Preoperative CA19-9 levels predict disease-free survival and overall survival in pancreatic adenocarcinoma patients after resection
Introduction
Pancreatic adenocarcinoma (PAC) is one of the most deadly and devastating cancers. Pancreatic cancer is rated the sixth and the seventh leading cause of cancer death in China and worldwide, respectively (1,2). The 5-year survival rate of PAC is approximately 8% (3). Only around 20% of cases are surgically resectable. For such patients, the median survival time ranges from 25 to 30 months, while the 5-year survival rate is merely 20% (4).
Various adjuvant therapies, including chemotherapy (CT) with or without radiotherapy (RT), have been extensively investigated over the past few decades to improve the survival of patients with early-stage PAC. Randomized clinical trials have demonstrated improved survival with adjuvant CT (5-8). However, additional adjuvant RT has been controversial, considering the conflicting results of several studies (9-11). Although adjuvant therapies can improve survival for some patients, the overall survival (OS) and disease-free survival (DFS) are still unmet clinical need. The mixed results of survival benefit from adjuvant CT may be explained by the high heterogeneity of PAC. Multiple studies have determined several important prognostic factors, including surgical margins and lymphovascular invasion (12-16). But none of these factors could predict recurrence and survival before surgery. CA19-9, the most clinically useful and valuable tumor biomarker in PAC, was found to be associated with tumor burden and respectability (17), as well as recurrence and survival (18-20). Therefore, we hypothesized that preoperative serum CA19-9 levels may be a helpful biomarker to identify the highly aggressive PAC subgroup, those of which might benefit more from postoperative CT. This study aims to investigate the association between preoperative serum CA19-9 levels and clinical prognosis, including DFS and OS in patients with resectable PAC.
Methods
Study cohort
From January 01, 2015 to June 30, 2017, the clinical data of 421 patients who underwent radical resection were retrieved from the medical records of the PLA General Hospital database. Available data included demographics, pathological data from resected specimens, preoperative serum CA19-9 levels, types of treatment, and the date of disease recurrence/metastasis and/or death. The pathological stage was re-determined according to the 8th edition of the American Joint Committee on Cancer (AJCC) Staging Manual (9). None of the patients had received preoperative treatment. The inclusion criteria were: (I) age >18 years; (II) radical resection of pancreatic cancer (R0 or R1); (III) histologically confirmed PAC without distant metastasis; (IV) WHO Performance Status 0 to 2. The exclusion criteria were: (I) loss of follow-up; (II) palliative or R2 resection; and (III) lack of data on preoperative CA19-9 levels. This retrospective study was approved by the ethics committee of the Chinese People’s Liberation Army (PLA) General Hospital.
Adjuvant therapy and follow-up
Adjuvant CT was administered within 3 months after surgery. Eight cycles of gemcitabine (GEM) plus S-1 or GEM monotherapy or S-1 monotherapy were planned for postoperative patients. GEM was administered at 1,000 mg/m2 intravenously on days 1 and 8, S-1 was given twice a day orally at 80–120 mg/d on days 1 through 14 of each 21-day cycle. Contrast enhanced computer tomography were scheduled every 3 months within 3 years after the surgery, and every 6 months thereafter. All patients were tracked for survival status until June 30, 2018.
CA19-9 testing method
The serum levels of CA19-9 were detected using standard electrochemiluminescence immunoassays. According to the manufacturer’s instructions, the recommended upper limit of normal CA19-9 levels is 37 U/mL. Preoperative CA19-9 levels were measured in patients within 30 days before surgery.
Statistical analysis
Disease recurrence included local/regional recurrence (recurrence in the pancreatic bed, root of mesentery, soft tissues, or lymph nodes adjacent to the pancreatic bed) and distant failure (spreading of tumor to the liver, lungs, or other distant organs). DFS was defined as the time from the date of surgery to the date of first recurrence/metastasis or to the date of last follow-up (in patients without recurrence/metastasis). OS was defined as the time from the date of surgery to the date of death or the date of last follow-up (if death did not occur).
Pearson’s chi-square test was performed to test the correlation of preoperative serum CA19-9 levels with other variables. Survival was estimated using Kaplan-Meier survival curves, and compared using the log-rank test. The Cox proportional hazards ratio (HR) was used to identify the impact of variables on DFS and OS. A P<0.05 was considered to be statistically significant. Data were analyzed using the SPSS v22.0 (IBM, Armonk, NY, USA).
Results
Clinicopathologic features and the correlation with preoperative CA19-9 levels
Of the 421 patients examined, 67 were excluded for the following reasons: lost to follow-up (N=55), lack of data on serum CA19-9 levels (N=10) and R2 surgical margins (N=2) (Figure S1). In a total of 354 eligible patients, the median age of patients was 60 years, and 224 (63.3%) patients were males. Performance status were 0, 1 and 2 score in 76 (21.5%), 170 (48.0%) and 108 (30.5%) patients, respectively. Surgical margins were identified as R1 (presence of tumor cells within 1 mm of ink) or R0 (absence of tumor cells within 1 mm of ink) in 17 (4.8%) and 337 (95.2%) patients, respectively. Patients with stage I, II and III were 168 (47.5%), 158 (44.6%) and 28 (7.9%), respectively. A total of 119 (33.6%) patients received at least 2 cycles of adjuvant CT (median 6 cycles, range from 2 to 8 cycles), including 25 patients who received chemoradiation therapy, and 235 (66.4%) patients did not receive adjuvant therapies. In 119 patients received adjuvant CT, 49 (41.2%) of them received less than 6 cycles of CT. Patients were classified into three groups according to their CA19-9 levels (range from 0.4–6,641 U/mL): G1 (≤87 U/mL), G2 (87–322 U/mL) and G3 (>322 U/mL) (in tertiles, 118 patients per group). The correlations between preoperative CA19-9 levels in the serum and baseline characteristics of 354 patients are detailed in Table S1. The proportion of patients who underwent R1 resection in patients with preoperative CA19-9 levels >322 U/mL were 8.5%, significantly higher than those with preoperative CA19-9 levels ≤87 U/mL (0.8%) and those with preoperative CA19-9 levels of 87–322 U/mL (5.1%) (P=0.023). There was no correlation observed between preoperative CA19-9 levels and other baseline characteristics.
Survival analysis
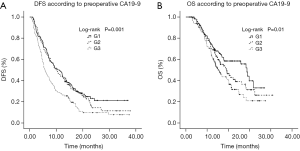
The median follow-up time was 22.7 months (range, 6.5 to 41.8 months). By June 30, 2018, the median DFS and OS of 354 patients were 8.6 and 19.4 months, respectively. The 1- and 3-year recurrence rates were 75% and 86%, respectively. The 1- and 3-year survival rates were 52% and 28%, respectively. Patients with higher preoperative CA19-9 levels had lower survival rates. The median DFS for G1 vs. G2 vs. G3 were 10.6 vs. 9.7 vs. 5.7 months, respectively (Figure 1A). The differences in DFS were significant between the G1 and G3 patients (P<0.001), as well as between the G2 and G3 patients (P=0.003). The median OS for G1 vs. G2 vs. G3 were 28.8 vs. 19.0 vs. 15.5 months, respectively (Figure 1B). The differences in OS were significant between the G1 and G3 (P=0.003), but not between the G2 and G3 (P=0.13).
A multivariable Cox proportional hazards model was developed adjusted by the co-variables with P<0.05 in the univariable analysis (Table 1). Multivariable analysis showed that performance status, tumor grade, surgical margins, TNM stage, preoperative CA19-9 levels and adjuvant CT were independent predictors of DFS. The performance status, TNM stage, preoperative CA19-9 levels and adjuvant CT were independent predictors of OS. Patients who received adjuvant CT had a significantly decreased risk of recurrence [HR 0.46; 95% confidence interval (CI), 0.36–0.61, P<0.001] and death (HR 0.40; 95% CI, 0.28–0.57, P<0.001) compared to those who did not receive adjuvant CT. Patients in the G1 group had a significantly lower risk of recurrence (HR 0.59; 95% CI, 0.44–0.79) and death (HR 0.59; 95% CI, 0.40–0.87, P<0.001) compared to patients in the G3 group. Moreover, patients in the G2 group had a significantly lower risk of recurrence (HR 0.59; 95% CI, 0.44–0.80, P=0.001) compared to patients in the G3 group. The median OS in the G2 group was much longer than that observed in the G3 group (19.0 vs. 15.5 months), although the difference was only borderline significant (HR 0.77; 95% CI, 0.54–1.10, P=0.15). In order to avoid the confounding effect of hyperbilirubinemia, we reanalyzed the data after excluding 72 patients with a serum bilirubin >2 mg/dL at the time of CA19-9 measurement. The results were similar (Table S2).
Table 1
| Variables | DFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age (years) | 0.39 | 0.18 | |||||||||
| ≤60 | 1 (ref) | NA | NA | 1 (ref) | NA | NA | |||||
| >60 | 0.90 (0.71–1.14) | 1.22 (0.91–1.63) | |||||||||
| Gender | 0.22 | 0.69 | |||||||||
| Male | 1 (ref) | NA | NA | 1 (ref) | NA | NA | |||||
| Female | 0.86 (0.67–1.10) | 0.94 (0.69–1.28) | |||||||||
| Performance status | |||||||||||
| 0 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| 1 | 1.83 (1.31–2.55) | <0.001* | 1.83 (1.31–2.57) | <0.001* | 1.63 (1.08–2.46) | 0.02* | 1.54 (1.02–2.34) | 0.041* | |||
| 2 | 2.52 (1.78–3.58) | <0.001* | 2.73 (1.90–3.92) | <0.001* | 1.71 (1.10–2.66) | 0.017* | 1.78 (1.13–2.80) | 0.013* | |||
| Location | 0.5 | 0.26 | |||||||||
| Head and neck | 1 (ref) | NA | NA | 1 (ref) | NA | NA | |||||
| Body and tail | 0.92 (0.72–1.18) | 0.84 (0.61–1.14) | |||||||||
| Tumor grade | 0.001* | 0.002* | 0.089 | ||||||||
| Well-moderate | 1 (ref) | 1 (ref) | 1 (ref) | NA | NA | ||||||
| Poor | 1.49 (1.18–1.89) | 1.45 (1.14–1.85) | 1.29 (0.96–1.73) | ||||||||
| Surgical margin | 0.012* | 0.01* | 0.001* | 0.001* | |||||||
| R0 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| R1 | 1.96 (1.16–3.30) | 2.60 (1.51–4.50) | 2.56 (1.48–4.42) | 2.60 (1.48–4.56) | |||||||
| Chemotherapy | <0.001* | <0.001* | <0.001* | <0.001* | |||||||
| No | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| Yes | 0.60 (0.46–0.77) | 0.46 (0.36–0.61) | 0.44 (0.32–0.62) | 0.40 (0.28–0.57) | |||||||
| TNM stage | |||||||||||
| III | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| II | 0.46 (0.30–0.70) | <0.001* | 0.60 (0.39–0.93) | 0.022* | 0.40 (0.25–0.65) | <0.001* | 0.51 (0.31–0.84) | 0.008* | |||
| I | 0.40 (0.26–0.60) | <0.001* | 0.42 (0.28–0.65) | <0.001* | 0.35 (0.21–0.56) | <0.001* | 0.34 (0.21–0.56) | <0.001* | |||
| Preoperative CA19–9 | |||||||||||
| G3 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| G2 | 0.66 (0.49–0.87) | 0.003* | 0.59 (0.44–0.80) | 0.001* | 0.77 (0.55–1.08) | 0.13 | 0.77 (0.54–1.10) | 0.15 | |||
| G1 | 0.60 (0.45–0.80) | <0.001* | 0.59 (0.44–0.79) | <0.001* | 0.57 (0.40–0.83) | 0.003* | 0.59 (0.40–0.87) | 0.007* | |||
Multivariable hazard ratio of DFS was adjusted for: Performance status; Tumor grade; Surgical margin; Chemotherapy; TNM stage; Preoperative CA19-9. Multivariable hazard ratio of OS was adjusted for: Performance status; Surgical margin; Chemotherapy; TNM stage; Preoperative CA19-9. *, statistically significant. CI, confidence interval; DFS, disease-free survival; HR, hazards ratio; G1, CA19-9 ≤87 U/mL; G2, CA19-9 87-322 U/mL; G3, CA19-9 >322 U/mL. NA, not applicable; OS, overall survival; PAC, pancreatic adenocarcinoma; TNM stage, staging based on AJCC staging system 8th edition.
Subgroup analysis
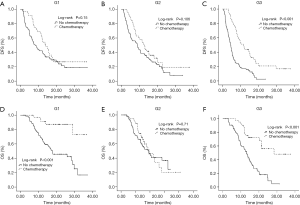
We also examined the prognostic effect of adjuvant CT while in strata of preoperative CA19-9 levels. The multivariable-adjusted HR of DFS for patients in group G3 with CT was 0.32 (95% CI, 0.21–0.49; P<0.001). A similar, although attenuated, differential association of CT with DFS through preoperative CA19-9 was observed in the other two groups (G1: multivariable-adjusted HR, 0.54; 95% CI, 0.33–0.86; P=0.01; G2: multivariable-adjusted HR, 0.63; 95% CI, 0.40–0.97; P=0.037) (Table 2, Figure 2). However, a similar result was not observed in OS. This may be attributed to the influence of several factors on the OS (e.g., multimodal treatment strategy after metastasis), which were not analyzed in this study. We also observed the similar differential association between CT and DFS through preoperative CA19-9 in 282 patients with a serum bilirubin <2 mg/dL (Table S3). In order to strengthen the result, we conducted a sensitivity analysis by excluding 25 patients received adjuvant chemoradiation therapy. A decreasing HR of recurrence risk was also observed in the higher preoperative CA 19-9 group in patients treated with CT only (Table S4).
Table 2
| Chemotherapy and CA19-9 levels | DFS | OS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DFS (mon) | Univariable analysis | Multivariable analysis | OS (mon) | Univariable analysis | Multivariable analysis | ||||||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||||
| G1: ≤87 U/mL | 0.14 | 0.01* | <0.001* | <0.001* | |||||||||
| No chemotherapy | 8.2 | 1 (ref) | 1 (ref) | 17.8 | 1 (ref) | 1 (ref) | |||||||
| Chemotherapy | 13.9 | 0.71 (0.44–1.12) | 0.54 (0.33–0.86) | NR | 0.19 (0.07–0.47) | 0.17 (0.07–0.44) | |||||||
| G2: 87–322 U/mL | 0.096 | 0.037* | 0.77 | 0.502 | |||||||||
| No chemotherapy | 9.0 | 1 (ref) | 1 (ref) | 19.0 | 1 (ref) | 1 (ref) | |||||||
| Chemotherapy | 12.7 | 0.69 (0.45–1.07) | 0.63 (0.40–0.97) | 19.4 | 0.93 (0.55–1.55) | 0.84 (0.49–1.41) | |||||||
| G3: >322 U/mL | <0.001* | <0.001* | <0.001* | <0.001* | |||||||||
| No chemotherapy | 4.5 | 1 (ref) | 1 (ref) | 13.2 | 1 (ref) | 1 (ref) | |||||||
| Chemotherapy | 11.5 | 0.38 (0.25–0.57) | 0.32 (0.21–0.49) | 28.4 | 0.29 (0.16–0.51) | 0.29 (0.17–0.51) | |||||||
Multivariable hazard ratio of DFS was adjusted for: Performance status; Tumor grade; Surgical margin; Chemotherapy; TNM stage; Preoperative CA19-9. Multivariable hazard ratio of OS was adjusted for: Performance status; Surgical margin; Chemotherapy; TNM stage; Preoperative CA19-9. *, statistically significant. CI, confidence interval; DFS, disease-free survival; HR, hazards ratio; NR, not reached; OS, overall survival; PAC, pancreatic adenocarcinoma.
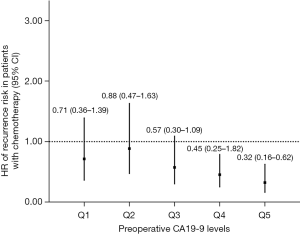
We also conducted sensitivity analysis, excluding patients with normal preoperative CA19-9 levels (≤37 U/mL, N=66). Patients (N=288) were divided into five groups (Q1–Q5) according to their CA19-9 levels (in quintile, 57/58 patients per group). As shown in Table 3, CT was significantly associated with decreased recurrence risk in Q4 and Q5 groups (CA19-9 >324 U/mL), but not in Q1, Q2 and Q3 groups. A trend of decreasing HR of recurrence risk was observed in the higher preoperative CA 19-9 group treated with CT (Figure 3). Since longer DFS after CT administration may represent better response to CT indirectly, it seems that the patients with higher preoperative CA19-9 levels may benefit more from adjuvant CT, especially in patients with CA19-9 levels >200 U/mL.
Table 3
| CA19–9 levels and chemotherapy | No. (%) | HR (95% CI) | P value |
|---|---|---|---|
| Q1: 37.1–96 U/mL | 57 | 0.32 | |
| Chemotherapy | 17 (29.8) | 0.71 (0.36–1.39) | |
| No chemotherapy | 40 (70.2) | 1 (ref) | |
| Q2: 96.1–199 U/mL | 58 | 0.68 | |
| Chemotherapy | 19 (32.8) | 0.88 (0.47–1.63) | |
| No chemotherapy | 39 (67.2) | 1 (ref) | |
| Q3: 199.1–324 U/mL | 58 | 0.09 | |
| Chemotherapy | 23 (39.7) | 0.57 (0.30–1.09) | |
| No chemotherapy | 35 (60.3) | 1 (ref) | |
| Q4: 324.1–758 U/mL | 58 | 0.009* | |
| Chemotherapy | 22 (37.9) | 0.45 (0.25–0.82) | |
| No chemotherapy | 36 (62.1) | 1 (ref) | |
| Q5: 758.1–6,641 U/mL | 57 | 0.001* | |
| Chemotherapy | 17 (29.8) | 0.32 (0.16–0.62) | |
| No chemotherapy | 40 (70.2) | 1 (ref) |
*, statistically significant. CI, confidence interval; DFS, disease-free survival; HR, hazards ratio; PAC, pancreatic adenocarcinoma.
In addition, the correlation between number of adjuvant CT cycles and survival was analyzed in 119 patients received at least 2 cycles of adjuvant CT (Table S5). The median DFS of patients received ≥6 cycles of CT was longer than that observed in patients received <6 cycles of CT (13.9 vs. 11.5 months), although the difference was not significant (HR 0.89; 95% CI, 0.58–1.38, P=0.61).
Discussion
This study showed that the median DFS and OS of patients with resectable PAC were 8.6 and 19.4 months, respectively. The median DFS in our study was consistent with the CONKO-001 trial (the median DFS: 13.4 vs. 6.7 months in GEM vs. observation group) (8). However, the DFS and OS in our study were much poorer compared to recent clinical trials, e.g., ESPAC04 trial (the median DFS: 13.1 vs. 13.9 months in GEM vs. GEM + capecitabine group) (21), JASPAC01 trial (the median DFS: 11.3 vs. 22.9 months in GEM vs. S-1 group) (22,23) and PRODIGE24 trial (the median DFS: 12.8 vs. 21.6 months in GEM vs. FOLFIRINOX group) (24). It may partially attributes to the high proportion (66.4%) of patients not receiving adjuvant CT in our study. Another reason may be that at least 10% of patients had a postoperative CA19-9 level higher than 180 U/mL within 12 weeks after surgery in our study, whereas those patients with postoperative CA19-9 higher than 180 U/mL were excluded in some recent trials.
Due to the inherent biological aggressiveness of pancreatic cancer, a significant number of patients present with metastatic disease shortly after curative surgery. Only few patients achieve longer survival or even cure. It would be helpful to make adjust treatment strategy if we can identify patients with an aggressive tumor and worse prognosis before surgery. The CA19-9 tumor antigen was initially defined in colorectal cancer (25) and is currently the most widely used clinical biomarker in pancreatic cancer. Preoperative CA19-9 is the only biomarker for resectable PAC approved by the Food and Drug Administration of the USA. Ferrone et al., show that preoperative CA19-9 levels correspond to pathologic stage. They concluded that higher CA19-9 levels should raise suspicion of a more extensive tumor burden (19). In our study, the proportion of stage III in G3 group (CA19-9 levels >322 U/mL) was the highest, although no remarkable correlation between preoperative CA19-9 and TNM stage. It might suggest that the current pathologic stage may not be perfect, which could not represent tumor invasiveness and biological behavior. We found that high preoperative CA19-9 level was positivly correlated with high probability of R1 surgical margin. Thus, carefully confirmation of surgical margins or extended resection during surgery may be needed for those patients with high CA19-9. Our results also showed that elevated CA19-9 levels were associated with increased risk of recurrence and death, which is consistent with Bergquist’s study using NCDB data. They found that elevated preoperative CA19-9 levels were associated with decreased survival in patients with early stage (I/II) disease (26).
Since 1997, randomized clinical trials have demonstrated improved survival with adjuvant CT (27). In recent years, GEM + Capetabine, S-1 and FOLFIRINOX have become better selections of adjuvant therapy for PAC compared with GEM monotherapy. GEM + Capetabine and FOLFIRINOX are preferred regiments according to NCCN guidelines. However, we found that FOLFIRINOX is not practically manageable due to its toxicity and poor compliance. S-1 has shown non-inferiority to GEM as adjuvant CT in Asian patients according to JASPAC01 trial. Moreover, GEM + S1 regiment is superior to GEM monotherapy in GEST trial. Hence, GEM, S1 and GEM + S1 are evidence supported and more widely used regiments in our hospital. Although our study showed that patients receiving adjuvant CT were associated with a 54% decrease in the risk of recurrence and a 60% decrease in the risk of death compared to those without CT, the DFS remained short compared to recent trials. The main reasons may include: (I) higher proportion patients did not receive adjuvant CT (66.4%) due to surgery-related side effects, economic factors or patient preference; (II) unselected patients according to postoperative CA19-9. Multiple studies have reported that a decrease in CA19-9 level in patients with unresectable pancreatic cancer was associated with response to therapy (28-30). But few studies have reported the association between preoperative CA19-9 level and response to therapy in resectable disease. Our data indicated that patients in higher preoperative CA19-9 group could benefit more and achieved a more significantly decreased risk of recurrence (HR 0.32) from adjuvant CT. Furthermore, similar results were observed in sensitivity analyses by excluding patients with normal CA19-9 levels, or patients with a serum bilirubin >2 mg/dL at the time of CA19-9 measurement. According to these results, we concluded that CA19-9 value of 322 U/mL may act as a candidate of cutoff value for identifying patients who could benefit more from adjuvant CT, even more extensive adjuvant therapies.
This study had the following limitations. We did not detect Lewis antigen of red cell phenotyping which is indispensable for expression of the CA19-9 antigen. However, it has been reported that the CA19-9 nonsecretors and normal-level patients achieve equivalent survival (26). Moreover, heterogeneity of patients was inevitable due to the features of retrospective study. For example, only 33.6% of patients received CT, including 25 patients who received chemoradiation therapy. However, the number of patients received adjuvant CT were homogeneous in G1, G2 and G3 group (Table S1). Furthermore, we conducted a sensitivity analysis excluding patients received chemoradiation therapy and drew a similar result (Table S4).
Conclusions
In conclusion, our data suggest that higher preoperative serum CA19-9 levels was associated with poorer DFS and OS, as well as a higher probability of R1 resection in PAC patients had radical resection. We also observed a trend of decreasing HR of recurrence risk in the higher preoperative CA 19-9 group treated with CT. These results suggest that the patients with higher preoperative CA19-9 levels may benefit more from adjuvant CT, especially in the patients with CA19-9 levels >322 U/mL. Preoperative CA19-9 might be a helpful biomarker to identify a highly aggressive PAC subgroup, which might benefit more from postoperative CT. It can also be combined with TNM stage to classify PAC patients and make surgery and adjuvant treatment strategy more accurately.
Table S1
| Variables | Total, n (%) | Preoperative CA19–9, n (%) | P value | ||
|---|---|---|---|---|---|
| G1: ≤87 U/mL | G2: 87~322 U/mL | G3: >322 U/mL | |||
| No. of patients | 118 | 118 | 118 | ||
| Age (years) | 0.43 | ||||
| ≤60 | 189 (53.4) | 63 (53.4) | 58 (49.2) | 68 (57.6) | |
| >60 | 165 (46.6) | 55 (46.6) | 60 (50.8) | 50 (42.4) | |
| Gender | 0.64 | ||||
| Male | 224 (63.3) | 71 (60.2) | 75 (63.6) | 78 (66.1) | |
| Female | 130 (36.7) | 47 (39.8) | 43 (36.4) | 40 (33.9) | |
| Performance status | 0.12 | ||||
| 0 | 76 (21.5) | 34 (28.8) | 22 (18.6) | 20 (16.9) | |
| 1 | 170 (48.0) | 56 (47.5) | 57 (48.3) | 57 (48.3) | |
| 2 | 108 (30.5) | 28 (23.7) | 39 (33.1) | 41 (34.7) | |
| Location | 0.4 | ||||
| Head and neck | 236 (66.7) | 82 (69.5) | 81 (68.6) | 73 (61.9) | |
| Body and tail | 118 (33.3) | 36 (30.5) | 37 (31.4) | 45 (38.1) | |
| Tumor grade | 0.3 | ||||
| Well-moderate | 206 (58.2) | 73 (61.9) | 71 (60.2) | 62 (52.5) | |
| Poor | 148 (41.8) | 45 (38.1) | 47 (39.8) | 56 (47.5) | |
| Surgical margin | 0.023* | ||||
| R0 | 337 (95.2) | 117 (99.2) | 112 (94.9) | 108 (91.5) | |
| R1 | 17 (4.8) | 1 (0.8) | 6 (5.1) | 10 (8.5) | |
| TNM stage | 0.11 | ||||
| I | 168 (47.5) | 49 (41.5) | 63 (53.4) | 56 (47.4) | |
| II | 158 (44.6) | 61 (51.7) | 49 (41.5) | 48 (40.7) | |
| III | 28 (7.9) | 8 (6.8) | 6 (5.1) | 14 (11.9) | |
| Chemotherapy | 0.52 | ||||
| Yes | 119 (33.6) | 35 (29.7) | 43 (36.4) | 41 (34.7) | |
| No | 235 (66.4) | 83 (70.3) | 75 (63.6) | 77 (65.3) | |
*, statistically significant. PAC, pancreatic adenocarcinoma.
Table S2
| Variables | DFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age (years) | 0.78 | 0.049* | 0.21 | ||||||||
| ≤60 | 1 (ref) | NA | NA | 1 (ref) | 1 (ref) | ||||||
| >60 | 0.96 (0.74–1.25) | 1.40 (1.00–1.95) | 1.25 (0.88–1.77) | ||||||||
| Gender | 0.38 | ||||||||||
| Male | 1 (ref) | NA | NA | 1 (ref) | 0.92 | NA | NA | ||||
| Female | 0.88 (0.67–1.17) | 0.98 (0.69–1.40) | |||||||||
| Performance status | |||||||||||
| 0 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| 1 | 1.84 (1.27–2.68) | 0.001* | 1.81 (1.23–2.66) | 0.002* | 1.67 (1.04–2.69) | 0.033* | 1.55 (0.96–2.52) | 0.076 | |||
| 2 | 2.36 (1.58–3.53) | <0.001* | 2.46 (1.62–3.75) | <0.001* | 1.67 (0.99–2.80) | 0.055 | 1.66 (0.97–2.85) | 0.065 | |||
| Location | 0.69 | 0.62 | |||||||||
| Head and neck | 1 (ref) | NA | NA | 1 (ref) | NA | NA | |||||
| Body and tail | 0.95 (0.73–1.24) | 0.92 (0.66–1.29) | |||||||||
| Tumor grade | 0.002* | 0.008 | 0.11 | ||||||||
| Well-moderate | 1 (ref) | 1 (ref) | 1 (ref) | NA | NA | ||||||
| Poor | 1.51 (1.16–1.96) | 1.44 (1.10–1.89) | 1.32 (0.94–1.84) | ||||||||
| Surgical margin | 0.026* | 0.009* | 0.004* | 0.011* | |||||||
| R0 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| R1 | 1.94 (1.08–3.49) | 2.27 (1.23–4.19) | 2.51 (1.35–4.66) | 2.29 (1.21–4.33) | |||||||
| Chemotherapy | <0.001* | <0.001* | <0.001* | <0.001* | |||||||
| No | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| Yes | 0.54 (0.41–0.72) | 0.44 (0.33–0.59) | 0.42 (0.29–0.62) | 0.39 (0.26–0.58) | |||||||
| TNM stage | |||||||||||
| III | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| II | 0.44 (0.28–0.70) | 0.001* | 0.62 (0.39–1.00) | 0.051 | 0.38 (0.23–0.65) | <0.001* | 0.51 (0.30–0.88) | 0.016* | |||
| I | 0.37 (0.23–0.59) | <0.001* | 0.42 (0.26–0.68) | <0.001* | 0.32 (0.19–0.54) | <0.001* | 0.33 (0.19–0.58) | <0.001* | |||
| Preoperative CA19–9 | |||||||||||
| G3 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| G2 | 0.68 (0.49–0.94) | 0.018* | 0.68 (0.49–0.94) | 0.021* | 0.86 (0.58–1.26) | 0.43 | 0.92 (0.62–1.37) | 0.68 | |||
| G1 | 0.60 (0.43–0.83) | 0.002* | 0.61 (0.43–0.85) | 0.004* | 0.56 (0.37–0.86) | 0.008* | 0.58 (0.37–0.91) | 0.017* | |||
*, statistically significant. CI, confidence interval; DFS, disease-free survival; HR, hazards ratio; G1, CA19-9≤87 U/mL; G2, CA19-9 87-322 U/mL; G3, CA19-9>322 U/mL; NA, not applicable; OS, overall survival; PAC, pancreatic adenocarcinoma; TNM stage, staging based on AJCC staging system 8th edition.
Table S3
| Chemotherapy and CA19-9 levels | DFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| G1: ≤87 U/mL | 0.14 | 0.021* | 0.003* | 0.003* | |||||||
| No chemotherapy | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| Chemotherapy | 0.68 (0.41–1.14) | 0.54 (0.32–0.91) | 0.24 (0.09–0.62) | 0.24 (0.09–0.61) | |||||||
| G2: 87–322 U/mL | 0.047* | 0.014* | 0.50 | 0.30 | |||||||
| No chemotherapy | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| Chemotherapy | 0.62 (0.39–0.99) | 0.55 (0.34–0.88) | 0.82 (0.47–1.44) | 0.74 (0.42–1.31) | |||||||
| G3: >322 U/mL | <0.001* | <0.001* | <0.001* | <0.001* | |||||||
| No chemotherapy | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| Chemotherapy | 0.31 (0.19–0.52) | 0.30 (0.18–0.50) | 0.21 (0.10–0.44) | 0.23 (0.11–0.47) | |||||||
*, statistically significant. Multivariable hazard ratio of DFS was adjusted for: Performance status; Tumor grade; Surgical margin; Chemotherapy; TNM stage; Preoperative CA19-9. Multivariable hazard ratio of OS was adjusted for: Performance status; Surgical margin; Chemotherapy; TNM stage; Preoperative CA19-9. HR, hazards ratio; CI, confidence interval; DFS, disease-free survival; OS, overall survival.
Table S4
| Chemotherapy and CA19-9 levels | DFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| G1: ≤87 U/mL | 0.17 | 0.012* | 0.001* | 0.001* | |||||||
| No chemotherapy | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| Chemotherapy | 0.70 (0.42–1.16) | 0.51 (0.31–0.86) | 0.19 (0.07–0.53) | 0.18 (0.06–0.49) | |||||||
| G2: 87–322 U/mL | 0.09 | 0.036* | 0.92 | 0.66 | |||||||
| No chemotherapy | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| Chemotherapy | 0.67 (0.41–1.07) | 0.60 (0.37–0.97) | 0.97 (0.57–1.67) | 0.89 (0.51–1.53) | |||||||
| G3: >322 U/mL | <0.001* | <0.001* | <0.001* | <0.001* | |||||||
| No chemotherapy | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |||||||
| Chemotherapy | 0.32 (0.20–0.51) | 0.28 (0.18–0.46) | 0.22 (0.12–0.45) | 0.23 (0.12–0.46) | |||||||
Multivariable hazard ratio of DFS was adjusted for: Performance status; Tumor grade; Surgical margin; Chemotherapy; TNM stage; Preoperative CA19-9. Multivariable hazard ratio of OS was adjusted for: Performance status; Surgical margin; Chemotherapy; TNM stage; Preoperative CA19-9. *, statistically significant. HR, hazards ratio; CI, confidence interval; DFS, disease-free survival; OS, overall survival.
Table S5
| No. of chemo cycles | No. of patients, % | DFS | OS | |||||
|---|---|---|---|---|---|---|---|---|
| Months | HR (95% CI) | P valuea | Months | HR (95% CI) | P valuea | |||
| <6 cycles | 49 (41.2) | 11.5 | 1 (ref) | 0.61 | 28.4 | 1 (ref) | 0.59 | |
| ≥6 cycles | 70 (58.8) | 13.9 | 0.89 (0.58–1.38) | 28.1 | 0.84 (0.46–1.52) | |||
| 2 | 3 (2.5) | NA | NA | |||||
| 3 | 9 (7.6) | |||||||
| 4 | 33 (27.7) | |||||||
| 5 | 4 (3.4) | |||||||
| 6 | 49 (41.2) | |||||||
| 7 | 4 (3.4) | |||||||
| 8 | 17 (14.3) | |||||||
a, P value in univariable hazard ratio of DFS and OS. CI, confidence interval; DFS, disease-free survival; HR, hazards ratio; NA, not applicable; OS, overall survival; PAC, pancreatic adenocarcinoma.
Acknowledgments
We thank all staffs of the Department of Medical Oncology of the Chinese People’s Liberation Army General Hospital.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.04.24). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the retrospective nature of the study. This retrospective study was approved by the ethics committee of the Chinese People’s Liberation Army (PLA) General Hospital (No. S2018-041-01).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA 2016;66:115-32. [PubMed]
- Shi S, Hua J, Liang C, et al. Proposed Modification of the 8th Edition of the AJCC Staging System for Pancreatic Ductal Adenocarcinoma. Ann Surg 2019;269:944-50.
- Kamarajah SK, Burns WR, Frankel TL, et al. Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (SEER) Analysis. Ann Surg Oncol 2017;24:2023-30.
- Stocken DD, Büchler MW, Dervenis C, et al. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer 2005;92:1372-81. [Crossref] [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073-81. [Crossref] [PubMed]
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. [Crossref] [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 782-4. [Crossref] [PubMed]
- Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet (London, England) 2001;358:1576-85. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Kimura K, Amano R, Nakata B, et al. Clinical and pathological features of five-year survivors after pancreatectomy for pancreatic adenocarcinoma. World J Surg Oncol 2014;12:360. [Crossref] [PubMed]
- Wang J, Estrella JS, Peng L, et al. Histologic tumor involvement of superior mesenteric vein/portal vein predicts poor prognosis in patients with stage II pancreatic adenocarcinoma treated with neoadjuvant chemoradiation. Cancer 2012;118:3801-11. [Crossref] [PubMed]
- Yamamoto T, Yagi S, Kinoshita H, et al. Long-term survival after resection of pancreatic cancer: a single-center retrospective analysis. World J Gastroenterol 2015;21:262-8. [Crossref] [PubMed]
- Chang DK, Johns AL, Merrett ND, et al. Margin clearance and outcome in resected pancreatic cancer. J Clin Oncol 2009;27:2855-62. [Crossref] [PubMed]
- Osipov A, Naziri J, Hendifar A, et al. Impact of margin status and lymphadenectomy on clinical outcomes in resected pancreatic adenocarcinoma: implications for adjuvant radiotherapy. J Gastrointest Oncol 2016;7:239-47. [PubMed]
- Zhang S, Wang YM, Sun CD, et al. Clinical value of serum CA19-9 levels in evaluating resectability of pancreatic carcinoma. World J Gastroenterol 2008;14:3750. [Crossref] [PubMed]
- Nishio K, Kimura K, Amano R, et al. Preoperative predictors for early recurrence of resectable pancreatic cancer. World J Surg Oncol 2017;15:16. [Crossref] [PubMed]
- Ferrone CR, Finkelstein DM, Thayer SP, et al. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol 2006;24:2897-902. [Crossref] [PubMed]
- Sugiura T, Uesaka K, Kanemoto H, et al. Serum CA19-9 is a significant predictor among preoperative parameters for early recurrence after resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:977-85. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet (London, England) 2016;388:248-57. [Crossref] [PubMed]
- Maeda A, Boku N, Fukutomi A, et al. Randomized phase III trial of adjuvant chemotherapy with gemcitabine versus S-1 in patients with resected pancreatic cancer: Japan Adjuvant Study Group of Pancreatic Cancer (JASPAC-01). Jpn J Clin Oncol 2008;38:227-9. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. Unicancer GI PRODIGE 24/CCTG PA.6 trial: A multicenter international randomized phase III trial of adjuvant mFOLFIRINOX versus gemcitabine (gem) in patients with resected pancreatic ductal adenocarcinomas. J Clin Oncol 2018;36:LBA4001. [Crossref]
- Koprowski H, Steplewski Z, Mitchell K, et al. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet 1979;5:957-71. [Crossref] [PubMed]
- Bergquist JR, Puig CA, Shubert CR, et al. Carbohydrate Antigen 19-9 Elevation in Anatomically Resectable, Early Stage Pancreatic Cancer Is Independently Associated with Decreased Overall Survival and an Indication for Neoadjuvant Therapy: A National Cancer Database Study. J Am Coll Surg 2016;223:52-65. [Crossref] [PubMed]
- Neoptolemos JP, Baker P, Beger H, et al. Progress report. A randomized multicenter European study comparing adjuvant radiotherapy, 6-mo chemotherapy, and combination therapy vs no-adjuvant treatment in resectable pancreatic cancer (ESPAC-1). Int J Pancreatol 1997;21:97-104. [PubMed]
- Gogas H, Lofts FJ, Evans TR, et al. Are serial measurements of CA19-9 useful in predicting response to chemotherapy in patients with inoperable adenocarcinoma of the pancreas? Br J Cancer 1998;77:325-8. [Crossref] [PubMed]
- Berger AC, Meszoely IM, Ross EA, et al. Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol 2004;11:644-9. [Crossref] [PubMed]
- Luo G, Jin K, Guo M, et al. Patients with normal-range CA19-9 levels represent a distinct subgroup of pancreatic cancer patients. Oncol Lett 2017;13:881-6. [Crossref] [PubMed]