
Solamargine inhibits proliferation and promotes apoptosis of CM-319 human chordoma cells through suppression of notch pathway
Introduction
Chordoma, which is a rare clinical malignant bone tumor, has an incidence of less than one in a million, accounting for 1–4% of all primary bone tumors (1,2). Chordoma acts as the most common primary malignant tumor of the spine, except for the plasma cell tumor that often occurs in the sacrum (50%), skull base (35%) and spine (15%) (3). At present, the rest of notochord cells during embryonic development stage are considered as the source of chordoma so that chordoma cells have double manifestations of epithelial mesenchymal cells in many aspects (4). The main clinical manifestations of chordoma appear as local pain and nerve compression symptoms. In general, the development of chordoma is relatively slow and limited, compared with other malignant bone tumors (5). Though the surgical resection remains a preferred treatment of chordoma up to now, several studies also have shown that rational use of effective adjunctive methods could partially improve the survival rate of chordoma patients (6-8). Nevertheless, studies are hardly conducted on the inhibitory effect of chemotherapeutic drugs on the proliferation of chordoma cells (9,10).
Notch gene was first reported by Morgan in 1917 and has been successfully cloned in the mid-1980s through investigating the mutant type of fruit flies (11). There are four mature single transmembrane receptors including Notch1, Notch2, Notch3 and Notch 4 (12). Overexpression of Notch1 has been demonstrated to induce the rat mammary tumor generation, while studies also found that Notch1 strongly inhibited the growth of human cervical cancer cells and mice skin tumor cells (13,14). Notch signaling pathway is widely presented in embryos and adult individual tissues, and it plays an important role in the embryonic development and steady-state maintenance of adult tissues (15). It has been proved that the activation of Notch pathway could suppress the proliferation of tumor cells including prostate cancer, lung cancer, brain tumor and hepatocellular carcinoma (16-18). However, there are few reports considering the effects of Notch pathway in chordoma cells.
Solamargine (SM), which serves as a natural steroid alkaloid glycoside compound and cytotoxic agent, is isolated from the Chinese herb Solanum species. Several studies reported that SM had significant in vitro toxicity effects on cancer cells (19-21). For example, Munari et al. reported that SM had pronounced anti-proliferative activities with IC50 values ranging from 4.58 to 18.23 µg/mL. Especially, the IC50 of HepG2 was 4.58 µg/mL treated by SM (22). It has been reported that SM strengthened the human epidermal growth factor receptor 2 (HER2) expression, regulated the expression levels of factor associated suicide (Fas) and enhanced the sensitivity of lung cancer cells to trastuzumab and epirubicin (23,24). Moreover, the inhibitory effects of SM on lung cancer cells were possibly realized through affecting signal transducer and activator of transcription stat 3 (stat3) pathway (25). In the leukemia cells, SM was found to activate the lysosome-mitochondria pathway (26). However, the pharmacological activity and mechanism of SM in the proliferation and apoptosis of chordoma cells have not been studied yet.
In the present study, we investigated whether SM could serve as a novel therapeutic agent affected the growth and apoptosis of CM-319 cells. Furthermore, it was also fascinating to explore the crucial role of Notch pathway in the pathomechanism of chordoma.
Methods
Reagents and cells
The products utilized in cell culture were purchased from Gibco (Carlsbad, CA, USA). Antibodies were obtained from Abcam (Camb, UK). The CM-319 human chordoma cell line was obtained from the Cell bank of Chinese Academy of Sciences. The cells were cultured at 37 °C with 5% CO2 in RPMI-1640 medium (GENOM, Hangzhou, China) with 0.3 μL 2-mercaptoethanol and 10% fetal bovine serum (Thermo Fisher, USA) (27). CM-319 cell was a polygonal cell and expressed CD24, which was traditionally used as diagnostic markers of chordoma (28). The cell line was first derived from sacral chordoma (27).
Cell viability analysis
Cell counting kit-8 (CCK-8; Shanghai Beyotime Biotechnology Co., Ltd., Shanghai, China) assay was carried out to measure the cell viability. About 6×104 cells/ml of CM-319 cells in the logarithmic phase were seeded in the wells of 96-well plates and maintained in an incubator (at 37 °C, 5% CO2) for 12 h. After that, CM-319 cells were treated with different concentrations of SM (0, 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 µM) for 12, 24 and 48 h. Cells were then maintained in the incubator (37 °C, 5% CO2) for 12 h. Next, the cells were supplemented with 10 µL CCK reagent in each well, followed by being put in an incubator with 5% CO2 at 37 °C for 3 h. The absorbance at 450 nm was read by an enzyme labeling instrument (Bio-Rad Laboratories, Inc., USA). Cell viability was determined in terms of the proportion of cell survival, compared with control.
Apoptosis assay
Flow cytometry (FCM) was performed to determine cell apoptosis. CM-319 cells were harvested 48 hours after transfection. Then the cells were suspended in an incubation buffer at a density of 1×106 cells/mL. After that, the cells were incubated with Annexin V-FITC and propidium iodide (PI) at room temperature in the dark for 15 min. The proportion of apoptosis cell number was quantified by FACSCalibur (BD Biosciences, San Diego, CA, USA).
5(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling
CM-319 cells were suspended in phosphate buffered solution (PBS) at a final concentration of 1×106 cells/ml and 5 µM CFSE (Thermofisher, New York, USA) was supplemented into the cells. The cells were treated with 10 volumes of ice-cold dulbecco’s modified eagle medium (DMEM; Gibco) containing 10% fetal calf serum (FCS; Gibco) after incubation at 37 °C for 10 min. Next, the cells were maintained on ice for 5 min and then centrifuged for 1 min. After centrifugation, the cells were washed twice by culture medium and sowed into 24-well plates (about 1×105 cells per well). The cells were harvested and then treated with 0, 25, 50 and 100 µM SM, respectively.
Cell cycle analysis
After being digested by 0.25% trypsin (Beyotime, Shanghai, China), cells were seeded in the wells of 6-well plates at a final density of 1×107 cells/well and maintained for 24 h in an incubator (at 37 °C, 5% CO2). Four treatment groups were performed on the cells in 6-well plates and the cells were then incubated for 48 h. The cells were re-suspended after being washed by PBS and then centrifuged for 5 min at 1,000 rpm/min and the supernate was discarded. Pre-cooling 70% ethanol (1 mL) was added into the cells, which were then blown gently. After that, the cells were saved in a refrigerator at 4 °C overnight. After centrifugation, the ethanol was discarded and the cells were washed thrice by PBS. 500 µL PBS containing PI (50 µg/mL), RNase A (100 µg/mL) and Triton X-100 (0.2%) was added into the cells, which were then dyed at dark for 30 min. Cell cycle were measured by FCM.
Quantitative real-time reverse transcription PCR (qRT-PCR) analysis
The total RNA was extracted from CM-319 cells by TRIzol (Thermo Fisher Scientific Inc, New York, USA) following the instructions of manufacturers. Afterwards, two microliters of RNA were used for the cDNA synthesis with a first strand cDNA kit (Sigma, Munich, Germany) following the specification. SYBR Green Premix Reagent (TakaraBio Inc., Shiga, Japan) was utilized. qRT-PCR assay was carried out using ABI 7500 Thermocycler (Applied Biosystems, Foster City, CA, USA). The PCR cycles were under the following conditions: 10 min pretreatment at 94 °C, 95 °C for 5 s, 65 °C for 30 s (35 cycles), 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s, a final extension at 72 °C for 10 min and held at 4 °C. The primers were designed by Shanghai Sangon Biotech Co., Ltd. (Shanghai, China) and were as follows: Caspase-3, forward: 5'-GGCGCTCTGGTTTTCGTTAATA-3' and reverse: 5'-GCTGCATCGACATCTGTACC-3' (product: 246 bp); Caspase-8, forward: 5'-CTGGTCTGAAGGCTGGTTGT-3' and reverse: 5'-CAGGCTCAGGAACTTGAGGG-3' (product: 275 bp); Caspase-9, forward: 5'-CTGGCTTCGTTTCTGCGAAC-3' and reverse: 5'-GCACAGGGACCCACGTAAA-3' (product: 253 bp); Ki67, forward: 5'-CTGACCCTGATGAGAGTGAGGGA-3' and reverse: 5'-ACTCTGTAGGGTCGAGCAGG-3' (product: 736 bp); Cyclin D1, forward: 5'-TGAGGGACGCTTTGTCTGTC-3' and reverse: 5'-CTTCTGCTGGAAACATGCCG-3' (product: 223 bp); DLL1, forward: 5'-CACCGCTATGTGTGCGAGTG-3' and reverse: 5'-TTGGCCAGGTTGTTCATGGT-3' (product: 284 bp); DLL3, forward: 5'-CAACCTAAGGACGCAGGAGG-3' and reverse: 5'-CACGGACAGAATCGAGGAAGG-3' (product: 217 bp); Notch1, forward: 5'-CCACCCCTCCTAGTTTGGGA-3' and reverse: 5'-TGGCATGACACACAACAGAC-3' (product: 140 bp); HES1, forward: 5'-GGCTAAGGTGTTTGGAGGCT-3' and reverse: 5'-GGTGGGTTGGGGAGTTTAGG-3' (product: 250 bp); glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward: 5'- AATGGGCAGCCGTTAGGAAA-3' and reverse: 5'-GCGCCCAATACGACCAAATC-3' (product: 168 bp). GAPDH was used as the control for the input RNA level.
Western blot analysis
Cell lysates were lysed by RIPA buffer (Sigma-Aldrich, USA) and moved into tubes for later use. After being washed with PBS twice, the proteins from cells were extracted. Proteins were partitioned on a sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Next, the cells were moved to a PVDF membrane (Millipore, Billerica, MA, USA). Blots were carried out with anti-Caspase-3, dilution, 1:500, Abcam, ab13585; anti-Caspase-8, dilution, 1:1,000, Abcam, ab32397; anti-Caspase-9, dilution, 1:2,000, Abcam, ab202068; anti-Ki67, dilution, 1:5,000, Abcam, ab92742; anti-Cyclin D1, dilution, 1:10,000, Abcam, ab134175; anti-DLL1, dilution, 1:500, Abcam, ab10554; anti-DLL3, dilution, 1:500, Abcam, ab63707; anti-Notch1, dilution, 1:500, Abcam, ab65297; anti-HES1, dilution, 1:500, Abcam, ab108937; anti-GAPDH, dilution, 1:10,000, Abcam, ab181602. After that, horseradish peroxidase-conjugated secondary antibodies (bs-0293M; BIOSS, Beijing, China) were added to the cells and incubated at room temperature for 1 h. The bolts were analyzed by chemiluminescent reagents (Millipore, Billerica, MA, USA).
Statistical analysis
The results in our study were shown as mean ± SEM of at least three independent experiments. Data were analyzed by Student’s t-test, or one-way analysis of variance (ANOVA). The statistical significance was defined as P<0.05.
Results
SM distinctly suppressed the cell viability and proliferation of CM-319 cells
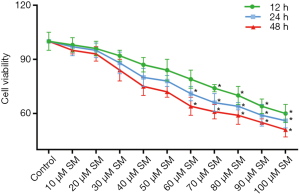
Previous study has already used CM-319 cell lines to carry out several experiments on the resistance to chemotherapy and radiotherapy in chordoma research. Therefore, CM-319 cell lines were also selected to perform this experiment (27,29). CCK-8 assay results revealed that the cell activity of CM-319 cells treated with 60, 70, 80, 90 and 100 µM SM was distinctly lower than that in control (Figure 1; P<0.05). Based on the processing time, we found that the trends of cell viability of the cells with 48 h of treatment were more obvious than those with 12 and 24 h. Moreover, as shown in CFSE results, the cell proliferation assessment of CM-319 cells treated with different concentrations of SM was obviously lower than that in control, especially with the treatment of 100 µM (Figure 2; P<0.01). These outcomes revealed that SM could reduce the cell activity and proliferation capacity of CM-319 cells. All together, we confirmed that SM led to a significant low cell viability and proliferation capacity of CM-319 cells in a dose-dependent manner.
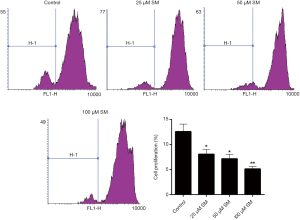
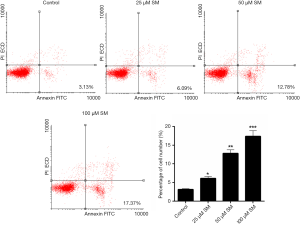
SM significantly induced the CM-319 cells apoptosis
As FCM data shown in Figure 3, the proportion of apoptosis cell number in control was 3.13%. However, after being treated with SM in 25, 50 and 100 µM, the apoptosis rate of CM-319 cells was enhanced to 6.09%, 12.78% and 17.37%, respectively. The percentages of apoptosis cell number in SM treatment groups were significantly higher than those in control (25 µM, P<0.05; 50 µM, P<0.01; 100 µM, P<0.001), indicating that SM enhanced the apoptosis capacity of CM-319 cells.
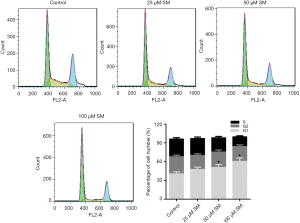
The cell cycle of CM-319 cells was blocked by SM in G1 phase
We evaluated the cell cycle of CM-319 treated with different concentrations of SM, and the results were revealed in Figure 4. We found that the G1 phase of CM-319 cells in control group was 40.78%. After treating with different concentrations of SM, the G1 phase of CM-319 cells increased from 47.89% to 60.75%. However, the G2 and S phases of CM-319 cells in four groups had no significant difference. Altogether, it was indicated that SM markedly increased the G1 phase of CM-319 cells in a dose-dependent manner (P<0.05). Therefore, we could draw a conclusion that the cell cycle of CM-319 cells was blocked by SM in G1 phase.
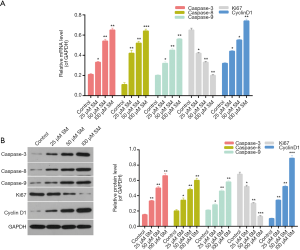
The expression levels of proliferation-, apoptosis-, and cell cycle-associated genes and proteins in CM-319 cells treated by SM
To investigate the mechanism of SM affecting the proliferation, apoptosis and cell cycle of CM-319 cells, we therefore measured the expression levels of Caspase-3/8/9, Ki67 and Cyclin D1 in CM-319 cells from each group. According to the qRT-PCR results, we found that the expression levels of Caspase-3, Caspase-8 and Caspase-9 in CM-319 cells were significantly enhanced by treating the cells with different concentrations of SM (Figure 5A; 50 µM, P<0.01). However, compared with control, a sharp decrease in Ki67 expression in CM-319 cells treated with different concentrations of SM was observed (Figure 5A; 25 µM, P<0.05; 50 and 100 µM, P<0.01). The expression level of Cyclin D1 in CM-319 cells was found to be distinctly strengthened by treating the cells with SM (Figure 5A; 25 and 50 µM, P<0.05; 100 µM, P<0.01). Moreover, western blot data also revealed the similar outcomes. It was indicated that SM markedly up-regulated the expression levels of Caspase-3, Caspase-8, Caspase-9 and Cyclin D1, while down-regulating the Ki67 expression in CM-319 cells (Figure 5B; 50 µM, P<0.01). These conclusions suggested that SM affected the proliferation, apoptosis and cell cycle of CM-319 cells through regulating the expression levels of Caspase-3/8/9, Ki67 and Cyclin D1 in CM-319 cells.
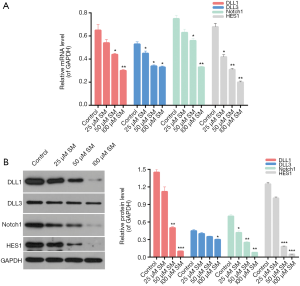
SM down-regulated the Notch pathway in CM-319 cells
We assessed the level of Notch pathway in CM-319 cells treated with different concentrations of SM, and the expression levels of delta-like 1 (DLL1), DLL3, Notch1 and hairy and enhancer of split-1 (HES1) were measured in our study. The qRT-PCR data revealed that the expression levels of DLL1 (50 µM, P<0.05; 100 µM, P<0.01), DLL3 (P<0.05), Notch1 (50 µM, P<0.05; 100 µM, P<0.01) and HES1 (25 µM, P<0.05; 50 and 100 µM, P<0.01) in CM-319 cells treated with different concentrations of SM were significantly lower than those in control (Figure 6A). These results indicated that SM could reduce the DLL1 (50 µM, P<0.01; 100 µM, P<0.001), DLL3 (100 µM, P<0.05), Notch1 (25 µM, P<0.05; 50 and 100 µM, P<0.01) and HES1 (50 and 100 µM, P<0.001) expressions in CM-319 cells. Additionally, compared with control, the protein expressions of DLL1, DLL3, Notch1 and HES1 in CM-319 cells were markedly reduced by treating the cells with different concentrations of SM (Figure 6B). Therefore, we confirmed that SM could significantly down-regulate the expression level of Notch pathway in CM-319 cells. Altogether, it was reasonable to conclude that SM suppressed the proliferation and promoted the apoptosis of CM-319 cells via down-regulating the Notch pathway in CM-319 cells.
Discussion
On account of the low sensitivity of chordoma in radiotherapy and chemotherapy, surgical resection is still the preferred method for treating chordoma (30). However, the local recurrence of chordoma remains a major problem in the treatment of chordoma (31). Chondrosarcoma and malignant fibrous histiocytoma structure can often be found in the tissues from frequentative recurrence of chordoma, indicating that the recurrence of chordoma caused enhanced malignancy and poor prognosis (2). Due to the high recurrence rate of chordoma and its insensitivity to conventional radiotherapy and chemotherapy, drug and molecular targeted therapies are particularly important in treating chordoma.
SM is a steroidal glycoalkaloid separated from the Chinese herb Solanum. As a main active component or a preparation, SM is helpful for treating several diseases including keratosis, basal cell carcinomas and squamous cell carcinomas (32,33). Yang et al. reported that Solanum incanum extract (SR-T100) may be effectively and safely treated for keratosis through a phase III randomized double-blind vehicle-controlled parallel trial (34). Cisplatin can be toxic to a wide variety of cancers by inducing apoptosis via lethal DNA damage, however, it is associated with several mechanisms including intrinsic and acquired resistance. Moreover, high concentration of Cisplatin produces resistance to breast and lung cancer cells, and SM could enhance the cell death of several cell lines of breast and lung cancers in the presence of Cisplatin (35,36). In addition, SM not only displayed a significant cytotoxicity in the multidrug resistance (MDR) sublines, but also could inhibit the expressions of MDR-related genes including P-gp (37,38). It has been demonstrated that SM possessed the function of inhibiting the migration and invasion of human hepatocellular carcinoma cells (39). Furthermore, studies have reported that SM suppressed the growth of human tumor cells, involving colon cancer cells, prostate cancer cells, breast cancer cells and hepatoma cells (32,40-42). Nevertheless, the pharmacological activity and mechanism of SM in the growth and apoptosis of chordoma cells have not been investigated yet. Thus, we set out to explore whether SM positively affected chordoma cell proliferation and apoptosis. Firstly, we assessed the cell viability of CM-319 human chordoma cells treated with different concentrations of SM. It was confirmed that SM significantly reduced the cell activity of CM-319 cells, indicating that SM might possess the antitumor effects on chordoma. Secondly, we evaluated the anti-proliferation capacity of SM on CM-319 cells, and the results showed that SM distinctly suppressed the proliferation of CM-319 cells in a dose-dependent manner. In addition, we found that SM could significantly enhance the apoptosis ability of CM-319 cells, and it was revealed that SM arrested the cell cycle of CM-319 cells in the G1 phase. These results suggested that SM might serve as a novel therapeutic agent targeting the CM-319 cells. Furthermore, we investigated the mechanisms of SM affecting the proliferation, apoptosis and cell cycle of CM-319 cells. As shown in the results, SM significantly enhanced the expression levels of Caspase-3, Caspase-8, Caspase-9 and Cyclin D1, while down-regulating the Ki67 expression in CM-319 cells. These results further confirmed that SM suppressed the proliferation, induced the apoptosis, and affected the cell cycle of CM-319 through regulating the apoptosis- and cell cycle-associated genes and proteins.
Notch pathway, which is a signaling pathway conserved in evolution, participates in various physiological and pathological processes and plays an important role in cell proliferation, differentiation and apoptosis (43). In the development and progression of tumors, Notch pathway also exerts important effects (44). Nevertheless, Notch pathway plays different roles in different tumors. On one hand, Notch pathway acts as cancer suppressor gene, on the other hand, Notch pathway represents as oncogene (45). Therefore, it is necessary to determine the exact role of Notch pathway in the tumor before blocking or activating. In the current study, we envisioned whether SM affected the proliferation and apoptosis of chordoma cells via interposing the Notch signaling pathway. DLL1 and DLL3 acted as ligands of Notch (46), and HES1 was reported as a down-stream target of Notch (47). Previous researches have suggested that regulating the expression levels of these factors in Notch pathway is related to the proliferation or apoptosis of tumor cells (48-50). Nevertheless, few studies were conducted on the regulation of Notch pathway by SM in chordoma cells. In our study, we found that SM markedly reduced the expression levels of DLL1, DLL3, Notch1 and HES1 in CM-319 cells. It was indicated that SM might regulate the Notch pathway in CM-319, thereby affecting the proliferation and apoptosis of CM-319 cells.
Recently, Cornelius et al. reported photodynamic adjuvant therapy of chordoma using in vitro experiments (51). We believe that SM in combination with photodynamic therapy could be more useful in treatment of chordoma. Base on the results of in vitro experiments, the prospect of clinical application of SM is possible. However, human body is a complex environment, in vivo model is required to further study the effects of SM on chordoma and we are planning to take a related in vivo experiment.
Altogether, it was demonstrated that SM reduced the cell viability of CM-319 cells. SM inhibited the proliferation and induced the apoptosis of CM-319 cells through blocking the cell cycle of CM-319 cells in G1 phase and regulating the expression levels of Caspase-3/8/9 and Ki67. Furthermore, SM suppressed the Notch pathway in CM-319 cells.
Conclusions
In summary, we confirmed that SM suppressed the proliferation and enhanced the apoptosis ability of CM-319 cells via down-regulating the Notch pathway. These results suggested that SM might be a novel therapeutic agent and supported the utilization of SM in chordoma.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.03.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43-66. [Crossref] [PubMed]
- McMaster ML, Goldstein AM, Bromley CM, et al. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control 2001;12:1-11. [Crossref] [PubMed]
- Chugh R, Tawbi H, Lucas DR, et al. Chordoma: the nonsarcoma primary bone tumor. Oncologist 2007;12:1344-50. [Crossref] [PubMed]
- Van Gompel JJ, Janus JR. Chordoma and chondrosarcoma. Otolaryngol Clin North Am 2015;48:501-14. [Crossref] [PubMed]
- Yakkioui Y, van Overbeeke JJ, Santegoeds R, et al. Chordoma: the entity. Biochim Biophys Acta 2014;1846:655-69. [PubMed]
- Blanco Kiely JP, White BM. Dosimetric feasibility of single-energy proton modulated arc therapy for treatment of chordoma at the skull base. Acta Oncol 2016;55:1243-5. [Crossref] [PubMed]
- Scheipl S, Barnard M, Cottone L, et al. EGFR inhibitors identified as a potential treatment for chordoma in a focused compound screen. J Pathol 2016;239:320-34. [Crossref] [PubMed]
- Uhl M, Mattke M, Welzel T, et al. Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: first long-term results. Cancer 2014;120:3410-7. [Crossref] [PubMed]
- Gunes M, Gunaldi O, Tugcu B, et al. Intracranial chondrosarcoma: a case report and review of the literature. Minim Invasive Neurosurg 2009;52:238-41. [Crossref] [PubMed]
- Launay SG, Chetaille B, Medina F, et al. Efficacy of epidermal growth factor receptor targeting in advanced chordoma: case report and literature review. BMC Cancer 2011;11:423. [Crossref] [PubMed]
- Wharton KA, Johansen KM, Xu T, et al. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell 1985;43:567-81. [Crossref] [PubMed]
- Blaumueller CM, Qi H, Zagouras P, et al. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell 1997;90:281-91. [Crossref] [PubMed]
- Nicolas M, Wolfer A, Raj K, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet 2003;33:416-21. [Crossref] [PubMed]
- Talora C, Sgroi DC, Crum CP, et al. Specific down-modulation of Notch1 signaling in cervical cancer cells is required for sustained HPV-E6/E7 expression and late steps of malignant transformation. Genes Dev 2002;16:2252-63. [Crossref] [PubMed]
- Dumortier A, Wilson A, MacDonald HR, et al. Paradigms of notch signaling in mammals. Int J Hematol 2005;82:277-84. [Crossref] [PubMed]
- Croquelois A, Blindenbacher A, Terracciano L, et al. Inducible inactivation of Notch1 causes nodular regenerative hyperplasia in mice. Hepatology 2005;41:487-96. [Crossref] [PubMed]
- Shou J, Ross S, Koeppen H, et al. Dynamics of notch expression during murine prostate development and tumorigenesis. Cancer Res 2001;61:7291-7. [PubMed]
- Sriuranpong V, Borges MW, Ravi RK, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res 2001;61:3200-5. [PubMed]
- Sun L, Zhao Y, Yuan H, et al. Solamargine, a steroidal alkaloid glycoside, induces oncosis in human K562 leukemia and squamous cell carcinoma KB cells. Cancer Chemother Pharmacol 2011;67:813-21. [Crossref] [PubMed]
- Chen Y, Tang Q, Wu J, et al. Inactivation of PI3-K/Akt and reduction of SP1 and p65 expression increase the effect of solamargine on suppressing EP4 expression in human lung cancer cells. J Exp Clin Cancer Res 2015;34:154. [Crossref] [PubMed]
- Xie X, Zhu H, Yang H, et al. Solamargine triggers hepatoma cell death through apoptosis. Oncol Lett 2015;10:168-74. [Crossref] [PubMed]
- Munari CC, de Oliveira PF, Campos JC, et al. Antiproliferative activity of Solanum lycocarpum alkaloidic extract and their constituents, solamargine and solasonine, in tumor cell lines. J Nat Med 2014;68:236-41. [Crossref] [PubMed]
- Liang CH, Shiu LY, Chang LC, et al. Solamargine enhances HER2 expression and increases the susceptibility of human lung cancer H661 and H69 cells to trastuzumab and epirubicin. Chem Res Toxicol 2008;21:393-9. [Crossref] [PubMed]
- Liang CH, Shiu LY, Chang LC, et al. Solamargine upregulation of Fas, downregulation of HER2, and enhancement of cytotoxicity using epirubicin in NSCLC cells. Mol Nutr Food Res 2007;51:999-1005. [Crossref] [PubMed]
- Zhou Y, Tang Q, Zhao S, et al. Targeting signal transducer and activator of transcription 3 contributes to the solamargine-inhibited growth and -induced apoptosis of human lung cancer cells. Tumour Biol 2014;35:8169-78. [Crossref] [PubMed]
- Sun L, Zhao Y, Li X, et al. A lysosomal-mitochondrial death pathway is induced by solamargine in human K562 leukemia cells. Toxicol In Vitro 2010;24:1504-11. [Crossref] [PubMed]
- Zhang DZ, Ma BA, Fan QY, et al. Establishment and characteristics of a human chordoma cell line. Zhonghua Zhong Liu Za Zhi 2003;25:234-7. [PubMed]
- Oakley GJ, Fuhrer K, Seethala RR. Brachyury, SOX-9, and podoplanin, new markers in the skull base chordoma vs chondrosarcoma differential: a tissue microarray-based comparative analysis. Mod Pathol 2008;21:1461-9. [Crossref] [PubMed]
- Ji Z, Long H, Hu Y, et al. Expression of MDR1, HIF-1alpha and MRP1 in sacral chordoma and chordoma cell line CM-319. J Exp Clin Cancer Res 2010;29:158. [Crossref] [PubMed]
- Williams BJ, Raper DM, Godbout E, et al. Diagnosis and treatment of chordoma. J Natl Compr Canc Netw 2013;11:726-31. [Crossref] [PubMed]
- Varga PP, Szoverfi Z, Fisher CG, et al. Surgical treatment of sacral chordoma: prognostic variables for local recurrence and overall survival. Eur Spine J 2015;24:1092-101. [Crossref] [PubMed]
- Ding X, Zhu FS, Li M, et al. Induction of apoptosis in human hepatoma SMMC-7721 cells by solamargine from Solanum nigrum L. J Ethnopharmacol 2012;139:599-604. [Crossref] [PubMed]
- Cham BE, Daunter B, Evans RA. Topical treatment of malignant and premalignant skin lesions by very low concentrations of a standard mixture (BEC) of solasodine glycosides. Cancer Lett 1991;59:183-92. [Crossref] [PubMed]
- Yang CC, Wong TW, Lee CH, et al. Efficacy and safety of topical SR-T100 gel in treating actinic keratosis in Taiwan: A Phase III randomized double-blind vehicle-controlled parallel trial. J Dermatol Sci 2018;90:295-302. [Crossref] [PubMed]
- He J, Yu JJ, Xu Q, et al. Downregulation of ATG14 by EGR1-MIR152 sensitizes ovarian cancer cells to cisplatin-induced apoptosis by inhibiting cyto-protective autophagy. Autophagy 2015;11:373-84. [Crossref] [PubMed]
- Liu LF, Liang CH, Shiu LY, et al. Action of solamargine on human lung cancer cells--enhancement of the susceptibility of cancer cells to TNFs. FEBS Lett 2004;577:67-74. [Crossref] [PubMed]
- Li X, Zhao Y, Ji M, et al. Induction of actin disruption and downregulation of P-glycoprotein expression by solamargine in multidrug-resistant K562/A02 cells. Chin Med J (Engl) 2011;124:2038-44. [PubMed]
- Fu D, Roufogalis BD. Actin disruption inhibits endosomal traffic of P-glycoprotein-EGFP and resistance to daunorubicin accumulation. Am J Physiol Cell Physiol 2007;292:C1543-52. [Crossref] [PubMed]
- Sani IK, Marashi SH, Kalalinia F. Solamargine inhibits migration and invasion of human hepatocellular carcinoma cells through down-regulation of matrix metalloproteinases 2 and 9 expression and activity. Toxicol In Vitro 2015;29:893-900. [Crossref] [PubMed]
- Kalalinia F, Karimi-Sani I. Anticancer Properties of Solamargine: A Systematic Review. Phytother Res 2017;31:858-70. [Crossref] [PubMed]
- Kuo KW, Hsu SH, Li YP, et al. Anticancer activity evaluation of the solanum glycoalkaloid solamargine. Triggering apoptosis in human hepatoma cells. Biochem Pharmacol 2000;60:1865-73. [Crossref] [PubMed]
- Shiu LY, Chang LC, Liang CH, et al. Solamargine induces apoptosis and sensitizes breast cancer cells to cisplatin. Food Chem Toxicol 2007;45:2155-64. [Crossref] [PubMed]
- Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol 2000;228:151-65. [Crossref] [PubMed]
- Maillard I, Pear WS. Notch and cancer: best to avoid the ups and downs. Cancer Cell 2003;3:203-5. [Crossref] [PubMed]
- Sjolund J, Manetopoulos C, Stockhausen MT, et al. The Notch pathway in cancer: differentiation gone awry. Eur J Cancer 2005;41:2620-9. [Crossref] [PubMed]
- Geffers I, Serth K, Chapman G, et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J Cell Biol 2007;178:465-76. [Crossref] [PubMed]
- Ohtsuka T, Ishibashi M, Gradwohl G, et al. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J 1999;18:2196-207. [Crossref] [PubMed]
- Garzia L, Andolfo I, Cusanelli E, et al. MicroRNA-199b-5p impairs cancer stem cells through negative regulation of HES1 in medulloblastoma. PLoS One 2009;4:e4998. [Crossref] [PubMed]
- Wu F, Stutzman A, Mo YY. Notch signaling and its role in breast cancer. Front Biosci 2007;12:4370-83. [Crossref] [PubMed]
- Xu D, Hu J, Xu S, et al. Dll1/Notch activation accelerates multiple myeloma disease development by promoting CD138+ MM-cell proliferation. Leukemia 2012;26:1402-5. [Crossref] [PubMed]
- Cornelius JF, Eismann L, Ebbert L, et al. 5-Aminolevulinic acid-based photodynamic therapy of chordoma: In vitro experiments on a human tumor cell line. Photodiagnosis Photodyn Ther 2017;20:111-5. [Crossref] [PubMed]


