
Exosomes as critical mediators of cell-to-cell communication in cancer pathogenesis and their potential clinical application
Introduction
Cancer, a major public health problem worldwide, is one of the leading causes of death in humans. In the United States, a total of 1,660,290 new cancer cases and 580,350 cancer deaths were projected to occur in 2013 (1,2). It is estimated that almost 1,600 patients die from cancer per day, accounting for 25% of all deaths. Even though remarkable progress has been made in the field of cancer research, the early diagnosis and survival of cancer patients remains unsatisfactory. Surgery, chemotherapy and radiotherapy remain the mainstream treatments for various cancers. However, more efficacious and safe treatments are needed due to the high rate of incomplete removal of tumors, as well as problems with chemo- or radioresistance (3-5). Although considerable success has been achieved with regard to molecular targeted drug therapy for cancer and a series of monoclonal antibodies have been applied to clinical therapy, issues remain. For example, identification of unique targets and the eradication of all tumor cells are difficult due to cancer heterogeneity and a variety of subtypes. Moreover, the efficiency and accuracy of delivery of specific target molecules to cancer needs improvement (6). Tumor formation is a multistep process, and the causes of cancer are controversial at present. It is now widely understood that cancer originates from genetic instability and environment factors. From a molecular perspective, cancer is a genetic disease containing oncogenic and tumor suppressor mutations, which endows the cancer cell with properties of replicative immortality, active invasion and metastasis (7,8). Hanahan and Weinberg identified six hallmarks of cancer in 2000: (I) sustaining proliferative signaling; (II) evading growth suppressors; (III) enabling replicative immortality; (IV) activating invasion and metastasis; (V) inducing angiogenesis and (VI) resisting cell death (9). The mechanisms underlying cancer progression have not been thoroughly elucidated to date. Determining the biological basis of this process is an indispensable step for optimizing therapeutic strategies (10,11). Emerging evidence has shown that exosomes released by most cell types, including tumor cells, red blood cells, lymphocytes, platelets, and dendritic cells (DCs), have an important role in cell-cell communication, immune regulation, tumor cell mobility and metastasis, and in the processes of hypoxia-related vascular diseases, including myocardial infarction, stroke and venous thromboembolism (12-16). Depending on the cellular origin, exosomes contain various biologically active materials, including major histocompatibility complex (MHC) molecules, adhesion molecules, signal proteins, microRNAs (miRNAs) and mRNAs (17-19). These materials can be transferred to other cells by fusion of the exosomes with the recipient cell membrane. This transfer of proteins and RNAs results in changes in certain signal pathways, including PI3K/Akt, ERK1/2, Notch, Fas, TGF-β/Smad and Wnt, which may be a potential mechanism of cell-cell interaction (20-22). It is an effective way to protect target molecular integrity and to enhance the accuracy of delivery for exosomal cargo.
In this review, we will explore cancer biology by introducing exosomes and summarize the role of exosomes in cancer molecular behavior. Second, we will highlight the latest findings that connect exosomes with fundamental cancer characteristics. Finally, we will discuss the potential clinical applications of exosomes in cancer diagnosis, treatment and biomarkers for predicting prognosis.
Overview of exosomes
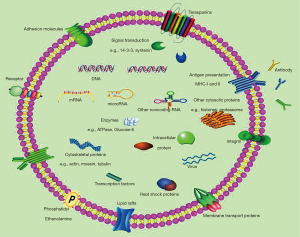
Extracellular vesicles can be divided into 3 primary classes based on their size: exosomes (20–100 nm), microvesicles (100–1,000 nm) and apoptotic bodies (1–5 µm). Exosomes have been the major focus of extracellular vesicle research. The term “exosome” was coined by Trams et al. in 1981 for “exfoliated membrane vesicles with 5'-nucleotidase activity” (23). Exosomes are distinguished from apoptotic bodies and microvesicles in term of their size, origin (endosomal or cell membrane), markers and composition. With spherical to cup-shaped nanoparticles and specific surface molecular markers, such as CD9 and CD63, exosomes are formed by the inward budding of endosomal membranes, thereby containing a variety of proteins, mRNAs and miRNAs (24-27). In addition to various cell or tissue specific materials, exosomes also contain certain common proteins, including cytoplasmic proteins (Hsp70 and Hsp90), cytoskeletal proteins (tubulin and actin), membrane fusion proteins (Rab GTPases) and membrane-associated proteins (CD9, CD81 and CD63) (28-30). These proteins could be used as markers for exosome isolation and identification. However, exclusive protein markers for exosomes are currently unknown. The material contained in exosomes is well protected to prevent degradation. For example, the RNA in exosomes is more stable than that in plasma and is not easily degraded by RNases. Exosomal RNA can be stored at −20 °C for more than 5 years, and the concentration is not decreased when compared with freshly prepared samples (31).
The signals and mechanisms underlying exosome formation and cargo sorting into exosomes have not been thoroughly elucidated to date. The present evidence shows that at least Endosomal Sorting Complexes Required for Transport (ESCRT) class proteins, tetraspanin CD63, specific glycan modification, the p53/TSAP6 pathway, and/or lipid-dependent mechanisms are involved in the formation of intraluminal vesicles in extracellular vesicles (32). Moreover, Rab-dependent trafficking mechanisms (Rab11, Rab27 and Rab35) have roles in exosome exocytosis and secretion (33) (Figure 1). Recipient cells internalize the foreign exosomes via multiple processes, including phagocytosis, clathrin-mediated endocytosis, macropinocytosis, and receptor-mediated and direct fusion (34,35). The factors that determine which and how a molecule is included or excluded in exosomes is under debate. It is reported that as a component of the COP9 signalosome regulatory complex, JAB1/CSN5 is involved in sorting proteins into exosomes (36). The introduction of exosomes provides a new molecular platform to further study cell-cell interaction, specific targeted cell selection, mechanisms of internalization and the potential of serving as a drug delivery system (37,38). Moreover, exosomes have been found in nearly all human body fluids, such as blood plasma, saliva, cerebrospinal fluid, urine, malignant ascites and semen (39-42), thereby implying that exosomes can be exploited as useful tools for cancer diagnosis and predictive biomarkers for cancer prognosis. It is interesting that the rate of exosomal release and content is different between healthy cell exosomes and tumor-derived exosomes. Numerous studies, including in vitro and in vivo studies, as well as clinical analysis, demonstrate that the number of exosomes increases significantly in cancer cells compared to normal cells. The distinct content of exosomes between the two groups (most notably miRNAs) may have important clinical significance (43,44).
Role of exosomes in cancer
The release of tumor-derived exosomes was first described in ovarian cancer patients (45). Currently, many types of tumors are reported to release exosomes, including cancers of the breast, pancreas, stomach, oral cavity, colorectum, brain, ovary, bladder, prostate, and melanomas (46-49). In this patient population, intact membrane exosomes isolated from blood plasma and effusion are found to express molecular markers that are identical to the tumor membrane. Tumor-derived exosomes could elicit a tolerogenic response and other biological process, such as platelet activation, mast cell degranulation, germinal center reaction, and potential engulfment of apoptotic cells (50). As a result of these effects, the exosomes induce a pro-inflammatory environment from macrophages and build a tumor-supportive microenvironment. The ability to evade immune recognition and suppress immune reactivity is crucial during cancer progression (51-53).
Pathological roles of exosomes in tumor molecular behavior
Several studies showed that exosomes could regulate gene expression in target cells and thereby influence their biological behavior. The contents of exosomes, composed of miRNAs, mRNAs, proteins, lipids and transcription factors, exert powerful effects in recipient cancer cells. miRNAs enter the recipient cell via exosomes and bind to the target mRNA sequence, thereby inhibiting the expression of certain genes (25). There is a strong correlation in mRNA and miRNA signals between tumor cells and exosomes. MiRNA expression is dysregulated in most cancers, and different cancer types have different exosome miRNAs signatures. A chip microarray analysis showed that the expression levels of hsa-miR-24-3p, hsa-miR-891a, hsa-miR-106a-5p, hsa-miR-20a-5p, and hsa-miR-1908 in nasopharyngeal carcinoma tumor-derived exosomes were higher than in exosomes from a healthy control group. These five exosomal miRNA clusters can regulate many signaling pathways, including mitogen-activated protein kinase (MAPK), the PI3K-Akt signaling pathway, the TGF-beta signaling pathway and ubiquitin-mediated proteolysis. These miRNAs can regulate the proliferation and differentiation of cells via phosphorylation of ERK and STAT proteins through the MAPK pathway (54). Bronisz et al. reported that miR-1 could serve as an orchestrator of exosome function and glioblastoma (GBM) growth and invasion. MiR-1 is transferred between GBM cells by exosome transport. Overexpression of miR-1 in GBM cells can block exosome stimulation of angiogenesis, invasion and neurosphere formation in recipient cells. Moreover, miR-1 overexpression affects the exosome protein cargo by regulating ANXA2 expression (55). The miRNAs that are tumor-specific can be used as diagnostic and prognostic biomarkers.
Exosomes alter the content and behavior of the recipient cell via transferring bioactive molecules to recipient cells close to or distant from the original cells. For example, colon cancer-derived exosomes can transfer KRAS, EGFR, SRC family kinases, and integrins that are expressed in colon cancer to other cells, thereby inducing the invasive ability of recipient cells (56). Tumor derived-exosomes promote the growth and proliferation of cancers such as breast cancer, lung cancer, pancreatic cancer, gastric cancer and hepatocellular carcinoma (24,57-60). Various signal pathways are involved in the process, such as activation of PI3K/Akt, MAPK/extracellular-regulated protein kinase and transforming growth factor β activated kinase-1-associated signaling (61,62).
Invasion and metastasis are the main characteristics of malignant tumors, and the poor prognosis of most cancers is attributed to this malignant behavior. During tumor invasion, tumor-derived exosomes serve as an important messenger between the cancer and the metastatic microenvironment (63). Gastrointestinal stromal tumor cells invade the interstitial stroma by secreting exosomes containing oncogenic protein tyrosine kinase. These released exosomes trigger the phenotypic conversion of progenitor smooth muscle cells to tumor-promoting cells and simulate stromal cells to produce matrix metalloproteinases 1 (MMP1) (63). To metastasize, cancer cells must acquire the ability to migrate and implant in both local and distant sites, which is a complicated process that involves various types of intercellular communication. Exosomes facilitate cancer cell metastasis via discarding tumor suppressor miRNAs, such as miR23b, or preparing sentinel lymph nodes and creating a permissive environment at potential metastatic sites (24). The precise underlying mechanisms have not been thoroughly elucidated to date, and further study is needed.
Extracellular communication
Cell communication is classified as paracrine, endocrine, exocrine or synaptic. These traditional modalities, considered receptor-medicated events, passively diffuse signaling molecules secreted by cells into recipient cells. However, when a more complex “message” needs to be transferred to an adjacent or distant cell, exosomes are employed. The characteristics of exosome-mediated communication are that the messages can be delivered to multiple specific locations (64,65). Several proteins, such as Rab proteins (for instance, Rab27A/B) and tetraspanin (for instance, CD9, CD63, CD81, and CD82), are involved in this process. These proteins are also recognized as exosomal markers (66). Exosomes secreted by a tumor can bind to neighboring cells or to the extracellular matrix or can be passively transported through the bloodstream and other body fluid. The half-life of exosomes is very short in the circulation, and up to 90% of exosomes are removed within 5 minutes after infusion (67). Numerous factors determine the biodistribution of exosomes in vivo, including original cells, route of delivery and targeting condition. The recipient cells absorb exosomes by membrane fusion, endocytosis, or receptor-mediated internalization.
Exosome-mediated immune response
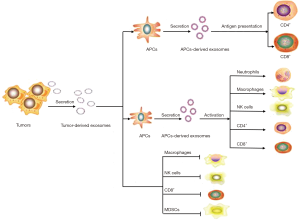
Exosomes derived from B-cells and DCs have the ability to induce antigen-specific T- and B-cell responses, which are considered as attractive immune modulators in fighting cancer. There is a clinical trial showing that DC-derived exosomes enhanced antitumor immunity in patients with advanced non-small cell lung cancer via increasing NKp30-dependent natural killer (NK) cell functions (68). Lu et al. reported that DC-derived exosomes elicited tumor regression and improved the tumor microenvironment by increasing CD8+ T lymphocytes, the levels of IFN-γ and interleukin-2, and decreasing CD25+Foxp3+ regulatory T (Treg) cells and the level of interleukin-10 and TGF-β in tumor sites (69). Studies indicated that NK cell-derived exosomes have cytotoxic effects on melanoma cells through presenting perforin and FasL, both of which are associated with apoptosis (70). The role of tumor-derived exosomes in the immune response is controversial, and the underlying mechanisms are complicated. First, several studies revealed that tumor-derived exosomes have an immunosuppressive effect and lead to tumor escape and loss of tumor immune surveillance, constructing favorable conditions for tumor growth. The underlying mechanisms are primarily classified into two parts: one is inhibition of immune cell effectors, and the other is activation of inhibitor immune cells (71). Exosomes can directly suppress cytotoxic T lymphocyte and NK-cell anti-tumor responses. Tumor-derived exosomes contain membrane-associated TGF-β, which blocks IL-2-mediated activation of NK cells and reduces the expression of NK cell activating receptor (NKG2D). It is also reported that tumor-derived exosomes have the ability to inhibit cytotoxic T-cell function via transfer of FasL and TRAIL or increased T-cell ROS content (72,73). Exosomes released from certain cancer cell types contain neither FasL nor TRAIL (74), suggesting that the exosomes from tumors can partially suppress immune reactions by multiple mechanisms (Figure 2). Pancreatic cancer-derived exosomes can decrease HLA-DR expression on CD14+ monocytes through the production of reactive oxidative stress (ROS), thus promoting tumor growth by immunosuppression (75). Recently, Chen and Colleagues showed that metastatic melanomas release exosomes that carry programmed death-ligand 1 (PD-L1) on their surface. PD-L1 binds to PD-1 through its extracellular domain to inactivate CD8 T cells. Stimulation with interferon-γ (IFN-γ) increases the amount of PD-L1 on these exosomes, which suppresses the function of CD8 T cells and facilitates tumour growth. The study also demonstrated the level of circulating exosomal PD-L1 stratifies clinical responders to pembrolizumab and non-responders (76). Cancer exosomes modulate immune regulation in a dual manner. Several other studies show that tumor-derived exosomes present tumor antigens to DCs and effectively activate antitumor immune responses (77). Certain exosomes released from cancer cells carry tumor antigens specific for the tumor, such as CEA, HER2, mesothelin, CD24 and EpCAM, thereby activating a cytotoxic T cell reaction and inducing protective antitumor immune responses (78-80). Cancer exosomes could be a two-edged sword for immune regulation, and the functional role of exosomes in immune regulation is variable, dependent on the local microenvironment generated by the tumor itself.
Stimulating angiogenesis
Blood supply is crucial for cancer growth, providing oxygen and nutrients that support cell proliferation and promote metastasis. Therefore, a blockade of angiogenesis is a promising strategy for inhibiting cancer. However, drugs or antibodies that prevent tumor blood vessel formation have a limited effect. Because there is an imbalance in the supply and consumption of oxygen by tumor cells, most solid tumors are under hypoxia and secrete exosomes that facilitate tumor angiogenesis (81). The hypoxia-inducible factor (HIF) family of transcription factors is an important promoter of this process. HIF cause cancer cells to become accustomed to hypoxic stress. In addition, HIF is also involved in the regulation of cell survival, angiogenesis, apoptosis, adhesion and drug resistance. Exosomes are closely associated with hypoxia. Low doses of hypoxia stimulate the release of exosomes via miRNAs and protein transport in many cancer types, such as breast cancer, pancreatic cancer, ovarian cancer, prostate cancer and nasopharyngeal cancer. Meanwhile, hypoxia can activate pro-angiogenic pathways through cancer-associated exosome secretion. Kim et al. reported in 2002 that tumor-derived exosomes stimulated the migration of endothelial cells and the formation of new vessels through a sphingomyelin-mediated interaction (82). The underlying mechanisms of exosomes in promoting angiogenesis are complicated. Webber et al. reported that exosomes carrying TGF-β1 can trigger the differentiation of fibroblasts to myofibroblasts (52). Myofibroblasts are a key source of matrix-remodeling proteins within the tumor microenvironment and induce tumor angiogenesis. Chowdhury et al. showed that TGFβ-bearing prostate cancer exosomes impaired the classical adipogenic differentiation of bone-marrow mesenchymal stem cells (MSCs), skewing differentiation toward an alpha-smooth muscle actin (αSMA)-positive myofibroblastic cell. The myofibroblastic cell secreted high levels of vascular endothelial growth factor (VEGF)-A, HGF and matrix regulating factors (MMP-1, -3 and -13). The exosome-modulated MSCs had pro-angiogenic functions and enhanced tumor proliferation and invasiveness (53). Moreover, tumor-derived exosomes can drive the secretion of proangiogenic cytokines, such as interleukin-1 a (IL-1a), fibroblast growth factors (FGF), granulocyte colony-stimulating factor, tumor necrosis factor (TNF) alpha and VEGF. For example, cancer cells transfer EGFR to endothelial cells via exosomes, thereby activating the VEGF/VEGFR-2 pathway in endothelial cells and enhancing tumor angiogenesis (83). Zhuang G. and colleagues showed that melanoma-derived exosomes can deliver microRNA-9 (miR-9) to endothelial cells. The uptake of miR-9 by endothelial cells can promote endothelial cell migration and tumor angiogenesis via activating the JAK-STAT pathway (84). The process of angiogenesis mediated by exosomes makes the tumor cells adaptable to hypoxic conditions, thereby resulting in more aggressive phenotypes with poorer outcomes in a variety of cancers.
Metastatic niche and the microenvironment
The tumor microenvironment is composed of various cell types: fibroblasts, immune and endothelial cells, pericytes and bone marrow-derived cells, all surrounded by matrix components. It is well known that the tumor microenvironment has an essential impact on tumor initiation, progression, treatment response and, ultimately, prognosis. The complicated interaction between cancer cells and the microenvironment makes it difficult to identify the factors that participate in their interaction. A better understanding of the communication between cancer cells and the microenvironment (mostly including stromal fibroblast-like cells) may lead to progress in the management of cancer and improvements in prognosis. Exosomes are present in nearly all human body fluids, but they are particularly enriched in the tumor microenvironment, thereby implying that they may play a special role in the interaction between cancer cells and the microenvironment. Numerous studies show that cancer cell- derived exosomes can shape the tumor microenvironment by influencing the function of stromal fibroblast-like cells. It is reported that exosomes from breast cancer cells cause fibroblasts and mammary epithelial cells to acquire the characteristics of a transformed cell, including the ability to grow in low serum conditions and form colonies in soft agar (85). Fibroblasts treated with exosomes are reprogramed to secrete growth factors, cytokines, and extracellular matrix proteins, which are used to further promote primary cancer cell growth and survival (86). Exosomes are novel and powerful intercellular signal mediators between cancer cells and their microenvironment; exosomes not only act as initiators of carcinogenesis but also promote tumor-cell dissemination and metastasis via distinct cargo molecules (87). Cancer cell-derived exosomes contain Src tyrosine kinase, insulin-like growth factor 1 receptor (IGF-IR), and focal adhesion kinase (FAK), all of which are tumor-promoting signaling molecules (88). Src, which signals through FAK, plays a role in promoting cancer cell proliferation, metastasis, and angiogenesis. Src also enhances angiogenesis by crosstalk with IGF-IR. Tumor-derived exosomes also regulate tumor-associated macrophages, which have been considered to play a critical role in determining the prognosis of cancer. It is reported that exosomes secreted by p53-mutant colon cancer cells transport miR-1246 to peripheral macrophages, causing M0 and M2 macrophages to secrete large amounts of VEGF, IL-10, CCL2 and TGF-β (89). The enhanced expression of cytokines associated with macrophage recruits immunosuppressive Tregs and promote tumor progression. Exosomes of chronic lymphocytic leukemia cells contain a large amount of Y RNA HY4, which can activate the TLR pathway in monocytes to secrete a large amount of CCL2, CCL4 and IL-6, and high expression of PD-L1 to promote the development of chronic lymphocytic leukemia cells (90).
Metastasis is a critical step in the malignant progression of tumors and is responsible for the majority of cancer-related deaths. The process of metastasis is complex and involves tumor cell intravasation, transport and immune evasion within the circulatory system, arrest at a secondary site, extravasation and, finally, colonization and growth (91). A few of the more recent studies describe that roles for exosomes in promoting cancer metastasis begin with exosome-mediated tumor angiogenesis. Treatment of endothelial cells with exosomes derived from cancer cells stimulated their growth, migration, and ability to undergo tubulation by stimulating the production of VEGF, a major player in blood vessel formation. VEGF bound to VEGF receptors within the same cells and promoted angiogenesis. Feng et al. showed that exosomes from breast cancer cell lines contained a unique cross-linked form of VEGF (92). Certain types of cancer cells preferentially colonize and metastasize to specific organs. Kaplan and colleagues first proposed the term premetastatic niche in 2005 to describe the phenomenon where primary tumors promoted metastasis to distant organs and established supportive metastatic environments (93). A recent study showed that tumor exosomes may be involved in determining organ-specific metastasis. It is reported that tumor exosomal integrins can facilitate organotropic metastasis by fusing with organ-specific resident cells to establish a premetastatic niche by activating Src phosphorylation and pro-inflammatory S100 expression, which prepares a favorable premetastatic niche for further metastasis (94). Exosomal integrins may be used as organotropism biomarkers to predict cancer organ-specific metastasis. Costa-Silva et al. showed that macrophage migration inhibitory factor (MIF) in tumor exosomes determined liver premetastatic niche formation during pancreatic cancer metastasis. This process is driven by the TGFβ pathway, fibronectin production and deposition, and recruitment of bone marrow-derived macrophages to liver metastatic niches. The effects of pancreatic cancer-derived exosomes during liver premetastatic niche formation can be partially reverted by specific targeting of MIF, fibronectin and macrophages (95). This evidence demonstrates an important role for exosomes in dictating organ-specific metastasis.
Potential clinical applications for exosomes in cancer
Improved methods for the isolation and purification of exosomes facilitate the application of exosomes in clinical translation. Anosys SA, Inc. (France) has developed a high-quality methodology for exosome purification that satisfies the criteria for clinical application. Exosomes are demonstrated to be critically significant in cancer immune biology, angiogenesis, immune regulation, invasion and metastasis, and drug resistance, making them a potent candidate for cancer diagnosis and treatment (Table 1).
Table 1
| Value of exosomes in cancer | Type of cancer | Marker in exosomes | Conclusion |
|---|---|---|---|
| Diagnosis | Colorectal cancer (96) | Circulating exosomes in plasma | The level of exosomes in cancer patients is statistically higher than that in healthy controls, and the numbers of exosomes were associated with degree of tumor differentiation and overall survival |
| Prostate cancer (97) | Circulating exosomes in plasma | ||
| Colorectal cancer (98) | mir21, mir23a, mir-1229, mir-1246, mir-150 | The level of certain miRNAs in exosomes were upregulated in tumor group compared to those in healthy group | |
| Gastric cancer (99) | Linc00152 | The level of LINC00152 in exosomes was higher in plasma of gastric cancer patients compared to those in healthy group | |
| Pancreatic cancer (100) | GPC1 protein | GPC1+ exosomes can serve as pancreatic cancer-specific markers for early diagnosis and assessing the possibility of curative surgery | |
| Drug delivery platform | Multiple drug resistance cancer cells (101) | PTX | exoPTX has significant potential for the delivery of various chemotherapeutics to treat drug resistant cancers |
| Treatment | Pancreatic cancer cells (102) | Gw4869 | Inhibition of exosomes biogenesis blocks cancer cell derived exosomes oncogenic roles |
| Hematopoietic cell (103) | Calcium | Calcium is an important regulator of exosomes biogenesis | |
| Bladder cancer cells (104) | Heparin | Heparin suppress exosomes uptake in bladder cancer cells | |
| Immunotherapy | Melanoma patients (105) | Dendritic cell-derived exosomes | Exosomes from dendritic cell are relatively safe and have positive effects on patients with tumors |
| NSCLC (106) | Dendritic cell-derived exosomes |
GPC1, glypican-1; PTX, paclitaxel; NSCLC, non-small cell lung cancer; miRNAs, microRNAs.
Cancer cells secrete more exosomes than normal cells, and exosomes from cancer cells can be considered as a package of cancer-related molecules. These vesicles may serve as sensitive biomarker for cancer diagnosis. Emerging evidence suggests that exosomes in the blood are an indicator for some types of cancers. The level of exosomes in colorectal cancer and prostate cancer patients was statistically higher than that in healthy controls or in benign tumor groups, and the numbers of exosomes in the plasma were associated with the degree of tumor differentiation and overall survival (96,97). In colorectal cancer, the level of certain miRNAs in exosomes, including miR21, miR23a, miR-1229, miR-1246, miR-150 and miR-223, were upregulated in the tumor group compared to those in the healthy group. The number of miRNAs was significantly lower after tumor resection, indicating the diagnostic utility of miRNA level in exosomes (98). LncRNA is another kind of ncRNA found in exosomes. It is reported that the level of long intergenic nonprotein coding RNA 152 (LINC00152) was higher in plasma from gastric cancer patients compared to the healthy group (99). The serum exosomal HOTAIR lncRNA may be a potential diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma (107). Therefore, there are many types of lncRNA species in human blood exosomes that are potential regulators or biomarkers (108). Proteins carried by exosomes can also be used as biomarkers for cancer diagnosis. Using mass spectrometry analyses, Melo and colleagues identified a cell surface proteoglycan, glypican-1 (GPC1), which was specifically enriched in pancreatic cancer-derived exosomes. GPC1+ circulating exosomes had high specificity and sensitivity in distinguishing healthy subjects and patients with early- and late-stage pancreatic cancer. Furthermore, the levels of GPC1+ exosomes were associated with tumor burden and the survival of patients. GPC1+ exosomes may serve as pancreatic cancer-specific markers for early diagnosis and assessing the possibility of curative surgery (100). These data demonstrate that the level of exosomes can be considered as sensitive biomarkers for cancer diagnosis and prognosis.
Exosomes naturally function as intercellular messengers, demonstrating that exosomes have the ability to interact with cellular membranes and deliver their contents to target cells. Based on their origin, exosomes have a specific cell tropism. Therefore, exosomes can be used as a drug delivery platform for a potent chemotherapeutic agent to treat chemo-resistant cancer. A new exosome-based formulation of paclitaxel has been developed and achieved a potent anticancer effect in drug resistant cancers (101). The injection of engineered exosomes loaded with doxorubicin leads to significant inhibition of tumor growth in vivo. The membrane-permeability of exosomes makes it possible for them to deliver small molecules or drugs. This ability has attracted considerable attention to the use of exosomes as a therapeutic delivery vehicle in cancer treatment (109).
Tumor-derived exosomes contain many active molecules from their original tumors; therefore, it is proposed that exosomes could be used as a vehicle to enhance cytotoxic T-lymphocyte responses. Meanwhile, exosomes from DCs can be engineered into an efficacious cancer immunotherapy. Clinical trials show that such approaches are relatively safe and have positive effects on patients with tumors (110). A Phase I clinical trial was performed to assess the safety of DC-derived exosomes in melanoma patients. A total of 15 patients with metastatic melanoma (stage IIIB or IV) were recruited and injected with exosomes isolated from DCs. No serious toxicity was observed, and 5 out of 15 patients showed clinical efficacy (111). Another phase I clinical trial recruited 13 patients suffering from stage IIIb/IV NSCLC. These patients received DC-derived exosomes four times at weekly intervals. Only grade 1–2 toxicity was observed (reaction at site of injection, flu-like illness and peripheral arm pain) (106). DC-derived exosomes used as vaccines were well tolerated, and the adverse effects were mild, including reaction at the injection site, nausea, pain, fever and fatigue. Based on the initial results from the clinical studies, several other clinical trials have been initiated. Several of these trials assess the role of exosomes derived from DCs or tumors as vaccines in melanoma, colorectal cancer, malignant glioma and pancreatic cancer. Several studies evaluate the efficacy of exosomes from grape or DCs for drug-delivery in colon cancer or in head and neck cancer chemotherapy [summarized in (43)].
Numerous studies show that exosomes secreted from malignant cells have tumor-promoting effects. Thus, direct targeting of exosomes (inhibition of exosome biogenesis or reduction of exosome uptake) becomes a potential strategy for cancer treatment. GW4869, as an inhibitor of nSMase2, was found to inhibit exosome biogenesis in several cell lines (102). Calcium is another important regulator of exosome biogenesis by the increase of Ca2+ as the Na+/H+ exchanger (103). The uptake of exosomes by recipient cells can also be decreased to block its oncogenic roles. Heparin, a competitive inhibitor of cell surface receptors dependent on HSPG coreceptors, suppressed exosome uptake significantly in bladder cancer cells (104). However, these exosome-regulating reagents are limited to research use only, and the translational implication of exosome-targeted reagents requires further research.
Because exosomes are found in all biofluids, it is possible to correlate the circulating exosome profile to tumor progression and for disease risk stratification. It is reported that serum exosome concentration is positively correlated with tumor lymph node metastasis and shorter disease-free survival (112). Moreover, the isolation of exosomes from patient samples is relatively easy and minimally noninvasive; therefore, exosomes used as biomarkers for disease diagnosis and prognosis monitoring is feasible. Clinical trials are only beginning. Nicholas et al. found that compared to healthy people, there was a 5- to 10-fold increase in protein content in tumor exosomes derived from stage III and stage IV melanoma patients (105). Evidently, exosomes have emerged as biological agents that are central to cancer progression and predictive for prognosis. However, there are still several questions to answer. First, many properties and mechanisms of exosomes in cancer remain elusive. In fact, several results from exosomes from the same cell types are contradictory. These discrepancies may be caused by a variety of factors, such as differences in cell culture conditions and purification protocols. Therefore, establishing cost-effective, large-scale and standardized protocols for exosome production is needed for preclinical and clinical trials. Second, many exosomal proteins or miRNAs are proposed as biomarkers, and their value in cancer diagnosis and treatment need to be further characterized. Screening out real and specific tumor biomarkers in exosomes is still an important task. Further research on tumor-derived exosome content that represents the cell of origin is important. Based on expression profiles of exosomal molecules in response to therapies and monitoring exosomal activity, scientists may determine the mechanisms that alter tumor phenotype and identify the factors responsible for drug resistance. Further research is warranted to explore each tumor’s specific exosomal molecular profile and subtype, which is helpful in predicting prognosis and assessing the risk for future metastasis. Using exosomes for cancer diagnosis and treatment is still in development. Clarifying the role of tumor-derived exosomes in cancer progression may change aspects of cancer treatment, leading to personalized medicine.
Acknowledgments
Funding: The study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Cheng YT, Yang CC, Shyur LF. Phytomedicine-Modulating oxidative stress and the tumor microenvironment for cancer therapy. Pharmacol Res 2016;114:128-43. [Crossref] [PubMed]
- Teo PY, Cheng W, Hedrick JL, et al. Co-delivery of drugs and plasmid DNA for cancer therapy. Adv Drug Deliv Rev 2016;98:41-63. [Crossref] [PubMed]
- Stone JB, DeAngelis LM. Cancer-treatment-induced neurotoxicity--focus on newer treatments. Nat Rev Clin Oncol 2016;13:92-105. [Crossref] [PubMed]
- Sun Z, Shi K, Yang S, et al. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer 2018;17:147. [Crossref] [PubMed]
- Tumas J, Kvederaviciute K, Petrulionis M, et al. Metabolomics in pancreatic cancer biomarkers research. Med Oncol 2016;33:133. [Crossref] [PubMed]
- Johnson CH, Spilker ME, Goetz L, et al. Metabolite and Microbiome Interplay in Cancer Immunotherapy. Cancer Res 2016;76:6146-52. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70. [Crossref] [PubMed]
- Strickaert A, Saiselet M, Dom G, et al. Cancer heterogeneity is not compatible with one unique cancer cell metabolic map. Oncogene 2017;36:2637-42. [Crossref] [PubMed]
- Santoni M, Piva F, Scarpelli M, et al. The origin of prostate metastases: emerging insights. Cancer Metastasis Rev 2015;34:765-73. [Crossref] [PubMed]
- Qian Z, Shen Q, Yang X, et al. The Role of Extracellular Vesicles: An Epigenetic View of the Cancer Microenvironment. Biomed Res Int 2015;2015:649161. [Crossref] [PubMed]
- Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009;9:581-93. [Crossref] [PubMed]
- Peinado H, Alečković M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012;18:883-91. [Crossref] [PubMed]
- Svensson KJ, Belting M. Role of extracellular membrane vesicles in intercellular communication of the tumour microenvironment. Biochem Soc Trans 2013;41:273-76. [Crossref] [PubMed]
- Belting M, Christianson HC. Role of exosomes and microvesicles in hypoxia-associated tumour development and cardiovascular disease. J Intern Med 2015;278:251-63. [Crossref] [PubMed]
- Zhou M, Chen J, Zhou L, et al. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell Immunol 2014;292:65-9. [Crossref] [PubMed]
- Wang Z, Chen JQ, Liu JL, et al. Exosomes in tumor microenvironment: novel transporters and biomarkers. J Transl Med 2016;14:297. [Crossref] [PubMed]
- Villanueva MT. Microenvironment: small containers, important cargo. Nat Rev Cancer 2014;14:764. [Crossref] [PubMed]
- Dayan D, Salo T, Salo S, et al. Molecular crosstalk between cancer cells and tumor microenvironment components suggests potential targets for new therapeutic approaches in mobile tongue cancer. Cancer Med 2012;1:128-40. [Crossref] [PubMed]
- Wang J, Hendrix A, Hernot S, et al. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood 2014;124:555-66. [Crossref] [PubMed]
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-9. [Crossref] [PubMed]
- Trams EG, Lauter CJ, Salem N Jr, et al. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 1981;645:63-70. [Crossref] [PubMed]
- Yu S, Cao H, Shen B, et al. Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget 2015;6:37151-68. [Crossref] [PubMed]
- Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol Metab 2017;28:3-18. [Crossref] [PubMed]
- Cai J, Wu G, Jose PA, et al. Functional transferred DNA within extracellular vesicles. Exp Cell Res 2016;349:179-83. [Crossref] [PubMed]
- Wang J, Faict S, Maes K, et al. Extracellular vesicle cross-talk in the bone marrow microenvironment: implications in multiple myeloma. Oncotarget 2016;7:38927-45. [PubMed]
- Kumar D, Gupta D, Shankar S, et al. Biomolecular characterization of exosomes released from cancer stem cells: Possible implications for biomarker and treatment of cancer. Oncotarget 2015;6:3280-91. [Crossref] [PubMed]
- Roma-Rodrigues C, Fernandes AR, Baptista PV. Exosome in tumour microenvironment: overview of the crosstalk between normal and cancer cells. Biomed Res Int 2014;2014:179486. [Crossref] [PubMed]
- Vlassov AV, Magdaleno S, Setterquist R, et al. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 2012;1820:940-8. [Crossref] [PubMed]
- Li M, Zeringer E, Barta T, et al. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos Trans R Soc Lond B Biol Sci 2014;369. [PubMed]
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014;30:255-89. [Crossref] [PubMed]
- Bell BM, Kirk ID, Hiltbrunner S, et al. Designer exosomes as next-generation cancer immunotherapy. Nanomedicine 2016;12:163-9. [Crossref] [PubMed]
- Greening DW, Gopal SK, Mathias RA, et al. Emerging roles of exosomes during epithelial-mesenchymal transition and cancer progression. Semin Cell Dev Biol 2015;40:60-71. [Crossref] [PubMed]
- Challagundla KB, Wise PM, Neviani P, et al. Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J Natl Cancer Inst 2015;107: [Crossref] [PubMed]
- Liu Y, Shah SV, Xiang X, et al. COP9-associated CSN5 regulates exosomal protein deubiquitination and sorting. Am J Pathol 2009;174:1415-25. [Crossref] [PubMed]
- Somasundaram R, Herlyn M. Melanoma exosomes: messengers of metastasis. Nat Med 2012;18:853-4. [Crossref] [PubMed]
- Yeo RW, Lai RC, Zhang B, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 2013;65:336-41. [Crossref] [PubMed]
- Lässer C, Alikhani VS, Ekström K, et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med 2011;9:9. [Crossref] [PubMed]
- Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 2004;101:13368-73. [Crossref] [PubMed]
- Vojtech L, Woo S, Hughes S, et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res 2014;42:7290-304. [Crossref] [PubMed]
- Andre F, Schartz NE, Movassagh M, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002;360:295-305. [Crossref] [PubMed]
- Milane L, Singh A, Mattheolabakis G, et al. Exosome mediated communication within the tumor microenvironment. J Control Release 2015;219:278-94. [Crossref] [PubMed]
- Rana S, Malinowska K, Zöller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia 2013;15:281-95. [Crossref] [PubMed]
- Taylor DD, Homesley HD, Doellgast GJ. “Membrane-associated” immunoglobulins in cyst and ascites fluids of ovarian cancer patients. Am J Reprod Immunol 1983;3:7-11. [Crossref] [PubMed]
- Zhang HG, Grizzle WE. Exosomes and cancer: a newly described pathway of immune suppression. Clin Cancer Res 2011;17:959-64. [Crossref] [PubMed]
- Stec M, Szatanek R, Baj-Krzyworzeka M, et al. Interactions of tumour-derived micro(nano)vesicles with human gastric cancer cells. J Transl Med 2015;13:376. [Crossref] [PubMed]
- Lugini L, Valtieri M, Federici C, et al. Exosomes from human colorectal cancer induce a tumor-like behavior in colonic mesenchymal stromal cells. Oncotarget 2016;7:50086-98. [Crossref] [PubMed]
- Luga V, Wrana JL. Tumor-stroma interaction: Revealing fibroblast-secreted exosomes as potent regulators of Wnt-planar cell polarity signaling in cancer metastasis. Cancer Res 2013;73:6843-7. [Crossref] [PubMed]
- Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol 2011;33:441-54. [Crossref] [PubMed]
- Park JE, Tan HS, Datta A, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics 2010;9:1085-99. [Crossref] [PubMed]
- Webber J, Steadman R, Mason MD, et al. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res 2010;70:9621-30. [Crossref] [PubMed]
- Chowdhury R, Webber JP, Gurney M, et al. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 2015;6:715-31. [Crossref] [PubMed]
- Ye SB, Li ZL, Luo DH, et al. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget 2014;5:5439-52. [Crossref] [PubMed]
- Bronisz A, Wang Y, Nowicki MO, et al. Extracellular vesicles modulate the glioblastoma microenvironment via a tumor suppression signaling network directed by miR-1. Cancer Res 2014;74:738-50. [Crossref] [PubMed]
- Demory Beckler M, Higginbotham JN, Franklin JL, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics 2013;12:343-55. [Crossref] [PubMed]
- Liu D, Li C, Trojanowicz B, et al. CD97 promotion of gastric carcinoma lymphatic metastasis is exosome dependent. Gastric Cancer 2016;19:754-66. [Crossref] [PubMed]
- Chen L, Charrier A, Zhou Y, et al. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology 2014;59:1118-29. [Crossref] [PubMed]
- Zhou W, Fong MY, Min Y, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014;25:501-15. [Crossref] [PubMed]
- Ding G, Zhou L, Qian Y, et al. Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212-3p. Oncotarget 2015;6:29877-88. [Crossref] [PubMed]
- Aoki H, Ohnishi H, Hama K, et al. Autocrine loop between TGF-beta1 and IL-1beta through Smad3- and ERK-dependent pathways in rat pancreatic stellate cells. Am J Physiol Cell Physiol 2006;290:C1100-8. [Crossref] [PubMed]
- Cho JA, Park H, Lim EH, et al. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol 2012;40:130-8. [PubMed]
- Atay S, Banskota S, Crow J, et al. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc Natl Acad Sci U S A 2014;111:711-6. [Crossref] [PubMed]
- Tan A, Rajadas J, Seifalian AM. Exosomes as nano-theranostic delivery platforms for gene therapy. Adv Drug Deliv Rev 2013;65:357-67. [Crossref] [PubMed]
- Li J, Liu K, Liu Y, et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat Immunol 2013;14:793-803. [Crossref] [PubMed]
- Kadota T, Yoshioka Y, Fujita Y, et al. Extracellular vesicles in lung cancer-From bench to bedside. Semin Cell Dev Biol 2017;67:39-47. [Crossref] [PubMed]
- Saunderson SC, Dunn AC, Crocker PR, et al. CD169 mediates the capture of exosomes in spleen and lymph node. Blood 2014;123:208-16. [Crossref] [PubMed]
- Besse B, Charrier M, Lapierre V, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2015;5:e1071008. [Crossref] [PubMed]
- Lu Z, Zuo B, Jing R, et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol 2017;67:739-48. [Crossref] [PubMed]
- Zhu L, Kalimuthu S, Gangadaran P, et al. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 2017;7:2732-45. [Crossref] [PubMed]
- Filipazzi P, Bürdek M, Villa A, et al. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin Cancer Biol 2012;22:342-9. [Crossref] [PubMed]
- Abusamra AJ, Zhong Z, Zheng X, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis 2005;35:169-73. [Crossref] [PubMed]
- Söderberg A, Barral AM, Söderström M, et al. Redox-signaling transmitted in trans to neighboring cells by melanoma-derived TNF-containing exosomes. Free Radic Biol Med 2007;43:90-9. [Crossref] [PubMed]
- Taylor DD, Akyol S, Gercel-Taylor C. Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol 2006;176:1534-42. [Crossref] [PubMed]
- Javeed N, Gustafson MP, Dutta SK, et al. Immunosuppressive CD14(+)HLA-DR(lo/neg) monocytes are elevated in pancreatic cancer and “primed” by tumor-derived exosomes. Oncoimmunology 2016;6:e1252013. [Crossref] [PubMed]
- Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018;560:382-6. [Crossref] [PubMed]
- Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 2001;7:297-303. [Crossref] [PubMed]
- Wu CJ, Biernacki M, Kutok JL, et al. Graft-versus-leukemia target antigens in chronic myelogenous leukemia are expressed on myeloid progenitor cells. Clin Cancer Res 2005;11:4504-11. [Crossref] [PubMed]
- Zech D, Rana S, Büchler MW, et al. Tumor-exosomes and leukocyte activation: an ambivalent crosstalk. Cell Commun Signal 2012;10:37. [Crossref] [PubMed]
- Berchem G, Noman MZ, Bosseler M, et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-β and miR23a transfer. Oncoimmunology 2015;5:e1062968. [Crossref] [PubMed]
- Umezu T, Tadokoro H, Azuma K, et al. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 2014;124:3748-57. [Crossref] [PubMed]
- Kim CW, Lee HM, Lee TH, et al. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res 2002;62:6312-7. [PubMed]
- Al-Nedawi K, Meehan B, Kerbel RS, et al. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A 2009;106:3794-9. [Crossref] [PubMed]
- Zhuang G, Wu X, Jiang Z, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J 2012;31:3513-23. [Crossref] [PubMed]
- Antonyak MA, Li B, Boroughs LK, et al. Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci U S A 2011;108:4852-7. [Crossref] [PubMed]
- Latifkar A, Cerione RA, Antonyak MA. Probing the mechanisms of extracellular vesicle biogenesis and function in cancer. Biochem Soc Trans 2018;46:1137-46. [Crossref] [PubMed]
- Xu B, Wang T. Intimate cross-talk between cancer cells and the tumor microenvironment of B-cell lymphomas: The key role of exosomes. Tumour Biol 2017;39:1010428317706227. [Crossref] [PubMed]
- DeRita RM, Zerlanko B, Singh A, et al. c-Src, Insulin-Like Growth Factor I Receptor, G-Protein-Coupled Receptor Kinases and Focal Adhesion Kinase are Enriched Into Prostate Cancer Cell Exosomes. J Cell Biochem 2017;118:66-73. [Crossref] [PubMed]
- Cooks T, Pateras IS, Jenkins LM, et al. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat Commun 2018;9:771. [Crossref] [PubMed]
- Haderk F, Schulz R, Iskar M, et al. Tumor-derived exosomes modulate PD-L1 expression in monocytes. Sci Immunol 2017;2: [Crossref] [PubMed]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002;2:563-72. [Crossref] [PubMed]
- Feng Q, Zhang C, Lum D, et al. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat Commun 2017;8:14450. [Crossref] [PubMed]
- Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005;438:820-7. [Crossref] [PubMed]
- Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015;527:329-35. [Crossref] [PubMed]
- Costa-Silva B, Aiello NM, Ocean AJ, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 2015;17:816-26. [Crossref] [PubMed]
- Silva J, Garcia V, Rodriguez M, et al. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer 2012;51:409-18. [Crossref] [PubMed]
- Tavoosidana G, Ronquist G, Darmanis S, et al. Multiple recognition assay reveals prostasomes as promising plasma biomarkers for prostate cancer. Proc Natl Acad Sci U S A 2011;108:8809-14. [Crossref] [PubMed]
- Ogata-Kawata H, Izumiya M, Kurioka D, et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS One 2014;9:e92921. [Crossref] [PubMed]
- Li Q, Shao Y, Zhang X, et al. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol 2015;36:2007-12. [Crossref] [PubMed]
- Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015;523:177-82. [Crossref] [PubMed]
- Kim MS, Haney MJ, Zhao Y, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016;12:655-64. [Crossref] [PubMed]
- Richards KE, Zeleniak AE, Fishel ML, et al. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770-8. [Crossref] [PubMed]
- Savina A, Furlán M, Vidal M, et al. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem 2003;278:20083-90. [Crossref] [PubMed]
- Franzen CA, Simms PE, Van Huis AF, et al. Characterization of uptake and internalization of exosomes by bladder cancer cells. Biomed Res Int 2014;2014:619829. [Crossref] [PubMed]
- Nicholas J. A new diagnostic tool with the potential to predict tumor metastasis. J Natl Cancer Inst 2013;105:371-2. [Crossref] [PubMed]
- Morse MA, Garst J, Osada T, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 2005;3:9. [Crossref] [PubMed]
- Wang J, Zhou Y, Lu J, et al. Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med Oncol 2014;31:148. [Crossref] [PubMed]
- Li S, Li Y, Chen B, et al. exoRBase: a database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res 2018;46:D106-12. [Crossref] [PubMed]
- Guo L, Guo N. Exosomes: Potent regulators of tumor malignancy and potential bio-tools in clinical application. Crit Rev Oncol Hematol 2015;95:346-58. [Crossref] [PubMed]
- Iero M, Valenti R, Huber V, et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ 2008;15:80-8. [Crossref] [PubMed]
- Escudier B, Dorval T, Chaput N, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med 2005;3:10. [Crossref] [PubMed]
- Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res 2011;71:3792-801. [Crossref] [PubMed]


