptosis Detection Kit according to the manufacturer’s protocol. Cells in 24-well plates were precultured for 24 h, and then treated with different drugs for 48 h at 37 °C with 5% CO2. Then, 5 µL of solutions were added into the cell cultures for 15 min in dark. Annexin V+ apoptotic cell percentage was detected using a FACS Calibur flow cytometer (BD Biosciences).
Statistical analysis
At least in triplicates were performed for all cellular experiments. Averaged data was presented as mean ± standard deviation (SD). GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA) was used for data statistical analyses. A one-way analysis of variance (ANOVA) and t-test was used for the analysis of differences among and between groups, respectively, with significant criteria of P value less than 0.05.
Results
Cell viability analysis
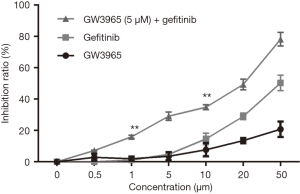
Figure 1 shows the influence of GW3965 and gefitinib on the cell viability of gefitinib-resistant PC9 cell line. The cell viability was gradually inhibited by GW3965 or gefitinib single and combined treatments, especially at ≥20 µM concentrations (P<0.05). Since treatment of the cells with 5 µM GW3965 had no significant effects on cell viability, we used this concentration for further analysis. Gefitinib induced higher inhibition ratio when there was GW3965 (at ≥20 µM). Increment in cell viability inhibition ratio was observed in GW3965 (5 µM) in combination with gefitinib treatment (≥1 µM) compared with gefitinib single treatment (P<0.05). These data suggested GW3965 overcame the obtained gefitinib-resistance in PC9 cells. With combination of GW3965 (5 µM), the half maximal inhibitory concentration (IC50) of gefitinib was 20 µM. Subsequent experiments were carried out at this concentration.
Cell autophagy analysis
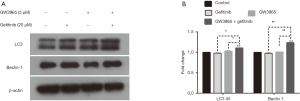
Figure 2A and B show the effect of single and combined treatments with GW3965 and gefitinib on cell autophagy. Single treatments with GW3965 or gefitinib alone did not influence the LC3 II/I ratio and expression of Beclin 1 protein (P>0.05 vs. control, Figure 2A and B). The combination of them, however, significantly upregulated LC3 II/I ratio and Beclin 1 protein fold change compared with control (P<0.01), showing the synergistic effect of GW3965 and gefitinib on activating the expression of autophagy related protein.
Autophagy is featured by the formation of autophagosomes and autolysosomes. To further confirm the accumulation of amphisomes and autolysosomes in gefitinib-resistant PC9 cell line, we conducted the AO staining analysis. As shown in Figure 3, GW3965 single treatment significantly increased AO staining in acidic vesicular organelles in PC9 cells (P<0.05), but treatment with gefitinib alone showed no obvious change (Figure 3A,B). Moreover, when treated with combination of GW3965 and gefitinib, the number of AO-stained cells increased dramatically in comparison with that of control cells and treatments with GW3965 or gefitinib alone.
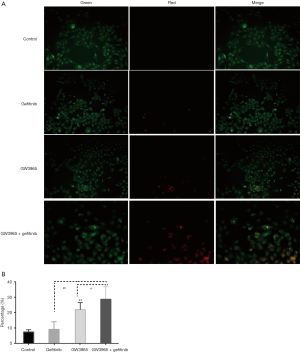
Taken together, these results suggested that GW3965 could significantly increase the autophagy level in gefitinib-resistant PC9 cell line with combined treatment with gefitinib.
Apoptosis analysis
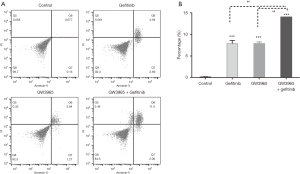
Figure 4A shows the result of cell apoptosis analysis using flow cytometric analysis. Obviously increased apoptotic cell percentages were found in cells treated with single (Gefitinib 7.64±0.27; GW3965 7.23±0.38) and combined agents (13.52±0.60) in comparison with control (0.22±0.03; P<0.001, Figure 4B). Difference was observed between single and combination drug treatments (P<0.01; Figure 4B). These results revealed that gefitinib (20 µM) and GW3965 (5 µM) single treatments increased the apoptosis of gefitinib-resistant PC9 cells, and GW3965 (5 µM) additional administration enhanced the effect of gefitinib (20 µM).
The effect of GW3965 and gefitinib on AMPK/Akt/mTOR signaling axis
Figure 5 shows the effect of GW3965 and gefitinib drug treatments on the expression of AMPK-mediated Akt/mTOR signaling axis. Synergistic effects of GW3965 and gefitinib on the inhibition of Akt, mTOR and AMPK expression were observed. Gefitinib and GW3965 single treatments upregulated p-AMPK (P<0.001), decreased p-Akt and p-mTOR (P<0.05), and the combination treatment enhanced all changes (P<0.001, Figure 5A,B). In comparison with gefitinib single treatment, significantly lower p-Akt and p-mTOR level (P<0.001) and higher p-AMPK level (P<0.05) were detected in cells under combination treatment with gefitinib and GW3965 (Figure 5A,B). We demonstrated that there were synergistic effects between gefitinib and GW3965 on activation of AMPK-mediated Akt and mTOR inhibition.
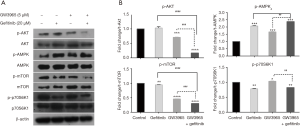
No enhanced downregulation was observed in p-p70S6K1 expression in cells with combination treatment comparing with gefitinib alone (P>0.05, Figure 5B). Moreover, the expression profile of p-AMPK protein was consistent with the apoptosis level in gefitinib-resistant PC9 cell line (Figures 4,5). In addition, we found that gefitinib and GW3965 single treatment decreased and increased p-p70S6K1 expression in PC9 cells, respectively, with contrary profiles to apoptosis and autophagy.
Discussion
Drug combinations have been widely used in treating complex diseases such as cancer. Several possible mechanisms have been proposed. For example, the parallel pathway inhibition model suggested that two drugs will be synergistic if they inhibit two proteins on parallel pathways essential for an observed phenotype (29). The mechanism of drug synergy is very complicated and their activities are highly dynamic. Our study demonstrated that both GW3965 and gefitinib could inhibit cell proliferation in gefitinib-resistant PC9 cell line. GW3965 and gefitinib induced cell autophagy and apoptosis, and activated AMPK-mediated Akt/mTOR signaling axis in vitro. We confirmed that the synergistic effect of GW3965 and gefitinib on the AMPK activation and inhibition of Akt/mTOR signaling axis and the promotion of cell autophagy and apoptosis in PC9 cells.
Elevated EGFR expression level has been identified as a prominent marker of various cancers, and it appears to confer solid and metastatic types, including NSCLC (30-33). EGFR mutations are dominant factors for the resistance and response to TKI therapy in NSCLC (1,3,34). EGFR modulates the proliferation, apoptosis, autophagy, migration and angiogenesis of cancer cells, which are essential for cancer growth, migration, solid growth and metastasis (10,13,16,30). Among these cellular behaviors, autophagy serves as a protective effect for EGFR-TKI resistance, although induced hyperautophagy is a beneficial therapeutic strategy for cancers (13,16).
It has been reported that EGFR-TKI induces autophagy and apoptosis in sensitive NSCLC lines, but not in resistant NSCLC lines (13,16,27). Zhao et al. reported that there was no response to gefitinib (500 nM) in gefitinib-resistant A549 cells (27). Gorzalczany et al. reported that H1299 cells showed a low level sensitivity to erlotinib (5 µM), while the administration of autophagy inducer rapamycin overcame it, showing enhanced sensitivity in H1299 cells to EGFR-TKI erlotinib (16). Li and his colleagues showed that erlotinib (0.1 µM, 0.2 µM) only induced autophagy in NSCLC sensitive cell lines (HCC827 and HCC4006, EGFR exon 19 del), but not in resistant cell lines (H358, wild type, and H1975 L858R/T790M) (13). In contrast, Li et al. enhanced cell sensitivity in NSCLC sensitive cell lines using an autophagy inhibitor chloroquine. In addition, they found the silencing of autophagy genes ATG5 and Beclin 1, which blocks the autophagy in sensitive cells, promoted cell sensitivity (13). These studies demonstrated that both inhibited and excessive elevated autophagy enhanced cancer cell sensitivity. In our present study, we detected the inhibited cell proliferation and increased apoptosis by gefitinib treatments (20 µM) in gefitinib-resistant PC9 cells. Consistent with previous studies, no significant autophagy changes were observed in our study.
Previous studies had shown that gefitinib-induced cell autophagy and apoptosis in NSCLC cells was associated with the activation of AMPK signaling pathway and inhibition of PI3K/Akt-mTOR signaling pathway (15,27). The hyperactivation of PI3K/Akt-mTOR signaling axis is prominent for tumor growth, proliferation, autophagy and survival and is common in cancer cells (23,27,35,36). The inhibition of AMPK, however, restored gefitinib-induced autophagy (15). AMPK is an energy-sensor which is activated by increased AMP/ATP(ADP) ratio, and is important for the induction of cell autophagy (37,38). AMPK triggers autophagy by directly activating ATG1 (ULK1) or inhibiting downstream PI3K/Akt-mTOR signaling pathway (Figure 6) (39,40). In addition, mTORC1-mediated inhibition of ATG1 was essential for autophagy regulation (41). In our present study, we demonstrated that gefitinib treatment induced p-AMPK expression, and inhibited p-Akt and p-mTOR expression, suggesting the inhibition of EGFR-PI3K/Akt-mTOR signaling pathway and upregulation of AMPK by gefitinib.
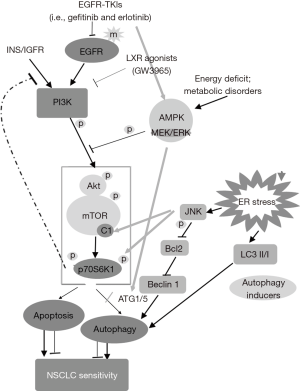
Several studies have proven that LXR agonists promoted cancer cell death and reserved gefitinib-resistance in NSCLC cell lines through inhibition of PI3K/AKT pathway downstream of EGFR (23-26). The synergistic effect of LXR ligands T0901317 and GW3965 on gefitinib have been reported in gefitinib-resistant NSCLC cells (25). T0901317 and GW3965 sensitized HCC827-8-1 cells to gefitinib by inhibiting Akt activation and enhancing cell apoptosis (25). In our present study, we demonstrated that GW3965 showed synergistic effects on gefitinib-inhibited PI3K/Akt-mTOR signaling pathway, enhanced AMPK activation, cell apoptosis and autophagy. These suggested GW3965 administration might overcome the acquired resistance to gefitinib in PC9 cell line. We found that the autophagy in PC9 cells was consistent with AMPK, and the apoptosis level was contrary to Akt/mTOR activation, respectively. These might demonstrate that the induced autophagy and apoptosis in gefitinib-treated cells was mediated by AMPK-ATG1/ULK1 activation and p70S6K1 inhibition (Figure 6), which was responsible for enhancing sensitivity to gefitinib in PC9 cells.
P70S6K1 is a substrate of mTORC1. c-Jun N-terminal kinase (JNK) coordinately regulates phosphorylation of p70S6K1 (42-44). JNK is a stress-activated pathway, and its activation directly enhances p70S6K1 phosphorylation and promotes the activation of mTORC1-dependent p70S6K1 (Figure 6) (44). Moreover, the JNK/Bcl-2/Beclin-1 signaling pathway was essential for endoplasmic reticulum stress-induced autophagy in cancer cells (Figure 6), without interaction with p70S6K1 (45-47). It has been reported that EGFR-TKIs (gefitinib and erlotinib) might induce ER stress in lung (48). Accordingly, we supposed that the potential of JNK/Bcl-2/Beclin-1 signaling-mediated autophagy and apoptosis by GW3965 treatment in PC9 cells (Figure 6, Grey lines), which might associate with Akt/mTOR, AMPK and p70S6K1 signaling pathways. All pathways might confer sensitivity to gefitinib-resistant PC9 cells, and were associated with the synergistic effect of GW3965 on overcoming the acquired resistance to gefitinib in PC9 cell line.
In summary, we confirmed the synergistic effect of LXR ligand GW3965 and gefitinib on the inhibition of PI3K/Akt/mTOR signaling pathway, and on cell apoptosis and autophagy. The synchronicity between AMPK activation and cell apoptosis and autophagy was also observed in our study. Thus, we concluded that the dominant roles of AMPK-ATG1, p70S6K1 and JNK mediated cell autophagy were essential for sensitivity in gefitinib-resistant PC9 cells. In addition, these signaling pathways were associated with the synergistic effect of GW3965 and gefitinib in PC9 cells on overcoming the gefitinib resistance.
Acknowledgments
We thank the central laboratory of Shanghai pulmonary hospital for the gift of the human NSCLC PC9 cell line.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2018.12.34). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Gazdar AF. Personalized medicine and inhibition of EGFR signaling in lung cancer. N Engl J Med 2009;361:1018-20. [Crossref] [PubMed]
- McDermott U, Pusapati RV, Christensen JG, et al. Acquired resistance of non-small cell lung cancer cells to MET kinase inhibition is mediated by a switch to epidermal growth factor receptor dependency. Cancer Res 2010;70:1625-34. [Crossref] [PubMed]
- Godin-Heymann N, Bryant I, Rivera MN, et al. Oncogenic activity of epidermal growth factor receptor kinase mutant alleles is enhanced by the T790M drug resistance mutation. Cancer Res 2007;67:7319-26. [Crossref] [PubMed]
- Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res 2008;68:9479-87. [Crossref] [PubMed]
- Yamamoto C, Basaki Y, Kawahara A, et al. Loss of PTEN expression by blocking nuclear translocation of EGR1 in gefitinib-resistant lung cancer cells harboring epidermal growth factor receptor-activating mutations. Cancer Res 2010;70:8715-25. [Crossref] [PubMed]
- Cui J, Hu YF, Feng XM, et al. EGFR inhibitors and autophagy in cancer treatment. Tumour Biol 2014;35:11701-9. [Crossref] [PubMed]
- Han W, Pan H, Chen Y, et al. EGFR tyrosine kinase inhibitors activate autophagy as a cytoprotective response in human lung cancer cells. PLoS One 2011;6:e18691. [Crossref] [PubMed]
- Sakuma Y, Matsukuma S, Nakamura Y, et al. Enhanced autophagy is required for survival in EGFR-independent EGFR-mutant lung adenocarcinoma cells. Lab Invest 2013;93:1137-46. [Crossref] [PubMed]
- Nihira K, Miki Y, Iida S, et al. An activation of LC3A-mediated autophagy contributes to de novo and acquired resistance to EGFR tyrosine kinase inhibitors in lung adenocarcinoma. J Pathol 2014;234:277-88. [PubMed]
- Li YY, Lam SK, Mak JC, et al. Erlotinib-induced autophagy in epidermal growth factor receptor mutated non-small cell lung cancer. Lung Cancer 2013;81:354-61. [Crossref] [PubMed]
- Fung C, Chen X, Grandis JR, et al. EGFR tyrosine kinase inhibition induces autophagy in cancer cells. Cancer Biol Ther 2012;13:1417-24. [Crossref] [PubMed]
- Xu Z, Hang J, Hu J, et al. Gefitinib, an EGFR tyrosine kinase inhibitor, activates autophagy through AMPK in human lung cancer cells. J BUON 2014;19:466-73. [PubMed]
- Gorzalczany Y, Gilad Y, Amihai D, et al. Combining an EGFR directed tyrosine kinase inhibitor with autophagy-inducing drugs: a beneficial strategy to combat non-small cell lung cancer. Cancer Lett 2011;310:207-15. [Crossref] [PubMed]
- Lee TG, Jeong EH, Kim SY, et al. The combination of irreversible EGFR TKIs and SAHA induces apoptosis and autophagy-mediated cell death to overcome acquired resistance in EGFR T790M-mutated lung cancer. Int J Cancer 2015;136:2717-29. [Crossref] [PubMed]
- Miller DH, Fischer AK, Chu KF, et al. T0901317 inhibits cisplatin-induced apoptosis in ovarian cancer cells. Int J Gynecol Cancer 2011;21:1350-6. [Crossref] [PubMed]
- Nguyen-Vu T, Vedin LL, Liu K, et al. Liver x receptor ligands disrupt breast cancer cell proliferation through an E2F-mediated mechanism. Breast Cancer Res 2013;15:R51. [Crossref] [PubMed]
- Candelaria NR, Addanki S, Zheng J, et al. Antiproliferative effects and mechanisms of liver X receptor ligands in pancreatic ductal adenocarcinoma cells. PLoS One 2014;9:e106289. [Crossref] [PubMed]
- Lo Sasso G, Bovenga F, Murzilli S, et al. Liver X receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology 2013;144:1497-507, 1507 e1-13.
- Komati R, Spadoni D, Zheng S, et al. Ligands of Therapeutic Utility for the Liver X Receptors. Molecules 2017;22: [Crossref] [PubMed]
- Guo D, Reinitz F, Youssef M, et al. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov 2011;1:442-56. [Crossref] [PubMed]
- Dufour J, Viennois E, De Boussac H, et al. Oxysterol receptors, AKT and prostate cancer. Curr Opin Pharmacol 2012;12:724-8. [Crossref] [PubMed]
- Wu Y, Yu DD, Hu Y, et al. LXR ligands sensitize EGFR-TKI-resistant human lung cancer cells in vitro by inhibiting Akt activation. Biochem Biophys Res Commun 2015;467:900-5. [Crossref] [PubMed]
- Koizumi F, Shimoyama T, Taguchi F, et al. Establishment of a human non-small cell lung cancer cell line resistant to gefitinib. Int J Cancer 2005;116:36-44. [Crossref] [PubMed]
- Zhao ZQ, Yu ZY, Li J, et al. Gefitinib induces lung cancer cell autophagy and apoptosis via blockade of the PI3K/AKT/mTOR pathway. Oncol Lett 2016;12:63-8. [Crossref] [PubMed]
- Deng QF, Su BO, Zhao YM, et al. Integrin beta1-mediated acquired gefitinib resistance in non-small cell lung cancer cells occurs via the phosphoinositide 3-kinase-dependent pathway. Oncol Lett 2016;11:535-42. [Crossref] [PubMed]
- Yeh PJ, Hegreness MJ, Aiden AP, et al. Drug interactions and the evolution of antibiotic resistance. Nat Rev Microbiol 2009;7:460-6. [Crossref] [PubMed]
- Normanno N, Tejpar S, Morgillo F, et al. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol 2009;6:519-27. [Crossref] [PubMed]
- Tol J, Dijkstra JR, Klomp M, et al. Markers for EGFR pathway activation as predictor of outcome in metastatic colorectal cancer patients treated with or without cetuximab. Eur J Cancer 2010;46:1997-2009. [Crossref] [PubMed]
- Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer 2001;37:S9-15. [Crossref] [PubMed]
- Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer 2001;37:S3-8. [Crossref] [PubMed]
- Sordella R, Bell DW, Haber DA, et al. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 2004;305:1163-7. [Crossref] [PubMed]
- Heras-Sandoval D, Perez-Rojas JM, Hernandez-Damian J, et al. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 2014;26:2694-701. [Crossref] [PubMed]
- Liu M, Li CM, Chen ZF, et al. Celecoxib regulates apoptosis and autophagy via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer cells. Int J Mol Med 2014;33:1451-8. [Crossref] [PubMed]
- Krieg S, Luscher B, Vervoorts J, et al. Studying the Role of AMPK in Autophagy. Methods Mol Biol 2018;1732:373-91. [Crossref] [PubMed]
- Fyffe FA, Hawley SA, Gray A, et al. Cell-Free Assays to Measure Effects of Regulatory Ligands on AMPK. Methods Mol Biol 2018;1732:69-86. [Crossref] [PubMed]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 2011;13:1016-23. [Crossref] [PubMed]
- Kim N, Jeong S, Jing K, et al. Docosahexaenoic Acid Induces Cell Death in Human Non-Small Cell Lung Cancer Cells by Repressing mTOR via AMPK Activation and PI3K/Akt Inhibition. Biomed Res Int 2015;2015:239764. [PubMed]
- Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 2010;22:132-9. [Crossref] [PubMed]
- Martin TD, Dennis MD, Gordon BS, et al. mTORC1 and JNK coordinate phosphorylation of the p70S6K1 autoinhibitory domain in skeletal muscle following functional overloading. Am J Physiol Endocrinol Metab 2014;306:E1397-405. [Crossref] [PubMed]
- Kimball SR, Gordon BS, Moyer JE, et al. Leucine Modulates mTORC1 Signaling by Acting Specifically to Alter the Phosphorylation Status of Sestrin2. The FASEB Journal 2016;30:1244.3-1244.3.
- Miller WP, Ravi S, Martin TD, et al. Activation of the Stress Response Kinase JNK (c-Jun N-terminal Kinase) Attenuates Insulin Action in Retina through a p70S6K1-dependent Mechanism. J Biol Chem 2017;292:1591-602. [Crossref] [PubMed]
- Cheng X, Liu H, Jiang CC, et al. Connecting endoplasmic reticulum stress to autophagy through IRE1/JNK/beclin-1 in breast cancer cells. Int J Mol Med 2014;34:772-81. [Crossref] [PubMed]
- Zhou YY, Li Y, Jiang WQ, et al. MAPK/JNK signalling: a potential autophagy regulation pathway. Biosci Rep 2015;35: [PubMed]
- Dhanasekaran DN, Reddy EP. JNK-signaling: A multiplexing hub in programmed cell death. Genes Cancer 2017;8:682-94. [PubMed]
- Koyama S, Omura T, Yonezawa A, et al. Gefitinib and Erlotinib Lead to Phosphorylation of Eukaryotic Initiation Factor 2 Alpha Independent of Epidermal Growth Factor Receptor in A549 Cells. PLoS One 2015;10:e0136176. [Crossref] [PubMed]


