
Treatment intensity in locoregionally advanced head and neck cancer: recent investigation leads to new questions
Introduction
This short review highlights a recent trial as an example of ongoing investigations of treatment intensification in locoregionally advanced squamous cell carcinoma of the head and neck (LRAHNC). Treatment intensification in LRAHNC is complicated by the fact that there are numerous methods of intensifying radiation treatment, chemotherapy, surgery and biological therapy for this disease. Treatment intensification and treatment optimization involves the appropriate integration of all treatment modalities. This review will focus on the non-surgical approaches of combining chemotherapy, targeted monoclonal antibody agents and radiation treatment as studied in the recent manuscript of Tao et al. (1). However, the timely integration of surgery in organ preservation strategies is the subject of many other reviews and studies (2-4). Finally, the rapid innovation of novel monoclonal antibody agents that target immune regulatory pathways is changing the landscape of treatment of head and neck cancer (5-7).
Report of GORTEC Trial 2007-01
Recently Tao et al. reported on the results of the GORTEC 2007-01 phase III randomized trial that examined two different intensities of treatment for LRAHNC (1). The trial included patients with stage III or IV (non-metastatic, locoregionally advanced head and neck cancer) squamous cell cancer of the oral cavity, oropharynx, hypopharynx or larynx. These patients were randomly assigned to standard once-daily (70 Gy/35) radiation treatment (RT) with weekly cetuximab [400 mg/m2/loading dose-one week prior to RT followed by weekly cetuximab (250 m/m2) during RT] vs. the more intense treatment in which patients received the same radiation treatment and cetuximab (RT-Cet) as noted above with the addition of concurrent chemotherapy (RT-Cet-CT) consisting of three cycles of carboplatin (70 mg/m2/d) and 5-fluroracil (5-FU) 600 mg/m2/d on days 1–4 with q3 week cycles. Randomization was performed by minimization (8) on centers, T-stage (TO-2 vs. T3-4) and N-stage (NO vs. N1-2). The more intense treatment of RT-Cet-CT resulted in an improvement in progression free survival (PFS, P=0.015) and locoregional control (LRC, P<0.001). It was noteworthy that these advantages, for the more intense regimen were observed in both the p16 positive (p16+) population as well as the p16 negative (p16−) population. However, the more intense regimen only demonstrated a suggestion of improved overall survival (P=0.11) compared to the less intense regimen. When assessing the trial of Tao et al., it is important to assess the control arm in this trial for its level of intensity. Should the control arm have employed altered fractionated RT since the trial that demonstrated superiority for RT-Cet over RT alone (9,10) suggested that the combination of RT-Cet may be most beneficial when altered fractionated RT was utilized? Also, was the combination treatment of RT-Cet the best control arm for this patient population? The results of GORTEC 2007-01 are important as they provide more information on the role of treatment intensity in this group of LRAHNC patients during a period in time when we have developed a better understanding of the advantageous and toxicities of intense concurrent treatments (11,12). The appropriate intensity of treatment for various groups of patients with LRAHNC remains the subject of ongoing investigation.
Radiation treatment (RT) fractionation and treatment intensity
The trial of Tao et al. utilized conventionally fractionated RT (once-daily) and this choice of RT fractionation deserves further dissection. In the 1980’s and 1990’s, the standard treatment for locoregionally advanced head and neck cancer consisted of RT alone. However, during that time frame, many investigations were being performed to explore combinations of chemotherapy and RT. Alternatively, many investigators were studying the subject of optimizing the RT alone regimen by exploring the use of altered RT fractionation. Various groups had developed different RT fractionation regimens and had explored these regimens in phase II studies. Subsequently, the Radiation Therapy Oncology Group (RTOG) decided to mount a large randomized trial (90-03) comparing four of the most commonly utilized fractionation regimens of the time period (Figure 1). The four regimens were: standard once-daily fractionation of 70 Gy/35 fractions, hyperfractionated twice-daily RT (HFX) of 81.6 Gy/68 twice-daily fractions/7 weeks, concomitant boost accelerated fractionation with 1.8 Gy daily fractions and the last 12 days included twice-daily fractionation with a second 1.5 Gy fraction (AFX-C, 72 Gy/42 fractions/6 weeks) or an accelerated fractionation regimen of 67.2 Gy/42 fractions/6 weeks with a 2-week break after 38.4 Gy (AFX-S) (13,14). The early reports of RTOG 90-03 suggested that both the AFX-C and HFX resulted in improved locoregional control (LRC) (13). Therefore, since AFX-C required fewer twice-daily treatments compared to HFX, the RTOG adopted the AFX-C as a standard regimen and employed it in future trials such as RTOG 01-29 [which randomized patients to standard once-daily RT with 3 cycles of cisplatin (100 mg/m2) every 3 weeks or AFX-C with two cycles of cisplatin (100 mg/m2) every 3 weeks (15)]. However, the final results of RTOG 90-03 (with censoring at 5 years) showed that the HFX regimen was the only regimen that was statistically superior to standard fractionation with respect to LRC and survival (14). It is notable that AFX-C showed strong trends for superiority over standard fractionation.
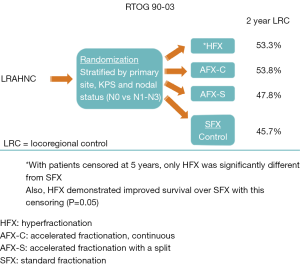
When the mature results of RTOG 90-03 were realized, fractionation regimens had evolved substantially because of the advent of intensity modulated radiotherapy (IMRT). IMRT regimens often incorporated a simultaneous integrated boost (higher daily dose to gross disease) with each daily treatment. The wide spread use of these IMRT techniques flourished in the oncology community while the trials that had explored various forms of altered fractionationed RT were maturing. Subsequently, the altered fractionation regimen of 6 fractions per week (16,17), which was popularized by the Danish, became very compatible with the simultaneous integrate boost techniques that employed IMRT. This 6 fractions/week regimen allowed for a more rapid delivery of treatment as well as the use of the popular IMRT technique of delivering different daily doses to various areas of the head and neck based on the presence or absence of gross disease and the risk of microscopic disease. Tao et al. employed the same standard once-daily RT fractionation in both arms as the study question centered on whether more intense systemic treatment would be beneficial. Therefore, in order to interpret these results, the question arises whether altered RT fractionation is beneficial when concomitant systemic agents are used. Additionally, the question of whether altered RT fractionation may be beneficial with only certain categories of systemic agents is relevant to the interpretation of this GORTEC 2007-01 study.
Concomitant chemoradiotherapy and treatment intensity
In addition to the above mentioned studies exploring radiation fractionation, the 1980’s and 1990’s were a period in which much investigation was undertaken regarding the addition of various chemotherapy regimens to the RT treatment backbone. During this time frame, many trials suggested that there may be an advantage to adding chemotherapy to radiotherapy, but the results were not initially consistent (18,19). In 2003, Adelstein et al. (20) published the results of the US Intergroup trial that randomized patients with LRAHNC to three different regimens: once-daily radiation alone (RT), RT with cisplatin—100 mg/m2 days 1, 22 and 43 vs. a split course RT arm with cisplatin and 5-FU. The comparison of the first two arms produced results that were practice changing. The addition of cisplatin to RT resulted in an improvement in the three year survival of patients from 23% to 37% (P=0.014). Following the publication of this intergroup trial, the regimen of once-daily RT with bolus cisplatin (q3 weeks) became an important standard treatment for patients with LRAHNC. However, there was still a question as to whether altered fractionation should be utilized when treating patients with concomitant chemoradiotherapy (CRT). This question was addressed in the RTOG 01-29 (15) study in which patients with LRAHNC were randomized to conventionally fractionated RT (once-daily) with three cycles of cisplatin (100 mg/m2), as in the Intergroup trial, vs. altered fractionated RT (AFX-C) with two cycles of cisplatin (100 mg/m2). The treatments produced comparable outcomes for locoregional control, survival and toxicity. Therefore, both regimens became commonly utilized. This study left an open question of whether AFX-C RT is necessary when only two cycles (compared to three cycles) of cisplatin are utilized (21).
Based on the results of the intergroup trial, and multiple other trials of concomitant cisplatin and RT, one can question the appropriateness of the control arm in GORTEC 2007-01. However, when the trial of Tao et al. was designed, RT and cetuximab had recently been shown to be superior to radiation alone and the magnitude of the survival benefit through the addition of cetuximab to radiation was comparable to the survival benefit of adding cisplatin to radiation. However, no trials existed that compared cetuximab/RT vs. cisplatin/RT. Recently, the RTOG and the De-ESCALATE group in the United Kingdom, completed trials comparing cisplatin/RT vs. cetuximab/RT for patients with HPV positive LRAHNC and the treatment of cisplatin/RT resulted in improved survival compared to cetuximab/RT in both trials (22,23). The RTOG trial utilized altered fractionation and the British trial utilized standard fractionation. (The RTOG trial will be discussed later in this review.) Additionally, it is reasonable to question whether the control arm that Tao et al. utilized was the most appropriate radiation fractionation based on the existing data at the time of their trial. The addition of cetuximab to radiation had demonstrated a significant survival advantage over RT alone for patients with LRAHNC (9,10). However, the randomized trial of these two treatments allowed for altered fractionated RT or standard once-daily fractionation. Radiation therapy fractionation was a stratification factor in the randomization procedure. The final results of the trial suggested that patients who received altered fractionated RT may have had the greatest benefit from the addition of cetuximab to RT (10). Therefore, it is possible that the control arm in the trial of Tao et al. was somewhat flawed. This potential problem reveals an important conundrum for trial designers. The results of RTOG 01-29 had shown that altered fractionated RT may not be necessary for concomitant RT-chemotherapy regimens but the concomitant cetuximab-RT data suggested that altered fractionated RT may be important when utilizing RT-cetuximab. The investigators would have been criticized for using two different RT regimens if they had chosen to use altered fractionated RT in just the RT-cetuximab arm.
HPV status and treatment intensity
Tao et al. demonstrated that treatment intensification improved LRC and PFS for the overall group of patients with LRAHNC. As noted previously, they also found that this benefit was evident for patients with p16+ disease as well as p16- disease. This finding, along with the aforementioned results of RTOG 1016, will be the subject of much future discussion because the last few years have seen many investigations into whether treatment can be de-intensified for patients with p16+ LRAHNC. Investigations have suggested that these patients may have less treatment-related toxicity with lower doses of radiation or chemotherapy and these dose reductions may not compromise the efficacy of treatment (21,24). Based on the results of Tao et al., and RTOG 1016, future investigations of de-intensification of therapy for patients with p16+ LRAHNC will require much consideration of the chosen treatments as well as the most appropriate subset of patients that should be eligible for these investigations. Investigators will need to approach these studies with much caution.
Treatment intensity with induction chemotherapy
Although the trial of Tao et al. did not study induction chemotherapy, this treatment has been a prominent investigative approach to the study of treatment intensification in LRAHNC. Enthusiasm greatly increased for the use of induction taxane-based chemotherapy (followed by definitive chemo-RT or RT alone regimens) when Posner et al., and Vermorken et al., published the results of studies demonstrating survival advantages for taxane-based (TPF: taxane, platinum, 5-FU) induction regimens compared to cisplatin-5FU induction regimens in the same issue of the New England Journal of Medicine (25,26). However, this enthusiasm waned when two trials of TPF induction chemotherapy vs. concomitant chemo-RT (27,28) showed no statistically significant difference in survival with only a suggestion of an advantage in survival for patients with very advanced disease (N2c–3, P=0.19) who received induction TPF. This latter finding was a subgroup analysis of a randomized trial (27). However, induction chemotherapy continues to be explored since recent results of an older laryngeal preservation trial have suggested that there may be more unexplained long-term deaths with concomitant chemo-RT compared to using induction chemotherapy followed by RT without concomitant chemotherapy (29). Forastiere et al. explored the long-term results of RTOG 91-11 in which patients with LRAHNC of the larynx/hypopharynx were randomized to RT alone, RT with concomitant cisplatin [as in the trial of Adelstein et al. (20)] or induction cisplatin/5-FU followed by RT without concomitant chemotherapy (29). The results of this trial showed that patients who received concomitant treatment of chemo-RT had decreased survival from causes other than cancer compared to the patients who had induction chemotherapy (death due to cancer was similar for the two groups). The results of this trial suggest that concomitant chemo-RT may lead to toxicities that are potentially fatal and may not be registered as related to treatment. This hypothesis has been strengthened by a report that compiled results from several large trials that employed concomitant chemo-RT and demonstrated that 43% of patients had a severe late toxicity. An alternative hypothesis, regarding the results of RTOG 91-11, is that there could have been an imbalance between the treatment arms with respect to other patient characteristics that accounted for this survival difference.
The results of RTOG 91-11 call upon investigators to continue to examine the toxicities of concomitant chemo-RT in LRAHNC. Perhaps there are methods to employ induction chemotherapy (± biologics) followed by RT (± selected concomitant treatments) that can lead to a decrease in the long term toxicities. The example study of Tao et al. did not examine these issues, but long-term follow-up of studies that have examined treatment intensification are warranted in order to examine late toxicities of these approaches. Future novel treatment combinations may include induction chemotherapy in order to avoid potential use of concomitant regimens that are associated with an increase in late toxicity.
Future directions
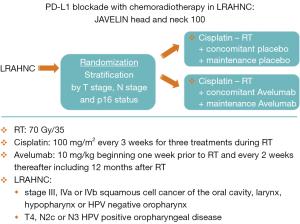
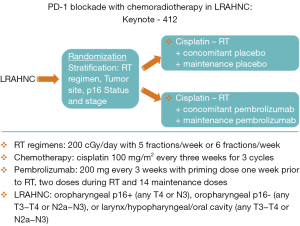
During the last few years, immunotherapy has developed as one of the most promising new therapeutics in head and neck cancer. The PD axis inhibitors are an example of the promise that these agents hold (7). These inhibitors prevent binding of PD1 (T cells) to PD-L1 (tumor cells) which has the result of decreasing the tumor’s capacity for escaping immune surveillance. Checkmate 141 was an early study of the use of a PD1 inhibitor, nivolumab, for patients with recurrent or metastatic head and neck cancer who had failed a prior cisplatin-based therapy (30). Patients who received nivolumab showed an improvement in survival compared to patients treated with standard chemotherapy. Following the demonstration that these PD Axis inhibitors had activity in the metastatic setting, there has been much interest in exploring the use of these agents in the curative setting. Laboratory investigations at The University of Chicago group showed that PD-L1 inhibition resulted in radiosensitization using in vivo colon cancer and breast cancer models (31). Subsequently, work from the University of Colorado demonstrated similar findings with an in vivo head and neck cancer model (32). Based on the documented activity of PD Axis inhibitors in metastatic head and neck cancer, as well as their radiosensitizing properties, investigators began to incorporate these agents into curative regimens utilizing these agents in combination with RT for head and neck cancer. For example, the group from Florence, Italy is performing a phase I/II trial that adds durvalumab (a monoclonal antibody that targets PD-L1) to the treatment RT-Cet for patients with LRAHNC (33). The enthusiasm for employing these agents for curative LRAHNC has resulted in the initiation of large randomized trials shortly after these agents were found to be effective in the recurrent/metastatic setting. The Javelin Head and Neck 100 study (Figure 2) is a large ongoing randomized trial of cisplatin-RT vs. avelumab (an inhibitor of PD-L1) with cisplatin-RT in patients with LRAHNC (34). Likewise Keynote 412 (Figure 3) is another large randomized trial of cisplatin-RT vs. pembrolizumab (an inhibitor of PD-1) with cisplatin-RT in patients with LRAHNC (35). It will be exciting to see the results of these ongoing trials following the completion of enrollment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor San-Gang Wu (Department of Radiation Oncology, Xiamen Cancer Center, the First affiliated Hospital of Xiamen University, Xiamen, China).
Conflicts of Interest: JA Bonner, MD: Occassional consultant/honoraria for Bristol-Myers Squibb Company, Eli Lilly and Company, Merck Serono, and Cel-Sci. DH Boggs, MD: Received honoraria and research support from Varian Medical System Inc. Received research support from Novocure Inc.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tao Y, Auperin A, Sire C, et al. Improved Outcome by Adding Concurrent Chemotherapy to Cetuximab and Radiotherapy for Locally Advanced Head and Neck Carcinomas: Results of the GORTEC 2007-01 Phase III Randomized Trial. J Clin Oncol 2018;JCO2017762518. [Epub ahead of print]. [PubMed]
- Wolf GT, Bellile E, Eisbruch A, et al. Survival Rates Using Individualized Bioselection Treatment Methods in Patients with Advanced Laryngeal Cancer. JAMA Otolaryngol Head Neck Surg 2017;143:355-66. [Crossref] [PubMed]
- Wolf GT. Reexamining the Treatment of Advanced Laryngeal Cancer: The VA Laryngeal Cancer Study Revisited. Head Neck 2010;32:7-14. [PubMed]
- Olsen KD. Reexamining the Treatment of Advanced Laryngeal Cancer. Head Neck 2010;32:1-7. [PubMed]
- Chin K. Industry Corner: Perspectives and Controversies. Ann Oncol 2017;28:1658-66. [Crossref] [PubMed]
- Atiq SO, Atiq OO, Atiq MO, et al. The Role Immunotherapy and Radiation Therapy in Tumor Chemosensitivity in Advanced Head and Neck Cancer. Am J Case Rep 2018;19:1241-4. [Crossref] [PubMed]
- Sim F, Leidner R, Bell RB. Immunotherapy for Head and Neck Cancer. Oral Maxillofac Surg Clin North Am 2019;31:85-100. [Crossref] [PubMed]
- Pocock SJ, Simon R. Sequential Treatment Assignment with Balancing for Prognostic Factors in the Controlled Clinical Trial. Biometrics 1975;31:103-15. [Crossref] [PubMed]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy Plus Cetuximab for Squamous Cell Carcinoma of the Head and Neck. N Engl J Med 2006;354:567-78. [Crossref] [PubMed]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy Plus Cetuximab for Locoregionally Advanced Head and Neck Cancer: 5-Year Survival Data From a Phase 3 Randomised Trial, and Relation Between Cetuximab-Induced Rash and Survival. Lancet Oncol 2010;11:21-8. [Crossref] [PubMed]
- Machtay M, Moughan J, Trotti A, et al. Factors Associated with Severe Late Toxicity After Concurrent Chemoradiation for Locally Advanced Head and Neck Cancer: An RTOG Analysis. J Clin Oncol 2008;26:3582-9. [Crossref] [PubMed]
- Argiris A, Brockstein BE, Haraf DJ, et al. Competing Causes of Death and Second Primary Tumors in Patients with Locoregionally Advanced Head and Neck Cancer Treated with Chemoradiotherapy. Clin Cancer Res 2004;10:1956-62. [Crossref] [PubMed]
- Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) Phase III Randomized Study to Compare Hyperfractionation and Two Variants of Accelerated Fractionation to Standard Fractionation Radiotherapy for Head and Neck Squamous Cell Carcinomas: First Report of RTOG 9003. Int J Radiat Oncol Biol Phys 2000;48:7-16. [Crossref] [PubMed]
- Beitler JJ, Zhang Q, Fu KK, et al. Final Results of Local-Regional Control and Late Toxicity of RTOG 90-03; A Randomized Trial of Altered Fractionation Radiation for Locally Advanced Head and Neck Cancer. Int J Radiat Oncol Biol Phys 2014;89:13-20. [Crossref] [PubMed]
- Nguyen-Tan PF, Zhang Q, Ang KK, et al. Randomized Phase III Trial to Test Accelerated Versus Standard Fractionation in Combination with Concurrent Cisplatin for Head and Neck Carcinomas in the Radiation Therapy Oncology Group 0129 Trial: Long-Term Report of Efficacy and Toxicity. J Clin Oncol 2014;32:3858-66. [Crossref] [PubMed]
- Overgaard J, Hansen HS, Specht L, et al. Five Compared with Six Fractions Per Week of Conventional Radiotherapy of Squamous-Cell Carcinoma of Head and Neck: DAHANCA 6 & 7 Randomised Controlled Trial. Lancet 2003;362:933-40. [Crossref] [PubMed]
- Overgaard J, Mohanti BK, Begum N, et al. Five Versus Six Fractions of Radiotherapy Per Week for Squamous-Cell Carcinoma of the Head and Neck (IAEA-ACC Study): A Randomised, Multicentre Trial. Lancet Oncol 2010;11:553-60. [Crossref] [PubMed]
- Munro AJ. An Overview of Randomised Controlled Trials of Adjuvant Chemotherapy in Head and Neck Cancer. Br J Cancer 1995;71:83-91. [Crossref] [PubMed]
- Pignon JP, le Maitre A, Maillard E, et al. Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC): An Update on 93 Randomised Trials and 17,346 Patients. Radiother Oncol 2009;92:4-14. [Crossref] [PubMed]
- Adelstein DJ, Li Y, Adams GL, et al. An Intergroup Phase III Comparison of Standard Radiation Therapy and Two Schedules of Concurrent Chemoradiotherapy in Patients with Unresectable Squamous Cell Head and Neck Cancer. J Clin Oncol 2003;21:92-8. [Crossref] [PubMed]
- Spreafico A, Huang SH, Xu W, et al. Impact of Cisplatin Dose Intensity on Human Papillomavirus-Related and Unrelated Locally Advanced Head and Neck Squamous Cell Carcinoma. Eur J Cancer 2016;67:174-82. [Crossref] [PubMed]
- Gillison ML, Trotti AM, Harris J, et al. Radiotherapy Plus Cetuximab or Cisplatin in Human Papillomavirus-Positive Oropharyngeal Cancer (NRG Oncology RTOG 1016): A Randomised, Multicentre, Non-Inferiority Trial. Lancet 2019;393:40-50. [Crossref] [PubMed]
- Mehanna H, Robinson M, Hartley A, et al. Radiotherapy Plus Cisplatin or Cetuximab in Low-Risk Human Papillomavirus-Positive Oropharyngeal Cancer (De-ESCALaTE HPV): An Open-Label Randomised Controlled Phase 3 Trial. Lancet 2019;393:51-60. [Crossref] [PubMed]
- Marur S, Li S, Cmelak AJ, et al. E1308: Phase II Trial of Induction Chemotherapy Followed by Reduced-Dose Radiation and Weekly Cetuximab in Patients with HPV Associated Resectable Squamous Cell Carcinoma of the Oropharynx – EGOG- ACRIN Cancer Research Group. J Clin Oncol 2017;35:490-7. [Crossref] [PubMed]
- Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and Fluorouracil Alone or with Docetaxel in Head and Neck Cancer. N Engl J Med 2007;357:1705-15. [Crossref] [PubMed]
- Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, Fluorouracil and Docetaxel in Unresectable Head and Neck Cancer. N Engl J Med 2007;357:1695-704. [Crossref] [PubMed]
- Cohen EE, Karrison TG, Kocherginsky M, et al. Phase III Randomized Trial of Induction Chemotherapy in Patients with N2 or N3 Locally Advanced Head and Neck Cancer. J Clin Oncol 2014;32:2735-43. [Crossref] [PubMed]
- Haddad R, O’Neill A, Rabinowits G, et al. Induction Chemotherapy Followed by Concurrent Chemoradiotherapy (Sequential Chemoradiotherapy) versus Concurrent Chemoradiotherapy Alone in Locally Advanced Head and Neck Cancer (PARADIGM): A Randomised Phase 3 Trial. Lancet Oncol 2013;14:257-64. [Crossref] [PubMed]
- Forastiere AA, Zhang Q, Weber RS, et al. Long-Term Results of RTOG 91-11: A Comparison of Three Nonsurgical Treatment Strategies to Preserve the Larynx in Patients with Locally Advanced Larynx Cancer. J Clin Oncol 2013;31:845-52. [Crossref] [PubMed]
- Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for Recurrent Squamous- Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375:1856-67. [Crossref] [PubMed]
- Deng L, Liang H, Burnette B, et al. Irradiation and Anti-PD-L1 Treatment Synergistically Promote Antitumor Immunity in Mice. J Clin Invest 2014;124:687-95. [Crossref] [PubMed]
- Oweida A, Lennon S, Calame D, et al. Ionizing Radiation Sensitizes Tumors to PD-L1 Immune Checkpoint Blockade in Orthotopic Murine Head and Neck Squamous Cell Carcinoma. Oncoimmunology 2017;6:e1356153. [Crossref] [PubMed]
- Bonomo P, Desideri I, Loi M, et al. Anti PD-L1 DUrvalumab Combined with Cetuximab and Radiotherapy in Locally Advanced Squamous Cell Carcinoma of the Head and Neck: A Phase I/II Study (DUCRO). Clin Transl Radiat Oncol 2018;9:42-7. [Crossref] [PubMed]
- National Institutes of Health. Available online: http://clinicaltrials.gov/ct2/show/NCT02952586. Accessed: January 7 2019.
- National Institutes of Health. Available online: http://clinicaltrials.gov/ct2/show/NCT03040999. Accessed: January 7, 2019.


