Successful treatment of giant mesenteric fibromatosis with surgery and tamoxifen: case report
Introduction
Aggressive fibromatosis (AF), also known as desmoid-type fibromatosis, originates from deep soft tissues, frequently invades the adjacent muscle or adipose tissue, is histologically classified as mesenchymal tumor, and is characterized by fibroblast hyperplasia (1). MF is a type of AF that develops in the small mesenteric region, retroperitoneal mesocolon, gastric colonic ligament, and omentum (2). MF was first reported by Stout in 1954 (3). The morbidity rates associated with AF range from 2.0% to 4.0% per million people annually, whereas the male/female ratio is approximately 5:7 (4). However, only 10–20% of cases with AF present with MF (5). The etiopathogenesis of MF includes lesions secondary to trauma, such as pregnancy or childbirth in some cases; disorders of the endocrine system; or abnormality of the Y chromosome (6,7). Choi et al. elucidated the relationship between genetic factors and Gardner syndrome in aggressive fibroids, and found that approximately one-third of Gardner syndrome patients subsequently developed intra-abdominal aggressive fibroids, whereas only 2% of patients with intra-abdominal aggressive fibroids had Gardner syndrome (8). Although surgical resection is the primary treatment approach, non-surgical therapy is also crucially beneficial for patients, given the high recurrence rate (9).
Case presentation
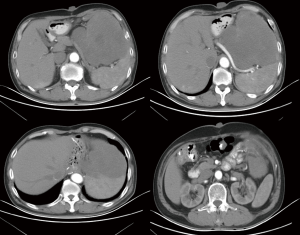
A 65-year-old man was admitted to the northern Jiangsu People’s Hospital (Yangzhou, China) on July 14, 2017, and presented with a 4-month history of lower abdominal swelling, which had gradually progressed to nausea and vomiting. Body examination revealed a non-tender mass in the upper abdomen. Computed tomography (CT) showed a 16 cm × 12 cm space-occupying lesion in the left upper abdomen (Figure 1). The lesion was considered to be a gastrointestinal stromal tumor (GIST) according to the imaging characteristics; however, given that the patient had undergone proximal gastrectomy for gastric carcinoma 3 years previously, its likelihood as recurrence and metastasis of the gastric cancer could not be completely eliminated.
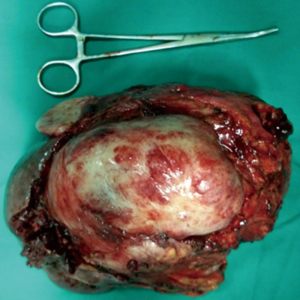
The tumor was large and located adjacent to the spleen; hence, radical removal was challenging. Our initial therapeutic strategy involved imatinib chemotherapy if the tumor was confirmed as a GIST. We performed CT-guided needle biopsy, and detected spindle cells under pathological examination, which suggests that the tumor may be derived from nervous tissue. Due to the presence of compression symptoms, the patient underwent surgery on August 2, 2017. The tumor originated from the surface of the transverse colon and adhered to the spleen. The tumor was completely resected via splenectomy and segmental transverse colectomy (Figure 2). The operation time was 200 min, intraoperative blood loss was 1,000 mL, and the amount of erythrocytes and plasma transfused was 450 and 400 mL, respectively.
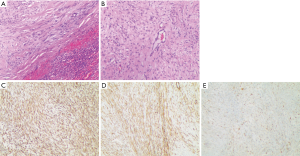
Neoplasm tissues were fixed in formalin, made into paraffin sections, and stained with hematoxylin and eosin. HE staining showed that abundant cells were arranged in a fascicle and whirlpool manner. Mitotic figures and interstitial mucous changes were also observed in some regions (Figure 3A,B). The pathological diagnosis was MF. Immunohistochemistry indicated vimentin(+), SMA(+), and β-catenin(+) (Figure 3C,D,E). The patient developed left pleural effusions on the 10th day after the operation and was treated accordingly.
A genetic test was performed to ensure accurate treatment, given the reported aggressiveness and high risk of recurrence associated with the tumor. It was only mildly sensitive to imatinib, moderately sensitive to interferon, and extremely sensitive to tamoxifen. No symptoms of abdominal discomfort or signs of recurrence signs via CT were noted, and the patient regularly received tamoxifen and careful follow-up for 1 year.
Discussion
The differential diagnosis of MF with GIST, smooth muscle tumor, leiomyosarcoma, neurofibromatosis, and schwannoma is challenging (6). MF is slow-growing and asymptomatic in the pre-clinical duration of disease. Patients may present with symptoms such as abdominal mass, abdominal distension, abdominal pain, black feces, incomplete intestinal obstruction, hydrocephalus, fever with the progression of MF. Previous medical history, including pregnancy, trauma, estrogen use, and Gardner syndrome, are significant for diagnosis (10).
The CT characteristics of MF include a single mass with contrast enhancement and heterogeneous density infiltrating the surrounding tissues and mesenteric blood vessels surrounding the lesion. In fact, the mesenteric blood vessels surrounding the lesion is a representative image, and can offer significant information for clinical diagnosis (11). However, abdominal CT examinations are limited to intestinal arteries, respiratory motion, and gastrointestinal contents formed by artifacts. The rate of space is insufficient, and the clinical diagnosis is comparatively limited (12). Abdominal ultrasonography can be performed under dynamic conditions, and can accurately indicate the intestinal wall, mesentery, and abdominal wall; Puncture biopsy with ultrasound-guided is inexpensive and feasible. Comprehensive consideration of multiple auxiliary examinations for each potentially diagnosed patients could improve the accuracy of the diagnosis (13). Pathological examination is the gold standard for MF diagnosis, and always reveals certain common features such as lumps of hard texture, rough parts, and gray and braided structure. The proliferation of fibroblasts and collagen fibers can also be observed under a microscope. Moreover, the morphology of cells, which are arranged in a parallel, wavy, or bundled manner, remains consistent in various patients. The cytoplasm is also stained a rich pink color, and chromatin staining indicates its sparse or vacuolar nature, along with a small nucleolus, rare mitotic and specificity. The tumor invades the surrounding intestinal wall, including smooth muscle and adipose tissue. In cases where the tumor is necrotic, focal mucinous degeneration, lymphocytic infiltration, and vasodilation can be observed (14).
It is difficult to distinguish between MF, GIST, and normal mesentery through microscopic pathological examination alone. Immunohistochemistry can help in the differential diagnosis. The strong positive expression of vimentin and SMA in mesenteric fibroma indicates that the section of fusiform tumor cells includes myofibroblasts (15). In certain cases, desmin protein is expressed. β-catenin is a key factor in the Wnt pathway, and its positive expression is important for the diagnosis and differential diagnosis of MF. MF usually presents with an inactivated APC gene in the Wnt pathway, which prevents the degradation of β-catenin and promotes accumulation in the tumor cell (16).
The non-surgical approach also plays a key role in the treatment for MF. The trauma caused by surgery may be the initial reason for developing AF. Some evidence suggests that surgery can lead to myofiber injury, hemorrhage, and even hematoma formation, which could promote the development of tumors; however, the underlying mechanism remains unclear (15). Our patient had a history of radical surgery for proximal gastric cancer, which could have induced the occurrence of MF.
The diffuse invasive growth pattern of the tumor could contribute to the high recurrence rate after surgery, and hence, postoperative adjuvant therapy is particularly important in these cases. Moreover, in cases where the tumor is unresectable, non-surgical treatment may be preferred. Several studies have indicated that comprehensive treatment is effective for MF. Non-surgical treatments, hormone therapy (tamoxifen) (17), Nonsteroidal anti-inflammatory drugs (NSAIDs), low-dose interferon, systemic chemotherapy, imatinib, and doxorubicin are reportedly beneficial for MF (18). Postoperative local radiotherapy may also be used, although the efficacy and side effects are unclear. A case of MF along with hydronephrosis and Gardner syndrome was treated with cyclooxygenase-2 inhibitor, and the patient was cured without requiring surgery (19).
Seinen et al. reported four different treatment strategies for AF: surgery alone, surgery with adjuvant radiotherapy, radiotherapy alone, and a wait-and-see approach (20). The findings indicated that the observation group had a lower local recurrence rate than the surgery alone group (P=0.001). Therefore, we ensured regular post-operative follow-up for our patient due to the high likelihood of recurrence. Multiple recurrences can lead to expansion of the range of lesions and may affect various important organs. Precision treatment could benefit these patients, with the continuous advancement in medical technology.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.05.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Efthimiopoulos GA, Chatzifotiou D, Drogouti M, et al. Primary asymptomatic desmoid tumor of the mesentery. Am J Case Rep 2015;16:160-3. [Crossref] [PubMed]
- Wronski M, Ziarkiewicz-Wroblewska B, Slodkowski M, et al. Mesenteric fibromatosis with intestinal involvement mimicking a gastrointestinal stromal tumour. Radiol Oncol 2011;45:59-63. [Crossref] [PubMed]
- Stout AP. Juvenile fibromatoses. Cancer 1954;7:953-78. [Crossref] [PubMed]
- van Broekhoven DL, Grünhagen DJ, den Bakker MA, et al. Time trends in the incidence and treatment of extra-abdominal and abdominal aggressive fibromatosis: a population-based study. Ann Surg Oncol 2015;22:2817-23. [Crossref] [PubMed]
- Bn A, Cd JK, Ps S, et al. Giant aggressive mesenteric fibromatosis- a case report. J Clin Diagn Res 2015;9:PD07-8. [PubMed]
- Dumont AG, Rink L, Godwin AK, et al. A nonrandom association of gastrointestinal stromal tumor (GIST) and desmoid tumor (deep fibromatosis): case series of 28 patients. Ann Oncol 2012;23:1335-40. [Crossref] [PubMed]
- Reitamo JJ, Scheinin TM, Häyry P. The desmoid syndrome. New aspects in the cause, pathogenesis and treatment of the desmoid tumor. Am J Surg 1986;151:230-7. [Crossref] [PubMed]
- Choi JY, Kang KM, Kim BS, et al. Mesenteric fibromatosis causing ureteral stenosis. Korean J Urol 2010;51:501-4. [Crossref] [PubMed]
- Chu Y, Guo Q, Wu D. Mesenteric fibromatosis after resection for gastrointestinal stromal tumor of stomach: A case report. Medicine (Baltimore) 2017;96:e8792. [Crossref] [PubMed]
- Mahajan A, Singh M, Varma A, et al. Mesenteric fibromatosis presenting as a diagnostic dilemma: a rare differential diagnosis of right iliac fossa mass in an eleven year old-a rare case report. Case Rep Surg 2013;2013:569578. [Crossref] [PubMed]
- Zhu H, Chen H, Zhang S, et al. Intra-abdominal fibromatosis: differentiation from gastrointestinal stromal tumour based on biphasic contrast-enhanced CT findings. Clin Radiol 2013;68:1133-9. [Crossref] [PubMed]
- Sohn MH, Jeong YJ, Lim ST, et al. F-18 FDG PET/CT Findings of Spontaneous Mesenteric Fibromatosis in a Patient with Gardner's Syndrome. Nucl Med Mol Imaging 2011;45:156-7. [Crossref] [PubMed]
- Karbeet Radhakrishna G, Bhat PR, Shenoy RK, et al. Primary mesenteric fibromatosis: a case report with brief review of literature. Indian J Surg 2013;75:131-3. [Crossref] [PubMed]
- Coffin CM, Hornick JL, Zhou H, et al. Gardner fibroma: a clinicopathologic and immunohistochemical analysis of 45 patients with 57 fibromas. Am J Surg Pathol 2007;31:410-6. [Crossref] [PubMed]
- Zeng WG, Zhou ZX, Liang JW, et al. Prognostic factors for desmoid tumor: a surgical series of 233 patients at a single institution. Tumour Biol 2014;35:7513-21. [Crossref] [PubMed]
- Thway K, Abou Sherif S, Riddell AM, et al. Fibromatosis of the Sigmoid Colon With CTNNB1 (β-Catenin) Gene Mutation, Arising at the Site of Ileocolic Anastomosis for Resection of Gastrointestinal Stromal Tumor. Int J Surg Pathol 2016;24:264-8. [Crossref] [PubMed]
- Skapek SX, Anderson JR, Hill DA, et al. Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: results of a Children's Oncology Group (COG) phase II study. Pediatr Blood Cancer 2013;60:1108-12. [Crossref] [PubMed]
- Chao AS, Lai CH, Hsueh S, et al. Successful treatment of recurrent pelvic desmoid tumour with tamoxifen: case report. Hum Reprod 2000;15:311-3. [Crossref] [PubMed]
- Ng TY, Yang MD, Chen YF, et al. Resolution of hydronephrosis due to massive mesenteric fibromatosis using cyclo-oxygenase 2 inhibitors. Urology 2007;70:591.e3-4. [Crossref] [PubMed]
- Seinen JM, Niebling MG, Bastiaannet E, et al. Four different treatment strategies in aggressive fibromatosis: A systematic review. Clin Transl Radiat Oncol 2018;12:1-7. [Crossref] [PubMed]