
Effectiveness of crizotinib in a patient with mesenchymal-epithelial transition overexpression/fluorescence in situ hybridization-negative/next-generation sequencing-negative advanced lung adenocarcinoma: a case report
Introduction
In recent years, the tyrosine kinase receptor mesenchymal-epithelial transition (MET) has become a target in non-small cell lung cancer (NSCLC). Crizotinib, a potent tyrosine kinase inhibitor (TKI) of anaplastic lymphoma kinase (ALK) and ROS1, has demonstrated its remarkable therapeutic effect in patients with MET amplified or MET exon 14 skipping mutated advanced-stage non-small-cell lung cancer in several previous reports (1-5). Methods to detect MET alteration includes immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), next-generation sequencing (NGS) and reverse transcription polymerase chain reaction (RT-PCR). Few cases reported response to crizotinib in advanced NSCLC patients according to the results of MET IHC staining. In this article, we report a case of crizotinib effectiveness in a pretreated patient with advanced lung adenocarcinoma detected as MET overexpression/FISH-negative/NGS-negative.
Case presentation
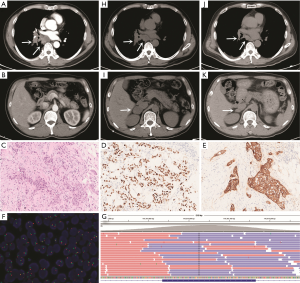
A 53-year-old male smoker visited our hospital with the chief complaint of causing cough and fever on March 12, 2018. Physical examination revealed enlarged lymph nodes in the left supraclavicular region. The patient underwent computed tomography (CT) scanning, which showed space-occupying lesions in the right lung and multiple enlarged lymph nodes in the mediastinum that were preliminarily determined to be lung adenocarcinoma by bronchoscopic examination and no distant metastatic lesions was detected (Figure 1A,B). Aspiration biopsy of the left supraclavicular lymph node was performed and histological examination revealed a lymph node invaded by adenocarcinoma cells which originated from primary adenocarcinoma of lung confirmed by IHC (Figure 1C,D). In addition, IHC revealed that the metastatic tumor expressed MET protein which was strong positive (score 3+ with SP44 from Ventana Medical Systems, USA) (Figure 1E). FISH analysis showed neither MET amplification nor ALK or ROS1 rearrangements (MET: Vysis MET SpectrumRed/CEP7 SpectrumGreen, Abbott Molecular, USA; ALK: Vysis ALK Dual Color, Abbott Molecular, USA; ROS1: Vysis ROS1 Dual color, Abbott Molecular, USA) (Figure 1F). We performed targeted NGS using biopsy tissue from left supraclavicular lymph node. Genomic DNA was profiled by using a capture-based targeted sequencing panel (Burning Rock Biotech, Guangzhou, China), including all exons in 56 genes and selected introns in MET, ALK, ROS1, RET, NTRK1, FGFR3 and FGFR1. Detailed procedure was reported in our previous study (6). The results of sequencing showed that no mutation was detected in MET gene (Figure 1G). The patient was treated with pemetrexed-carboplatin doublet chemotherapy initially on March 23, 2018. After two cycles of chemotherapy, the primary lesion maintained as stable disease (Figure 1H), but a metastatic tumor occurred in the right adrenal gland (Figure 1I). Considering strong positive staining of MET, we finally decided to administrate crizotinib 250 mg twice daily after multidisciplinary discussion on May 10, 2018. After one month of crizotinib treatment, the primary lesion and the metastatic tumor in the right adrenal gland both achieved a marked partial response (PR) (Figure 1J,K). The patient continued to receive crizotinib with no significant cancer progression as of December 2018.
Discussion
While crizotinib is recommended in advanced-stage NSCLC patients with MET amplification or MET exon 14 skipping mutation by NCCN guidelines, we report its effectiveness in an advanced lung adenocarcinoma considered MET IHC-positive/FISH-negative/NGS-negative.
As for detection of MET alteration, no gold standard method is widely accepted to determine appropriate patients with NSCLC who can benefit from crizotinib. The need for accurate detection of MET alteration has become much more important. Previous studies have described the correlation between MET amplification and MET protein expression, and revealed that almost all patients who displayed amplification of the MET gene also were positive for MET protein expression, while only half of the IHC positive patients had amplification (7,8). Therefore, for detection of MET alteration, many centers routinely applied IHC as initially screening tool due to its rapid and inexpensive advantage, and those with moderate or intense staining indicative of MET gene expression are then tested by FISH for confirmation of MET amplification.
Few studies reported the treatment efficacy of crizotinib in NSCLC patients with MET overexpression/FISH-negative. Li et al. demonstrated that 11 advanced NSCLC patients with de-novo overexpression of MET achieved PR to crizotinib, of which 3 patients showed MET IHC strong positive without MET amplification and ALK or ROS1 rearrangements (9). Our case also achieved PR to crizotinib based on MET IHC staining. However, the 3 patients in Li et al.’s study did not undergo gene sequencing to determine the MET exon 14 mutational status, and hence the effective treatment of crizotinib in these 3 patients may be attributed to the presence of MET exon 14 mutation. In view of the above-mentioned uncertainty, NGS was applied into our case and no MET exon 14 mutation was found, in addition to negative finding for MET amplification and ALK or ROS1 rearrangements.
We herein infer from this case that MET overexpression could be a biomarker for predicting efficacy of crizotinib. Further studies awaiting to be done for collecting more patients with MET overexpression for evaluating efficacy of crizotinib.
Acknowledgments
The authors thank Jiali Mu and Ruomei Dong for their technical assistance.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.03.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol 2011;6:942-6. [Crossref] [PubMed]
- Camidge RD, Ou S-HI, Shapiro G, et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer. J Clin Oncol 2014;32:abstr 8001.
- Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850-9. [Crossref] [PubMed]
- Paik PK, Drilon A, Fan PD, et al. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 2015;5:842-9. [Crossref] [PubMed]
- Awad MM, Oxnard GR, Jackman DM, et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and cMET overexpression. J Clin Oncol 2016;34:721-30. [Crossref] [PubMed]
- Li W, Zhang J, Guo L, et al. Combinational analysis of FISH and immunohistochemistry reveals rare genomic events in ALK fusion patterns in NSCLC that responds to crizotinib treatment. J Thorac Oncol 2017;12:94-101. [Crossref] [PubMed]
- Duan J, Yang X, Zhao J, et al. Correlation among genetic variations of c-MET in Chinese patients with non-small cell lung cancer. Oncotarget 2017;9:2660-7. [PubMed]
- Park S, Koh J, Kim DW, et al. MET amplification, protein expression, and mutations in pulmonary adenocarcinoma. Lung Cancer 2015;90:381-7. [Crossref] [PubMed]
- Li AN, Yang JJ, Zhang XC, et al. Crizotinib in advanced non-small-cell lung cancer with de novo c-Met overexpression. J Clin Oncol 2015;15:abstr 8090.


