
NUMB knockdown enhanced the anti-tumor role of cisplatin on ovarian cancer cells by inhibiting cell proliferation and epithelial-mesenchymal transition
Introduction
Ovarian cancer is the most fatal gynecological cancer, with a 5-year survival rate of 35% (1). Due to non-specific symptoms and the lack of effective screening methods, 70% of the patients were first diagnosed at advanced stage (2). The standard treatment of this disease is platinum-based chemotherapy and surgical removal of the tumor. Cisplatin is an anti-tumor agent that damages DNA and activates nuclear and cytoplasmic signaling, such as cell cycle, DNA damage repair, and cell apoptosis pathways (3). It is one of the most commonly used drugs for the treatment of ovarian cancer. Although ovarian cancer owns a high response rate to the initial cisplatin chemotherapy, resistance is common and eventually leads to treatment failure (4). Therefore, it is highly important to explore therapies to overcome the cisplatin resistance of ovarian cancer.
NUMB is a membrane-bound protein that has been initially recognized as a NOTCH signaling inhibitor (5). NUMB plays a crucial role in cell polarity maintenance, cell asymmetric division, cell adhesion and endocytosis during development (6,7). Six human NUMB isoforms have been identified (8,9). NUMB protein contains an amino-terminal phosphotyrosine-binding (PTB) domain, C-terminal proline-rich (PRR) and Eps15 homology regions. PRR domain contains Src homology binding sites involved in intracellular signal transduction and PTB domain is crucial for membrane localization (6). NUMB is also involved in tumor progression and acting on several signaling pathways, such as NOTCH, Hedgehog, WNT, P53 and EMT signaling pathways (10,11).
Loss of NUMB expression has been found in several human carcinomas (10,12-14). NUMB was reported to play roles in regulating sensitivity to chemotherapy in solid and hematological tumors. Kang et al. demonstrated that low expression of NUMB was associated with poor prognosis of epithelioid malignant pleural mesothelioma and overexpression of NUMB suppressed tumor cell proliferation and enhanced the sensitivity to cisplatin treatment (15). Additionally, NUMB inactivation was reported to confer resistance to imatinib in chronic myeloid leukemia cells (16). In contrast to the aforementioned studies that NUMB functions as a tumor suppressor, others indicated the oncogenic role of NUMB. Recent studies reported that NUMB expression was upregulated in human ovarian cancer (17,18). In addition, Bocci et al. showed that NUMB correlated with a worse survival in multiple independent ovarian datasets, confirming its relationship with increased cancer aggressiveness (19). Collectively, the role of NUMB in tumorigenesis is complex and varies in different contexts.
Accumulating evidence showed a crucial role of NUMB in the EMT process, which contributes to invasiveness and resistance to apoptosis and chemotherapy in multiply cancers (20). NUMB expression was reported to inhibit EMT in breast cancer via a dual mechanism: p53 activation (10) and NOTCH inhibition (21). However, NUMB expression was found to induce the EMT in the process of pulmonary fibrosis and renal fibrosis (22,23). The role of NUMB in ovarian cancer is unclear. Whether NUMB expression is involved in the sensitivity of cisplatin treatment in ovarian cancer need investigation.
In the present study, the effects of NUMB on the cell growth, apoptosis, EMT, and sensitivity to cisplatin in human ovarian cancer cells OVCAR-3 and SK-OV-3 were investigated. Our data suggest that NUMB acted as an oncogene in ovarian cancer and NUMB knockdown enhanced the anti-tumor role of cisplatin on ovarian carcinoma cells by inhibiting cell proliferation and EMT.
Methods
Reagents and antibodies
Cisplatin was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was freshly dissolved at 1 mg/mL in 0.9% NaCl and diluted in medium appropriately. Primary antibodies of NUMB, NOTCH1, E-cadherin, N-cadherin, Vimentin, Slug and Snail were purchased from Cell Signaling Technology (Danvers, MA, USA). Primary antibodies of β-actin and GAPDH and secondary antibodies of HRP-conjugated goat anti-mouse were purchased from ZSGB-BIO (Beijing, China). For Simon western blot analysis, secondary antibodies purchased from Protein Simple (San Jose, CA, USA)
Cell cultures
The human ovarian cancer cell lines (OVCAR-3 and SK-OV-3) were purchased from the American Type Culture Collection (ATCC) and were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS). All cell lines were identified by STRS (Microread Gene Technology Co., Ltd., Beijing, China).
Transfection and RNA silencing
A lentiviral vector expressing shNUMB (KD) was constructed (GeneChem Co. Ltd., Shanghai, China) to knockdown the NUMB expression. The targeting sequences of siRNA were (5'-ATACATAGCCATAATGATTGC-3') for NUMB, and (5'-UUCUCCGAACGUGUCACGUTT-3') for non-target control (NT), respectively. SiRNA were transferred into OVCAR-3 and SK-OV-3 cells with the Polybrene kit and puromycin was used to filtrate stable-infected cells.
Quantitative real-time PCR
Total RNA from the cells was extracted by using TRIzol reagent (Invitrogen, USA) according to manufacturer’s guideline. The mRNA level of NUMB was normalized to GAPDH using the 2−ΔΔCT method. Each reaction was performed in triplicate. The sequences of PCR primers are listed as follows: NUMB: forward, 5'-ACTTTTGATGCTAGTCGGACC-3'; reverse, 5'-GAAGTAGGAGAGGTGGGAGAG-3'; GAPDH: forward, 5'-ACCCAGAAGACTGTGGATGG-3'; reverse, 5'-TTCAGCTCAGGGATGACCTT-3'.
Simple Western system and traditional Western blot analysis
Simple Western blot analysis (ProteinSimple, California, USA) was performed by Simple Western system according to manufacturer’s guideline (https://www.proteinsimple.com.cn/wes.html). Briefly, the proteins were diluted to a final protein concentration of 1–3 µg/µL in a master mix containing internal fluorescent standards and DTT. The primary antibodies against N-cadherin, NOTCH1, Slug, Vimentin, E-cadherin, Snail and β-actin that diluted to 1:20–1:10 were used. Quantification of protein expression was determined automatically by the software in the system.
For traditional western blot analysis, the cells were lysed in RIPA buffer containing protease inhibitors. Forty µg of the protein lysates were electrophoretically separated on 10% SDS-PAGE gels and transferred to PVDF membranes. The membranes were blocked with 5% nonfat milk in Tris-buffered saline/0.1% Tween 20 for 1 h at room temperature and then incubated overnight at 4 °C with the primary antibodies. After incubation with the secondary antibody for 1 h at room temperature, the protein bands were detected using the ECL detection system (BD Biosciences). GAPDH was used as the loading controls.
Cell viability assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay was performed to measure the viability and IC50 value of cells after shRNA transfection or cisplatin treatment. SK-OV-3 and OVCAR-3 ovarian cancer cells (4×103) were seeded in the 96-well plate 24 h before transfection. For inhibition rate analysis, the cells were incubated with cisplatin (10 µM) and incubated for 48 h. For IC50 value analysis, 24 h after transfection, the cells were incubated with gradient concentrations of cisplatin (0.1, 1, 10, 100, 1,000 µM) and incubated for 48 h. Then the medium was added with 5 mg/mL of MTT reagent and incubated under 37 °C, 5% CO2 for 4 h. DMSO (100 µL) was added to each well and thoroughly mixed for 10 min. The optical density (OD) at 450 nm was determined by using the microplate reader. Inhibition rate = [1 − OD (KD)/OD (NT)] ×100%.
Cell cycle analysis
Cells were transfected using shNUMB or control-shRNA for 72 h, then cells were plated in 60-mm dishes with or without cisplatin (10 µM) for 48 h. After trypsinized, cells were resuspended in PI staining solution (Thermo Fisher) according to manufacturer’s protocols. The percentage of cells in each phase of the cell cycle was determined by FACS flow cytometer (BD Biosciences). Analyses were performed separately, in triplicate.
Apoptosis assay
Cells were transfected by shNUMB or control-shRNA for 72 h, and after that, cells were treated with or without cisplatin (10 µM) for 48 h. Apoptotic cells were detected by using an Annexin V-APC assay (Thermo Fisher) based on the manufacturer’s protocols. Analysis was performed by FACS flow cytometer (BD Biosciences).
Transwell assays
The Transwell migration and invasion assays were performed using 24-well plates (Corning, NY, USA) according to the manufacturer’s protocols. The quantification was performed as previously described (24).
Statistical analysis
The two-tailed Student’s t-test was performed to evaluate differences between two groups and P value <0.05 indicates statistically significance.
Results
NUMB knockdown enhanced the inhibitory effect of cisplatin on proliferation of OVCAR-3 and SK-OV-3 cells
We used lentiviral vector expressing short hairpin RNA (shRNA) to knock down the expression of NUMB (NT for non-target control and KD for shNUMB, respectively). The transfection efficiency was assessed by fluorescence microscope. As shown in Figure 1A, both OVCAR-3 and SK-OV-3 cells showed more than 90% transfection efficiency.
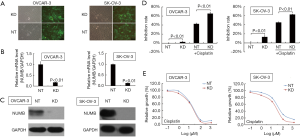
The efficiency of NUMB knockdown was also assessed on mRNA and protein levels by using qRT-PCR and Western blot analysis, respectively. As shown in Figure 1B,C, the level of NUMB mRNA and protein were decreased dramatically in OVCAR-3 and SK-OV-3 cells after shNUMB transfection.
To investigate the contribution of NUMB knockdown to cisplatin-sensitivity in ovarian cancer cells, ovarian cancer cells were transfected with NT or KD and treated with or without cisplatin. The growth inhibition rate of ovarian cancer cells was determined by MTT assays. NUMB knockdown markedly inhibited the growth of OVCAR-3 and SK-OV-3 cells (Figure 1D). In addition, with cisplatin treatment, NUMB knockdown showed higher growth inhibition rate compared to NT group in these two ovarian cancer cell lines. IC50 of cisplatin to NT group was 15.6 (95% confidence interval: 12.48–19.58) µM and IC50 of cisplatin to KD group was 16.5 (95% confidence interval: 13.41–20.41) µM in OVCAR-3 cells (P=0.545) (Figure 1E). In addition, IC50 of cisplatin to NT group was higher compared with the KD group (29.9 µM, 95% confidence interval: 22.6–33.6 µM vs. 22.0 µM, 95% confidence interval: 17.8–27.4 µM, P=0.011) in SK-OV-3 cells. Collectively, these data showed that NUMB knockdown decreased the IC50 of SK-OV-3 cells to cisplatin. However, no effects were found in OVCAR-3 cells.
NUMB knockdown increased the cisplatin-induced apoptosis in OVCAR-3 and SK-OV-3 cells
To investigate the antiproliferative mechanism of NUMB knockdown, cell cycle distributions and cell apoptosis were analyzed by FACS. Increased S populations and decreased G2/M populations suggested that cisplatin treatment induced the S arrest in both OVCAR-3 and SK-OV-3 cells (Figure 2A). However, NUMB knockdown showed no effects on cell cycle distributions in both cell lines in the absence or presence of cisplatin treatment.
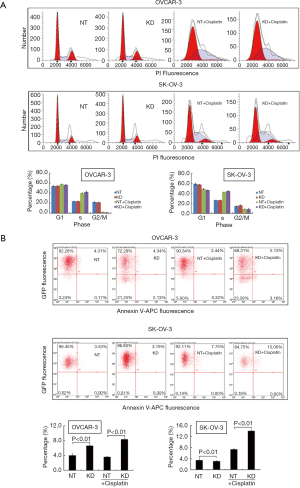
In addition, cell apoptosis analysis showed that NUMB knockdown increased the percentage of apoptosis cells compared with control group, with the percentage of 6.66% vs. 4.06% (P<0.001) in OVCAR-3 cells (Figure 2B). However, apoptosis rate was decreased after NUMB knockdown in SK-OV-3 cells, with the percentage of 3.09% vs. 3.45% (P=0.009). More importantly, in the presence of cisplatin, NUMB knockdown increased the apoptosis in both OVCAR-3 and SK-OV-3 cells, with the percentage of 8.40% vs. 3.64% (P<0.001) and 13.97% vs. 7.37% (P<0.001), respectively. These data indicated that NUMB knockdown inhibited proliferation of ovarian cancer cells by inducing apoptosis but not through cell cycle distributions.
NUMB knockdown inhibited migration and invasion of OVCAR-3 and SK-OV-3 cells
To investigate the effects of NUMB knockdown on cells migration and invasion abilities, we performed Transwell assays. As shown in Figure 3A, NUMB knockdown decreased migrated cells in both OVCAR-3 and SK-OV-3 cells, with 148 vs. 67 cells per filed (P<0.001) and 105 vs. 16 cells per field (P<0.001), respectively. However, in the presence of cisplatin, there was no cell migrated to the bottom compartment, indicating the strong inhibitory role of cisplatin on cell migration.
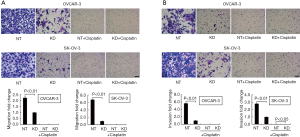
The similar pattern was obtained in Transwell invasion assay (Figure 3B), as NUMB knockdown decreased invaded cells in both OVCAR-3 and SK-OV-3 cells, with 55 vs. 10 cells per filed (P<0.001) and 121 vs. 43 cells per field (P<0.001), respectively. Moreover, in the presence of cisplatin, NUMB Knockdown further enhanced the inhibitory role of cisplatin on cell invasion in SK-OV-3 cells. Collectively, these data showed that NUMB knockdown inhibited migration and invasion of ovarian cancer cells.
NUMB knockdown decreased the expression of N-cadherin and Vimentin in SK-OV-3 cells
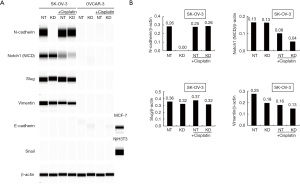
To determine whether NUMB knockdown and cisplatin treatment are associated with EMT regulation of ovarian cancer, we performed the western blot analysis to examine the expression of EMT-related markers in SK-OV-3 and OVCAR-3 cells. We found that NUMB knockdown dramatically decreased the expression of N-cadherin and Vimentin in SK-OV-3 cells, whereas it had no effects on Notch1 and Slug expression (Figure 4A,B). However, there was no detectable expression of E-cadherin and Snail in the cells. There was no detectable expression of all EMT-related markers that were tested in OVCAR-3 cells. Collectively, these data indicated that NUMB knockdown inhibited the migration and invasion of the ovarian cancer cells and enhanced the sensitivity to cisplatin of the cells through inhibiting the EMT.
Discussion
Platinum-based chemotherapy, causing DNA damage, resulting in cell cycle arrest and apoptosis, is the gold standard for the treatment of ovarian cancer (25). However, a significant proportion of tumors are resistant or obtain resistance to chemotherapy, which is considered the greatest barrier to successful treatment (26). EMT has been reported to contribute to tumor progression as well as resistance to cisplatin treatment in multiple cancers, including ovarian cancer (27,28).
NUMB is a determinant of cell fate and also a negative regulator of NOTCH signaling pathway (Figure 5A). Increasing evidence suggests that NUMB plays a crucial role in the EMT program. NUMB has been reported to interact with E-cadherin and N-cadherin to balance intracellular localization of these adhesion molecules (7,29). Additionally, NUMB suppressed E-cadherin expression and thus induced EMT in response to transforming growth factor (TGF-β1) signaling in renal fibrosis (23), while inhibited EMT by antagonizing NOTCH signaling in triple-negative breast cancer (21). Moreover, NUMB inactivation conferred resistance to imatinib in chronic myeloid leukemia cells (16) and NUMB overexpression enhanced sensitivity to cisplatin in epithelioid malignant pleural mesothelioma (15). However, the role of NUMB in the sensitivity to cisplatin in ovarian cancer has not been clarified yet.
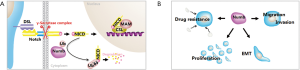
The present data showed that NUMB knockdown markedly inhibited the growth of ovarian cancer cells and enhanced the inhibitory effects of cisplatin on these cells. In addition, NUMB knockdown inhibited the abilities of migration and invasion of ovarian cancer cells. These data indicated that NUMB acted as an oncogene in ovarian cancer, which basically in accordance with the study showed that NUMB correlated with worse survival in ovarian cancer (19). However, NUMB overexpression was reported to suppress tumor cell growth in esophageal and breast cancer (14,30). NUMB protein contains an amino-terminal PTB domain, C-terminal proline-rich (PRR) and Eps15 homology regions. High expression of the NUMB PRR short (PRRS) isoform was reported to suppress NOTCH signaling, whereas high expression of PRR long (PRRL) isoform was reported to antagonize PRRS activity and increase the expression of NOTCH targeted genes (31,32). The opposite role of NUMB isoforms that played in NOTCH signaling pathway may mask relationships between NUMB and tumorigenesis. Collectively, these data were suggestive of an intimate link between NUMB and tumorigenesis but indicated that the relationship can vary widely in a context-dependent manner due to the different distribution of NUMB isoforms.
To further investigate the mechanism underlying the oncogenic role of NUMB in ovarian cancer, we performed the flow cytometry analysis and Western blot analysis. Our data indicated that cisplatin treatment induced the S arrest in ovarian cancer cells, whereas NUMB showed no effects on cell cycle distributions. Importantly, NUMB knockdown increased the apoptosis in the presence of cisplatin treatment. This finding is consistent with the studies showed that NUMB protected renal tubular cells from apoptosis (33). In addition, NUMB knockdown dramatically reduced the expressions of N-cadherin and Vimentin, which are markers of mesenchymal phenotype (34). However, E-cadherin was not detected in ovarian cancer cells. These data indicated that NUMB knockdown inhibited proliferation and enhanced the cisplatin sensitivity of ovarian cancer cells by inducing apoptosis and inhibiting EMT. Unexpectedly, NUMB knockdown had no effects on NOTCH1 expression. This may result from the combined effects of the PRRS isoform and PRRL isoform of NUMB on NOTCH signaling as mentioned above. Or NUMB exerted its role through P53 signaling rather than NOTCH signaling in ovarian cancer, which need to be further elucidated.
Conclusions
The present data demonstrated that NUMB acted as an oncogene in ovarian cancer and NUMB knockdown enhanced the anti-tumor role of cisplatin on ovarian cancer cells by inhibiting cell proliferation and EMT (Figure 5B). NUMB could be a potential target to increase the cisplatin sensitivity of ovarian cancer.
Acknowledgments
Funding: The research was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.01.35). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional Review Board approval was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kritsch D, Hoffmann F, Steinbach D, et al. Tribbles 2 mediates cisplatin sensitivity and DNA damage response in epithelial ovarian cancer. Int J Cancer 2017;141:1600-14. [Crossref] [PubMed]
- Selle F, Sevin E, Ray-Coquard I, et al. A phase II study of lenalidomide in platinum-sensitive recurrent ovarian carcinoma. Ann Oncol 2014;25:2191-6. [Crossref] [PubMed]
- Cepeda V, Fuertes M, Castilla J, et al. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem 2007;7:3-18. [Crossref] [PubMed]
- Marth C, Reimer D, Zeimet A. Front-line therapy of advanced epithelial ovarian cancer: standard treatment. Ann Oncol 2017;28:viii36-viii9.
- Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron 1996;17:27-41. [Crossref] [PubMed]
- Gulino A, Di Marcotullio L, Screpanti I. The multiple functions of Numb. Exp Cell Res 2010;316:900-6. [Crossref] [PubMed]
- Wang Z, Sandiford S, Wu C, et al. Numb regulates cell-cell adhesion and polarity in response to tyrosine kinase signalling. EMBO J 2009;28:2360-73. [Crossref] [PubMed]
- Verdi JM, Schmandt R, Bashirullah A, et al. Mammalian NUMB is an evolutionarily conserved signaling adapter protein that specifies cell fate. Curr Biol 1996;6:1134-45. [Crossref] [PubMed]
- Karaczyn A, Bani-Yaghoub M, Tremblay R, et al. Two novel human NUMB isoforms provide a potential link between development and cancer. Neural Dev 2010;5:31. [Crossref] [PubMed]
- Colaluca IN, Tosoni D, Nuciforo P, et al. NUMB controls p53 tumour suppressor activity. Nature 2008;451:76-80. [Crossref] [PubMed]
- Wang Z, Li Y, Kong D, et al. The role of Notch signaling pathway in epithelial-mesenchymal transition (EMT) during development and tumor aggressiveness. Curr Drug Targets 2010;11:745-51. [Crossref] [PubMed]
- Maiorano E, Favia G, Pece S, et al. Prognostic implications of NUMB immunoreactivity in salivary gland carcinomas. Int J Immunopathol Pharmacol 2007;20:779-89. [Crossref] [PubMed]
- Westhoff B, Colaluca I, D'Ario G, et al. Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci U S A 2009;106:22293-8. [Crossref] [PubMed]
- Hong J, Liu Z, Zhu H, et al. The tumor suppressive role of NUMB isoform 1 in esophageal squamous cell carcinoma. Oncotarget 2014;5:5602-14. [Crossref] [PubMed]
- Kang Y, Ding M, Tian G, et al. Overexpression of Numb suppresses tumor cell growth and enhances sensitivity to cisplatin in epithelioid malignant pleural mesothelioma. Oncol Rep 2013;30:313-9. [Crossref] [PubMed]
- García-Alegría E, Lafita-Navarro M, Aguado R, et al. NUMB inactivation confers resistance to imatinib in chronic myeloid leukemia cells. Cancer Lett 2016;375:92-9. [Crossref] [PubMed]
- Xu M, He HJ, Zhang H, et al. Expression and Clinical Significance of Numb and P53 Proteins in Epithelial Ovarian Carcinoma. Sichuan Da Xue Xue Bao Yi Xue Ban 2017;48:76-80. [PubMed]
- Jing H, Liu XY, Chen YL, et al. Expression Level of Membrane-associated Proteins Numb in Epithelial Ovarian Carcinoma and Its Relationship with Ovarian Cancer Stem Cell Markers CD117, CD133, ALDH1. Sichuan Da Xue Xue Bao Yi Xue Ban 2016;47:878-82. [PubMed]
- Bocci F, Jolly MK, Tripathi SC, et al. Numb prevents a complete epithelial-mesenchymal transition by modulating Notch signalling. J R Soc Interface 2017;14: [Crossref] [PubMed]
- Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010;29:4741-51. [Crossref] [PubMed]
- Zhang J, Shao X, Sun H, et al. NUMB negatively regulates the epithelial-mesenchymal transition of triple-negative breast cancer by antagonizing Notch signaling. Oncotarget 2016;7:61036-53. [PubMed]
- Zhang Z, Qu J, Zheng C, et al. Nrf2 antioxidant pathway suppresses Numb-mediated epithelial-mesenchymal transition during pulmonary fibrosis. Cell Death Dis 2018;9:83. [Crossref] [PubMed]
- Ding X, Ma M, Teng J, et al. Numb induces e-cadherin adhesion dissolution, cytoskeleton reorganization, and migration in tubular epithelial cells contributing to renal fibrosis. Curr Mol Med 2015;15:368-79. [Crossref] [PubMed]
- Liu Z, Qi S, Zhao X, et al. Metformin inhibits 17beta-estradiol-induced epithelial-to-mesenchymal transition via betaKlotho-related ERK1/2 signaling and AMPKalpha signaling in endometrial adenocarcinoma cells. Oncotarget 2016;7:21315-31. [PubMed]
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 2005;4:307-20. [Crossref] [PubMed]
- Borley J, Brown R. Epigenetic mechanisms and therapeutic targets of chemotherapy resistance in epithelial ovarian cancer. Ann Med 2015;47:359-69. [Crossref] [PubMed]
- Bugide S, Gonugunta V, Penugurti V, et al. HPIP promotes epithelial-mesenchymal transition and cisplatin resistance in ovarian cancer cells through PI3K/AKT pathway activation. Cell Oncol (Dordr) 2017;40:133-44. [Crossref] [PubMed]
- Haslehurst AM, Koti M, Dharsee M, et al. EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer 2012;12:91. [Crossref] [PubMed]
- Rasin MR, Gazula V, Breunig J, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci 2007;10:819-27. [Crossref] [PubMed]
- Tosoni D, Pambianco S, Ekalle Soppo B, et al. Pre-clinical validation of a selective anti-cancer stem cell therapy for Numb-deficient human breast cancers. EMBO Mol Med 2017;9:655-71. [Crossref] [PubMed]
- Bani-Yaghoub M, Kubu C, Cowling R, et al. A switch in numb isoforms is a critical step in cortical development. Dev Dyn 2007;236:696-705. [Crossref] [PubMed]
- Misquitta-Ali CM, Cheng E, O'Hanlon D, et al. Global profiling and molecular characterization of alternative splicing events misregulated in lung cancer. Mol Cell Biol 2011;31:138-50. [Crossref] [PubMed]
- Ding X, Ma M, Teng J, et al. Numb Protects Human Renal Tubular Epithelial Cells From Bovine Serum Albumin-Induced Apoptosis Through Antagonizing CHOP/PERK Pathway. J Cell Biochem 2016;117:163-71. [Crossref] [PubMed]
- Liu Z, Zhang H, Ding S, et al. βKlotho inhibits androgen/androgen receptor-associated epithelial-mesenchymal transition in prostate cancer through inactivation of ERK1/2 signaling. Oncol Rep 2018;40:217-25. [PubMed]


