
ShRNA-mediated silencing of hTERT promote apoptosis and senescence in human ovarian cancer cells
Introduction
The ends of eukaryotic chromosomes are usually capped by telomeres, which protect the chromosomes from damage and degradation (1). Besides, telomeres are essential for genomic integrity and cell viability (2). When the conventional DNA replication process can not completely synthesize chromosomal ends, proliferating cells that lacking de novo synthesis of telomeric DNA eventually enter a growth arrest state called replicated senescence (3,4). In addition, the synthesise and maintenance of telomeric DNA is dependent on telomerase activity (5), while human telomerase reverse transcriptase (hTERT) catalytic subunit hTERT seemed to be the rate-limiting factor for telomerase activity (6). hTERT has been found in many highly proliferating cells, such as early embryos, reproductive tissue in testis and ovary, stem cells (7). However, most hematopoiesis stem cell and somatic tissues contain undetectable levels of hTERT (8).
Ovarian cancer is one of the most deadly female malignant tumors in the world and is characterized by abnormal proliferation of ovarian cells, with a five-year survival rate is only about 35% (9). Tumor development in ovarian cancer is a complex process involving multiple factors and stages. In addition to genetic factors associated with carcinogenesis, telomere abnormalities also play a key role in cancer progression (10). hTERT is considered to be a rate-limiting factor for telomerase activity. In addition, hTERT is frequently expressed in the early stages of cancer development and allows cancer clones to bypass mitotic breakdown and replicative senescence (11). Thus, regulation of telomerase activity will have important effects on many developmental processes, including aging, proliferation, differentiation and tumorigenesis. Whether to prevent the progression of ovarian cancer by inactivating the hTERT gene is unclear.
In this study, we interfered with the hTERT gene by using a nude mice model of ovarian cancer transplantation. Then, we used immunohistochemistry staining and electron microscopy to detect the apoptotic phenotype of the cancerous tissues. We also investigated the senescence gene p53 and p21 expression in transplanted ovarian cancer tissues. Our experiments provide a theoretical basis and experimental basis for the clinical application of targeting hTERT gene therapy in ovarian cancer.
Methods
Animals
Four-week female BALB/c nude mice were provided by the Experimental Animal Center of Chongqing Medical University, body weight is about 14.2±2.1 g. The nude mice were cultured in SPF grade animal laboratory. Ovarian cancer cells A2780 and SKOV3 were obtained from ATCC. We cultured the cells at 37 °C, 5% CO2. After the cell confluence 80–90%. we collected the cells and adjusted the cell concentration to 5×107 cells/mL. We subcutaneous inject the 200 µL cells in the back of the mice neck. When the nodule diameter size is up to 0.5 cm, we recorded the size of the subcutaneous nodule and determined the xenografts.
All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Animal Experimentation Ethics Committee of the Chongqing Medical University.
Gene therapy assay by target hTERT
We constructed the pG2 plasmid containing the hTERT gene based on the molecular clone protocol. The xenograft mice models were divided into three groups, 6 mice per group were separately treated with 30 µg pGCR plus lipofectamine, 200 µL saline and 30 µg pG2 plus lipofectamine. We separately injected the control plasmid and hTERT plasmid and saline into three group mice every two days, and the total number of infections were 7 times. During these two weeks, we observed and recorded the mice’s spirit, diet and activates. We also recorded the size of the xenografts.
Immunohistochemistry
Immunohistochemistry stain experiments according to the protocol for two-step detection. Paraffin sections of xenografts and other mice organ tissues were cut into 4 µM slices. Then the slides were deparaffinized in xylene and rehydrated using descending concentrations of ethanol. Following, we performed antigen retrieval using 0.01 mol/L of citrate buffer (pH=6.0) in a microwave. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10min at room temperature. After washing with PBS, the sections were incubated with blocked serum for 1h. Tissue sections were then stained with primary monoclonal rabbit antibody against p53, p21 (Santa Cruz). Tissue slides were incubated with primary antibody overnight at 4 °C. The horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG treated the specimens at room temperature for about 30 min to 1 hour. Positive staining was visualized with DAB. The p53, p21, hTERT and apoptosis were evaluated by two pathologists blind record the results. The positive staining was counted in five separate specimens and statistical analysis was performed.
H&E staining
Xenograft tumors were immersed in 4% neutral formaldehyde and HE staining was completed according to previously described (12). According to the HE staining, Observe the cell phenotype in different groups. The remaining tumor tissue was stored in a −80 °C refrigerator.
Terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) assay of xenograft tissue sections
Paraffin-embedded tissue sections were deparaffinized, washed and permeabilized as described above according to the instructions for the in situ cell death assay kit. After treatment with 3% H2O2 treated 10 min at room temperature, we treated the slides with protease K at 37 °C for 10 min. After preparing the TUNEL reaction reagents, add 30 µL TUNEL reaction buffer and incubate at 37 °C for 60 min. After washing three times with PBS, add DAB for staining reaction and hematoxylin staining the nuclear, neutral gum sealing pieces and obtained the photography by microscopy.
Western blot analysis
The xenograft tissues were lysed by RIPA buffer and protein content was measured using Enhanced BCA Protein Assay kit. The tissue lysates were then separated on a SDS-PAGE gels, transferred to a polyvinylidene fluoride (PVDF) membranes, and blocked with 5% non-fat milk for 2 hr at room temperature. PVDF membranes were incubated with primary antibodies (The following antibodies β-tubulin and hTERT were purchased from Santa Cruz). Then the membranes were incubated with BeyoECL plus, and detected by ChemiDocTM XRS+ imaging system. Data was measured using ImageJ 1.48 u software. All experiments were repeated at least three times with similar results.
Statistical analysis
For all quantified data, the mean ± SEM is presented. Statistical significance between the two experimental groups was characterized as an asterisk, and comparisons were made using Student’s t-test. P value<0.05 was considered significant.
Results
Inject hTERT shRNA plasmids significantly decreased hTERT expression in A2780 and SKOV3 induced xenograft tumor
We constructed the transplantation tumor model and studied the function of hTERT inhibiting tumors. In this study, we constructed the hTERT shRNA plasmid and injected it into xenograft tumors plus lipofectamine. After two weeks, we detected hTERT expression in tumor tissues. As shown in Figure 1A,B, hTERT was significantly decreased in tumors. We repeated the experiment three times and confirmed that hTERT shRNA worked well in xenograft tumors (Figure 1C).
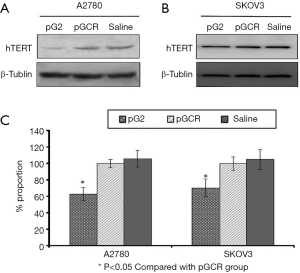
Knockdown expression of hTERT can significantly inhibit the ovarian tumor growth
To test whether hTERT plays a role in the inhibition of ovarian cancer, we constructed a nude transplantated mice model by injection of human ovarian cancer cell A2780 or SKOV3 cells. After xenograft formation, we injected the hTERT plasmids for 7 times in two weeks. As shown in Figure 2A, the results showed that the size of transplantated tumor became small after knocking down hTERT expression. Comparing the tumor size at different times, we can see that the tumors was significantly reduced after 6 days of injection of the pG2 plasmid. However, there was no significant change in the pGCR group and the saline group (Figure 2B,C). Moreover, we detected the weight of xenograft tumors and found that the pG2 group which inhibit the hTERT can obviously decreased tumor weight (Figure 2D,E).
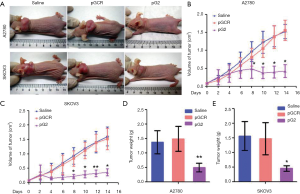
hTERT affect the tumor cell apoptosis and necrosis
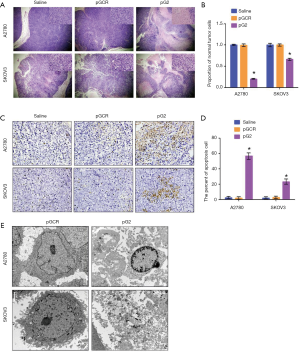
To investigated the role of hTERT in tumor proliferation, we stained xenograft tissue with H&E. As shown in Figure 3A,B, the results showed that knockdown of hTERT expression significantly increased tumor cell apoptosis and necrosis in A2780 and SKOV3 xenograft tumors. However, in the Saline and pGCR groups, tumor cells were phenotypically normal and well proliferated, the nuclear are clear and presented a large number of pathological spit phases. After TUNEL staining, as shown in Figure 3C,D, the percentage of positive TUNEL staining in the pG2 group was as high as 58.47%±4.75%, while in the saline and pGCR control group, the percentage of TUNEL positive was only 2.40%±1.41% and 1.89%±1.65%.Significant increase in the pG2 group in A2780 xenograft tumors. However, in SKOV3 xenograft tumor which lack p53 gene expression also showed that increased TUNEL positive cells, though compared with A2780 pG2 group, it is lower. To investigate the details of cell death, we examined the cell microstructure by transmission electron microscope (Figure 3E) and found that the apoptotic bodies in A2780 pG2 group and the nuclear membrane was pyknotic, it is classic apoptosis characters. However, in SKOV3 xenografts, the cells are necrotic, there are no apoptotic bodies, and the cells are destroyed. Above all, the results indicated that hTERT affects apoptosis dependent on the p53 pathway and induces necrosis in the absence of p53.
hTERT affect senescence by p53 pathway
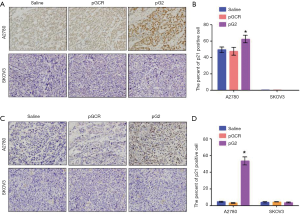
As we all known, p53 is a famous transcription factor, in addition to participating in the apoptosis pathway, p53 is also involved in the senescence pathway. Whether p53 affects cell aging in this mice model has not yet been known. To investigate this hypothesis, we stained the p53 downstream gene p21 and found that p21 was significantly increased after down-regulated hTERT expression in A2780 xenografts, whereas in p53 defected SKOV3 xenografts, there was no p21 expression changes (Figure 4). In the end, we concluded that the effect of hTERT on aging depends on the p53 pathway.
Discussion
Most of the tumor tissues have been identified by hTERT expression, and more than 85% of cancer cells contain genetic aberrations and hTERT overexpression (13). In this study, our data analysis led us to believe that hTERT is also increased in the patients with ovarian cancer. In our mice model studies, we found that hTERT affects ovarian cancer cell apoptosis and aging dependent on the p53 pathway. In addition, down-regulation of hTERT can also induce necrosis of p53-deficient ovarian cancer cells. hTERT can be used for the diagnosis and prognosis for ovarian cancer in the future, or be able to identity the risk of tumor progression and establish the appropriate therapy.
Down-regulation of p53 and necrotic pathways is well known in several cancers (14,15). In addition to the p53 mutation in most cancers, it has also been downregulated in cancer cells. In support of this point, current studies indicate that there is a significant correlation between hTERT and p53 induced apoptosis and senescence. Moreover, hTERT also significantly regulates necrosis in p53-deficient cancer cells. Although previous studies have shown that hTERT is considered to be an important factor in the development of ovarian cancer, and the Pi3K/AKT/mTOR pathway is involved in the carcinogenesis of hTERT regulation (16). Although hTERT expression does not directly cause cancer, it can contribute to the long-life span of cancer cells and subsequently increased tumor formation (17). Although there is no evidence that hTERT affects tumor cell survival depends on the transcription factor p53. In this study, we constructed two mice xenograft tumor mice models and confirmed our hypothesis. Although we do not have more evidence to support this mechanism or association between clinicopathological parameters and the individual expression of hTERT and p53. To date, data regarding the prognostic value of hTERT in ovarian cancer are inconclusive. In our study, we found that the therapeutic value of hTERT in affecting ovarian cancer cells survival.
Conclusions
We reported that the function of hTERT in ovarian xenograft tumor mice, the first data to document the therapeutic effect of down-regulated hTERT expression. Furthermore, high expression of hTERT in ovarian cancer may suggest that it may also be a potential prognostic marker of ovarian cancer.
Acknowledgments
We thank Miss Jing Chen (Chongqing Medical University) who provided purely technical help.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.03.17). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Animal Experimentation Ethics Committee of the Chongqing Medical University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Serakinci N, Tiber PM, Orun O. Chromatin modifications of hTERT gene in hTERT-immortalized human mesenchymal stem cells upon exposure to radiation. Eur J Med Genet 2018;61:288-93. [Crossref] [PubMed]
- Pandita TK, Benvenuto JA, Shay JW, et al. Effect of penclomedine (NSC-338720) on telomere fusions, chromatin blebbing, and cell viability with and without telomerase activity and abrogated p53 function. Biochem Pharmacol 1997;53:409-15. [Crossref] [PubMed]
- Shay JW. Telomeres and aging. Curr Opin Cell Biol 2018;52:1-7. [Crossref] [PubMed]
- Chilton W, O'Brien B, Charchar F. Telomeres, Aging and Exercise: Guilty by Association? Int J Mol Sci 2017;18. [PubMed]
- Mattarocci S, Reinert JK, Bunker RD, et al. Rif1 maintains telomeres and mediates DNA repair by encasing DNA ends. Nat Struct Mol Biol 2017;24:588-95. [Crossref] [PubMed]
- Maggisano V, Celano M, Lombardo GE, et al. Silencing of hTERT blocks growth and migration of anaplastic thyroid cancer cells. Mol Cell Endocrinol 2017;448:34-40. [Crossref] [PubMed]
- Jafri MA, Ansari SA, Alqahtani MH, et al. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med 2016;8:69. [Crossref] [PubMed]
- Shay JW, Wright WE. Hallmarks of telomeres in ageing research. J Pathol 2007;211:114-23. [Crossref] [PubMed]
- Cortez AJ, Tudrej P, Kujawa KA, et al. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol 2018;81:17-38. [Crossref] [PubMed]
- Martinez P, Blasco MA. Telomere-driven diseases and telomere-targeting therapies. J Cell Biol 2017;216:875-87. [Crossref] [PubMed]
- Kim W, Ludlow AT, Min J, et al. Regulation of the Human Telomerase Gene TERT by Telomere Position Effect-Over Long Distances (TPE-OLD): Implications for Aging and Cancer. PLoS Biol 2016;14:e2000016. [Crossref] [PubMed]
- Grosset AA, Loayza-Vega K, Adam-Granger E, et al. Hematoxylin and Eosin Counterstaining Protocol for Immunohistochemistry Interpretation and Diagnosis. Appl Immunohistochem. Mol Morphol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Zalewska-Ziob M, Dobija-Kubica K, Biernacki K, et al. Clinical and prognostic value of hTERT mRNA expression in patients with non-small-cell lung cancer. Acta Biochim Pol 2017;64:641-6. [Crossref] [PubMed]
- Ranjan A, Iwakuma T. Non-Canonical Cell Death Induced by p53. Int J Mol Sci 2016;17. [PubMed]
- Mohammad RM, Muqbil I, Lowe L, et al. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol 2015;35:S78-103. [Crossref] [PubMed]
- Lee YK, Chung HH, Kim JW, et al. Expression of phosphorylated Akt and hTERT is associated with prognosis of epithelial ovarian carcinoma. Int J Clin Exp Pathol 2015;8:14971-6. [PubMed]
- Maraei AA, Hatta AZ, Shiran MS, et al. Human telomerase reverse transcriptase expression in ovarian tumors. Indian J Pathol Microbiol 2012;55:187-91. [Crossref] [PubMed]


