
The maximum diameter of cervical lymph node was not a prognostic factor for local-regional advanced nasopharyngeal carcinoma treated with intensity modified radiotherapy
Introduction
Nasopharyngeal carcinoma (NPC) is the most commonly diagnosed head and neck malignancy in Southeast Asia, and radiation therapy is its mainstay treatment. Early stage NPC is usually treated with radiation therapy alone and concurrent chemoradiotherapy is the standard treatment modality for loco-regionally advanced diseases (1-5). Intensity modulated radiotherapy has become the standard of radiotherapy for NPC (6). In particular, the extensive use of intensity-modulated radiotherapy technology greatly improved the survival of patients and improved long-term quality of life (7,8). Cervical lymph node metastasis was an important prognostic factor (9). However, the prognosis of the maximum diameter of cervical lymph nodes before treatment has always been controversial (10-12). In two-dimensional radiotherapy era, a large sample of retrospective analysis founded that the maximum diameter of the lymph node after radiotherapy alone was not a prognostic factor for early NPC with cervical lymph node metastasis (11).
The aim of this report was to analyze the relationship between treatment outcomes and the maximum diameter of lymph nodes in advanced NPC treated with intensity modified radiotherapy before treatment according to an institutional protocol.
Methods
Study design and eligibility
This retrospective study was conducted in Ruijin Hospital of Shanghai Jiao Tong University. Data was obtained from the hospital’s medical records and the online data-management system. Patient information was anonymized and de-identified before analysis. Patients were deemed eligible when they started curative intent radiotherapy, with or without chemotherapy (Table 1). There were no medical interventions in the current study. Our study obtained ethics approval of Ruijin Hospital.
Table 1
| Characteristics | N (%) |
|---|---|
| Gender | |
| Male | 126 (77.3) |
| Female | 37 (22.7) |
| Age (year) | |
| Median [range] | 53 [13–84] |
| ≤60 | 125 (76.7) |
| >60 | 38 (23.3) |
| T-classification | |
| 1 | 11 (6.7) |
| 2 | 38 (23.3) |
| 3 | 63 (38.7) |
| 4 | 51 (31.3) |
| N-classification | |
| 0 | 10 (6.1) |
| 1 | 38 (23.3) |
| 2 | 78 (47.9) |
| 3 | 37 (22.7) |
| AJCC stage | |
| III | 84 (51.5) |
| IVa | 79 (48.5) |
| Dmax (cm) | |
| Median [range] | 2.9 [0–12] |
| ≤3 | 111 (68.1) |
| 3< Dmax ≤6 | 46 (28.2) |
| >6 | 6 (3.7) |
| Scheme | |
| Radiotherapy alone | 13 (8.0) |
| Combined chemotherapy | 150 (92.0) |
Patients and pretreatment evaluation
Between January 2012 and December 2017, 163 consecutive and non-selected histologically approved patients with locally advanced NPC were treated according to our institutional protocol at the Ruijin Hospital of Shanghai Jiao Tong University. Pretreatment evaluation consisted of a complete history and physical examination, indirect or fiber-optic endoscopic examination, complete blood counts, serum electrolytes, CT of the chest, magnetic resonance imaging (MRI) of the head and neck, ultrasound of liver and abdominal, Urinalysis, bone scan and dental evaluation. All MRI and clinical data were reviewed to minimize heterogeneity in restaging. The American Joint Committee on Cancer staging system (8th edition) was used for stage classification (13). All patients with positive lymph nodes in the cervical region according to the criteria described by van den Brekel et al., i.e., shortest axis of ≥11 mm in the jugulodigastric regions, or >10 mm in other cervical regions (14). In addition, a group of 3 or more lymph nodes of borderline in size is considered metastatic, and were included in this analysis. The maximum diameter of the lymph node was measured on the axial or coronal MRI image.
Treatment planning and delivery
All patients were immobilized in the supine position with a tailored head-shoulder thermoplastic mask, followed by a CT simulation. CT simulation (Brilliance Big Bore, Phillips, Amsterdam, Netherlands) was performed at a slice thickness of 3–5 mm from the head to 5 cm below the sternoclavicular joint. MR images were co-registered with CT images. The target volumes were delineated slice-by-slice using an individualized protocol that complies with the International Commission on Radiation Units and Measurements reports 50 and 62 as follows (15-17): the GTV of the primary tumor (GTV-P) included retropharyngeal lymph nodes, and the rest involved lymph nodes that were defined as GTV-LN. The GTV-P was delineated according to the pretreatment lesion as shown by MRI. Whereas post-induction chemotherapy volumes of involved neck lymph nodes were used for GTV-LN delineation. The planning target volume (PTV) of the primary tumor (PTV-G) and the lymph nodes (PTV-LN) were created by expanding a 3–5-mm margin around the GTV-P and the GTV-LN for setup variation and range uncertainties. Two clinical target volumes (CTVs)—CTV1 and CTV2—were then determined. These volumes represented high- and low-risk disease regions, respectively. The CTV1 included the nasopharyngeal cavity, posterior one-third of the nasal cavity and maxillary sinus, parapharyngeal space, pterygopalatine fossa, lateral pterygoid plate, skull base, prevertebral muscles, the whole clivus and sphenoid sinus, retropharyngeal nodal regions, and drainage area of the upper neck (levels II, III, and VA). Adjacent structures were included according to the scope of tumor invasion. The CTV2 included the drainage area of the lower neck (levels IV and VB), which were not outlined for patients with stage N0. If the lymph nodes were positive in the level IV or VB, the lower neck was included by the CTV1. PTV60 and PTV54 were created by expanding a 3–5-mm margin around the CTV1 and CTV2 to compensate for geometric uncertainties and patient movement. The OARs—including the brainstem, spinal cord, optic nerves, optic chiasm, eyeballs, lenses, temporal lobes, parotid glands, and larynx—were carefully outlined and expanded according to the RTOG 0225 protocol during optimization (18). Dose fractionation at discretion of the attending physician as follows: a total dose of 66–70.4 and 70.4 Gy in 30–32 fractions was usually prescribed for the PTV-G and PTV-LN, whereas 60 and 54 Gy in 30–32 fractions were prescribed to the PTVs of CTV1 and CTV2, or the dose prescriptions were 70 Gy for PTV-P at 2.12 Gy/fraction, 66 Gy for PTV-LN at 2 Gy/fraction and 60 and 54 Gy for PTVs of CTV1 and CTV2 delivered in 33 fractions, or the dose prescriptions were 70 Gy for PTV-P at 2 Gy/fraction, 66 Gy for PTV-LN and 60 and 54 Gy for PTVs of CTV1 and CTV2 delivered in 35 fractions. All patients received five daily fractions per week. The planning goal was to deliver at least 95% of the prescribed dose to 99% of the GTV without exceeding the dose tolerance of the critical neurological OARs. It was stipulated that not more than 110% of the prescribed dose should be outside of the PTV, no more than 5% of the PTV should receive more than 105% of the prescribed dose, and no more than 3% of the PTV should receive less than 93% of the prescribed dose. The dose received by each OAR was limited to a tolerance according to the RTOG 0225 protocol. By using the TPS, 7–9 fields of a 6-MV photon beam were evenly distributed around each patient’s head and neck.
Combined chemotherapy
Based on the treatment guidelines for NPC at our hospital, concurrent chemotherapy +/− induction chemotherapy or adjuvant chemotherapy was recommended to patients with stage III–IVa NPC. Induction chemotherapy was consisted of paclitaxel (135 mg/m2) or docetaxel (75 mg/m2) and cisplatin (75 mg/m2); every 3 weeks for 2–3 cycles. Concurrent chemotherapy was consisted of cisplatin administered every 3 weeks (100 mg/m2) or weekly (40 mg/m2) during RT.
Follow-up
All patients were evaluated weekly during treatment. After the completion of radiation therapy, the patients were followed up every 3 months during the first and second year, and every 6 months during the next 2–3 years, then once every year after that. MRI of the head and neck region, chest CT, abdominal ultrasound and physical examination were performed during follow-up session. MRI of the brain and liver, whole body bone scan or PET/CT were ordered when patients had indications.
Statistical analysis
All events were measured from the date of completion of radiation therapy until documented treatment failure or the last follow-up visit. The time to the first defining event was assessed for the following end-points: local failure-free rate (L-FFR—persistence/recurrence at nasopharynx/cervical lymph node), distant failure-free rate (D-FFR—disease metastasis at distant sites), overall survival (OS—death due to any cause) and disease free survival (DFS—staying free of disease after radiotherapy). Initial diagnosis of treatment failure was based on clinical and/or radiological examinations and pathology confirmation of local or regional recurrence were required. The rates of local and regional control, DFS, and OS rates were calculated with the Kaplan-Meier method, and the differences compared with the log-rank test. All statistical tests were two-sided and P values of 0.05 or less were considered significant. Multivariate analysis using the Cox proportional hazard model was performed to define independent predictors among various potential prognostic factors. The Statistical Package for the Social Sciences, version 22.0 (SPSS Inc.) was used for all statistical analysis.
Results
Treatment outcomes
The median follow-up time for the entire group was 31 months (range, 6.1 to 79.3 months). At the time of this analysis, a total of 6 cases experienced neck recurrence, 9 patients developed local recurrence in the primary area. In addition, 26 patients developed distant metastasis (Table 2).
Table 2
| Failure patterns | Number |
|---|---|
| Nasopharynx recurrence | 9 |
| Neck recurrence | 6 |
| Bone metastasis | 5 |
| Liver metastasis | 10 |
| Lung metastasis | 5 |
| Multiple metastasis | 6 |
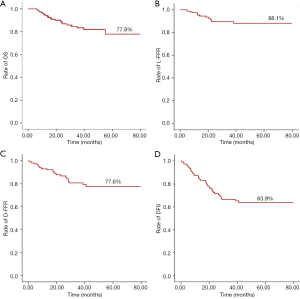
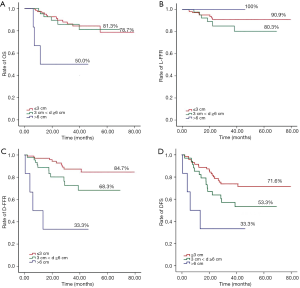
A total of 22 patients had deceased: 10 patients died from distant metastasis, 9 died from progression of local or regional disease after recurrence, and the causes of death of 3 additional cases were unknown (Table 3). The 3-year OS rate of whole cohort was 77.8%, and the 3-year L-FFR, D-FFR, and DFS rates were 88.1%, 77.6% and 63.9%, respectively (Figure 1). The median Dmax in our study was 2.9 cm (range, 0 to 12 cm). The 3-year OS rate (78.7% vs. 81.3% vs. 50.0%), L-FFR (90.9% vs. 80.3% vs. 100%), D-FFR (84.7% vs. 68.3% vs. 33.3%), and DFS rates (71.6% vs. 53.3% vs. 33.3%) of Dmax ≤3 cm, 3 cm < Dmax ≤6 cm, Dmax >6 cm (Figure 2).
Table 3
| Cause of death | Number |
|---|---|
| Total | 22 |
| Nasopharynx and neck recurrence | 9 |
| Distant metastasis | 10 |
| Unknown | 3 |
Prognostic factors
The value of various potential prognostic factors include age, T and N-classification, TNM stage, Dmax of LN on predicting L-FFR, D-FFR, DFS and OS were evaluated (Tables 4,5). Both uni- and multivariate analyses demonstrated that age and N-classification are the significant prognostic factor for predicting OS while Dmax, T and N-classification and AJCC-classification are the significant prognostic factor for predicting OS in univariate analyses. However, local or regional recurrence was not affected by any studied prognostic factor including Dmax, T-classification, AJCC-classification and chemotherapy.
Table 4
| Factor | OS | D-FFR | L-FFR | DFS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OS (%) | P value | D-FFR (%) | P value | L-FFR (%) | P value | DFS (%) | P value | ||||
| Age (year) | |||||||||||
| ≤60 | 90.6 | <0.001 | 80.1 | 0.202 | 91.8 | 0.004 | 71.2 | 0.001 | |||
| >60 | 24.9 | 64.5 | 75.1 | 35.1 | |||||||
| Dmax | |||||||||||
| ≤3 | 78.7 | <0.001 | 84.7 | <0.001 | 90.9 | 0.394 | 71.6 | 0.003 | |||
| 3–6 | 81.3 | 68.3 | 80.3 | 53.3 | |||||||
| >6 | 50.0 | 33.3 | 100.0 | 33.3 | |||||||
| T-classification | |||||||||||
| 1 | 90.9 | 0.048 | 90.9 | 0.501 | 100.0 | 0.075 | 80.0 | 0.038 | |||
| 2 | 90.6 | 84.7 | 95.8 | 78.6 | |||||||
| 3 | 86.8 | 78.9 | 89.8 | 66.9 | |||||||
| 4 | 47.8 | 60.6 | 76.0 | 41.1 | |||||||
| N-classification | |||||||||||
| 0 | 80.0 | 0.036 | 75.0 | 0.035 | 83.3 | 0.765 | 62.5 | 0.007 | |||
| 1 | 80.0 | 91.7 | 83.4 | 77.9 | |||||||
| 2 | 81.8 | 74.9 | 91.5 | 62.4 | |||||||
| 3 | 68.7 | 71.9 | 89.0 | 51.0 | |||||||
| AJCC | |||||||||||
| III | 91.7 | 0.001 | 85.3 | 0.016 | 91.9 | 0.119 | 74.8 | 0.004 | |||
| IVA | 55.4 | 67.0 | 83.2 | 49.5 | |||||||
| Scheme | |||||||||||
| RT alone | 62.5 | 0.140 | 100.0 | 0.198 | 66.7 | 0.031 | 51.4 | 0.598 | |||
| Combined chemotherapy | 78.8 | 76.4 | 89.7 | 64.6 | |||||||
OS, overall survival; D-FFR, distant failure-free rate; L-FFR, local failure-free rate; DFS, disease free survival.
Table 5
| Factor | B | SE | Sig. | Exp(B) | 95.0% Exp(B) CI |
|---|---|---|---|---|---|
| OS | |||||
| Age (year) | 1.885 | 0.466 | 0.001 | 6.583 | 2.643–16.400 |
| T-classification | 0.371 | 0.345 | 0.282 | 1.450 | 0.737–2.850 |
| N-classification | 0.868 | 0.392 | 0.027 | 2.383 | 1.104–5.141 |
| AJCC stage | 0.630 | 0.683 | 0.356 | 1.879 | 0.492–7.166 |
| Dmax | −0.309 | 0.505 | 0.542 | 0.735 | 0.273–1.978 |
| D-FFR | |||||
| N-classification | 0.282 | 0.333 | 0.398 | 1.325 | 0.690–2.546 |
| AJCC stage | 0.530 | 0.496 | 0.285 | 1.699 | 0.642–4.497 |
| Dmax | 0.884 | 0.452 | 0.051 | 2.422 | 0.998–5.877 |
| L-FFR | |||||
| T-classification | 0.794 | 0.412 | 0.054 | 2.211 | 0.987–4.955 |
| Scheme | −0.785 | 0.690 | 0.255 | 0.456 | 0.118–1.763 |
| Age (year) | 1.000 | 0.579 | 0.084 | 2.720 | 0.875–8.452 |
| DFS | |||||
| Age (year) | 1.003 | 0.352 | 0.004 | 2.726 | 1.368–5.433 |
| T-classification | 0.593 | 0.297 | 0.046 | 1.810 | 1.010–3.241 |
| N-classification | 0.647 | 0.305 | 0.034 | 1.909 | 1.050–3.470 |
| AJCC stage | −0.102 | 0.502 | 0.839 | 0.903 | 0.337–2.416 |
| Dmax | 0.318 | 0.376 | 0.397 | 1.375 | 0.658–2.874 |
OS, overall survival; D-FFR, distant failure-free rate; L-FFR, local failure-free rate; DFS, disease free survival.
Discussion
Radiation therapy is the mainstay treatment modality of non-metastatic NPC. Radiation therapy with or without chemotherapy for definitive treatment of NPC has produced an OS, local control, and regional rates of about 80%, 85%, and 90%, respectively (5,18,19). Nasopharynx has a rich lymphatic network and clinical evident cervical lymph adenopathy is seen in about 85% of the patients with nasopharyngeal cancer (20,21). Cervical lymph node status has always been an important therapeutic and prognostic factor in clinical staging. The influence of lymph node on prognosis includes size, location, unilateral and bilateral neck and lymph node capsule involvement and treatment scheme. In our study, local or regional recurrence was not affected by the maximum diameter of lymph node (P=0.542). The results suggested that multivariate analyses demonstrated the maximum diameter of lymph nodes had no significant effect on the prognosis. However, the prognosis of the maximum diameter of cervical lymph nodes before treatment has always been controversial (10-12). In a retrospective study by Gao (11), early nasopharyngeal cancer with unilateral neck lymph node metastasis, and the maximum diameter ≤6 cm receiving radiotherapy alone was selected. The results of this study showed that the maximum diameter of lymph nodes had no significant effect on the prognosis. It is possible that the intensity modulated radiotherapy significantly increased the local control rate, particularly combined with chemotherapy and the patients who failed in the initial treatment had better rescue measures, which significantly improved the prognosis of patients. Liao’s study suggested that the maximum diameter of cervical lymph nodes was related to the prognosis (22). But in that study, the measurement of the maximum diameter of lymph nodes was collected on axial imaging. For cervical lymph nodes with fusion, the maximum diameter may not be measured on axial but coronal. However, the fused lymph nodes are considered to have extrapsular invasion in clinical practice, which may also explain that the involvement of lymph node capsule has no significant influence on the prognosis in that study. In addition, when cervical lymph nodes exceed 6 cm, they are often classified as N3 in staging, which is closely related to the prognosis of patients, especially distant metastasis. In our study the Dmax is not the significant prognostic factor for predicting distant failure-free survival in multivariate analyses, but the P value is 0.051, which is close to 0.05. Due to the low proportion of patients with cervical lymph nodes exceeding 6 cm in our group, the number of regional failures N3 cases was too small (only 6 cases) to allow meaningful analysis.
The treatment failure patterns in this group were still the distant metastasis, followed by the local regional failure. The available clinical data suggested that patients with local-regional NPC could be treated with radiotherapy and induction chemotherapy as first line scheme to further improve the efficacy (23-25). Due to this ability of induction chemotherapy in the comprehensive treatment of local-regional advanced NPC, lymph nodes often shrink significantly after induction chemotherapy. The effect of the maximum diameter of cervical lymph nodes before and after chemotherapy on the prognosis of patients should be further explored.
Although a cohort and non-selected patients were included in the current study, the retrospective nature of this analysis certainly serves pitfalls of this study. Although most local advanced patients received chemotherapy, it was not protocolized and was used at discretion of the attending physician. However, multivariate analyses showed that chemotherapy was not a significant prognostic factor for predicting OS, D-FFR, DFS and L-FFR in this analysis.
Conclusions
In conclusion, in this retrospective study, we found that Dmax of cervical metastatic lymph nodes was not a prognostic factor in patients with local-regional advanced NPC treated with intensity modified radiotherapy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.04.22). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the retrospective nature of the study. Our study obtained ethics approval of Ruijin Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Al-Sarraf M, Leblanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998;16:1310-7. [Crossref] [PubMed]
- Chen QY, Wen YF, Guo L, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst 2011;103:1761-70. [Crossref] [PubMed]
- Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol 2005;23:6966-75. [Crossref] [PubMed]
- Colevas AD, Yom SS, Pfister DG, et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J Natl Compr Canc Netw 2018;16:479-90. [Crossref] [PubMed]
- Au KH, Ngan RKC, Ng AWY, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: A report of 3328 patients (HKNPCSG 1301 study). Oral Oncol 2018;77:16-21. [Crossref] [PubMed]
- Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys 2002;53:12-22. [Crossref] [PubMed]
- Huang TL, Chien CY, Tsai WL, et al. Long-term late toxicities and quality of life for survivors of nasopharyngeal carcinoma treated with intensity-modulated radiotherapy versus non-intensity-modulated radiotherapy. Head & Neck 2016;38:E1026-E1032. [Crossref] [PubMed]
- Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 2012;104:286-93. [Crossref] [PubMed]
- Mao YP, Liang SB, Liu LZ, et al. The N staging system in nasopharyngeal carcinoma with radiation therapy oncology group guidelines for lymph node levels based on magnetic resonance imaging. Clin Cancer Res 2008;14:7497-503. [Crossref] [PubMed]
- Mao YP, Tang LL, Chen L, et al. Prognostic factors and failure patterns in non-metastatic nasopharyngeal carcinoma after intensity-modulated radiotherapy. Chin J Cancer 2016;35:103. [Crossref] [PubMed]
- Gao YS, Xu TT, He XY, et al. Maximal diameter of lymph node is not a prognostic factor for early stage nasopharyngeal carcinoma treated by radiotherapy alone. China Oncology 2012;84:761-5.
- Pan JJ, Ng WT, Zong JF, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 2016;122:546-58.
- Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8 ed. New York: Springer International Publishing, 2017.
- van den Brekel MW, Stel HV, Castelijns JA, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology 1990;177:379-84. [Crossref] [PubMed]
- ICRU. Prescribing, recording and reporting photon beam therapy (Report 50). Bethesda USA 1993.
- ICRU. Prescribing, recording and reporting photon beam therapy (supplement to ICRU report 50) (Report 62). Bethesda USA 1999.
- Chinese nasopharyngeal carcinoma staging Committee. The consensus 2010 nasopharyngeal carcinoma IMRT target and dose expert design guidelines. Chin J Radiat Oncol 2011;20:267-9.
- Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol 2009;27:3684-90. [Crossref] [PubMed]
- Chen YP, Tang LL, Yang Q, et al. Induction chemotherapy plus concurrent chemoradiotherapy in endemic nasopharyngeal carcinoma: individual patient data pooled analysis of four randomized trials. Clin Cancer Res 2018;24:1824-33. [Crossref] [PubMed]
- Tang L, Mao Y, Liu L, et al. The volume to be irradiated during selective neck irradiation in nasopharyngeal carcinoma: analysis of the spread patterns in lymph nodes by magnetic resonance imaging. Cancer 2010;115:680-8. [Crossref] [PubMed]
- Wang X, Hu C, Ying H, et al. Patterns of lymph node metastasis from nasopharyngeal carcinoma based on the 2013 updated consensus guidelines for neck node levels. Radiother Oncol 2015;115:41-5. [Crossref] [PubMed]
- Liao X, Kang M, Xu M, et al. Relationship between positive lymph nodes and distant metastasis after intensity-modulated radiotherapy in patients with nasopharyngeal carcinoma. Chin J Radiat Oncol 2017;26:5.
- Kong L, Zhang Y, Hu C, et al. Effects of induction docetaxel, platinum, and fluorouracil chemotherapy in patients with stage III or IVA/B nasopharyngeal cancer treated with concurrent chemoradiation therapy: Final results of 2 parallel phase 2 clinical trials. Cancer 2017;123:2258-67. [Crossref] [PubMed]
- Kawahira M, Yokota T, Hamauchi S, et al. Survival benefit of adding docetaxel, cisplatin, and 5-fluorouracil induction chemotherapy to concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinoma with nodal Stage N2-3. Jpn J Clin Oncol 2017;47:705-12. [Crossref] [PubMed]
- Bae WK, Hwang JE, Shim HJ, et al. Phase II study of docetaxel, cisplatin, and 5-FU induction chemotherapy followed by chemoradiotherapy in locoregionally advanced nasopharyngeal cancer. Cancer Chemother Pharmacol 2010;65:589-95. [Crossref] [PubMed]


