
Efficacy of re-resection versus radiofrequency ablation for recurrent Barcelona Clinic Liver Cancer stage 0/A hepatocellular carcinoma (HCC) after resection for primary HCC
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common neoplasm worldwide and the third most common cause of cancer-related death (1,2). The reported 5-year survival rate of HCC patients ranges from 40% to 50% (3-5), and the 5-year recurrence rate in Eastern and Western countries ranges from 60% to 80% (6,7). Intrahepatic recurrence is the most common form of recurrent HCC and reported in up to 68% to 96% of patients (8). Because of the high recurrence rate, the long-term survival after hepatectomy is still unsatisfactory. An effective treatment strategy for recurrent HCC should prolong the survival of patients after resection for primary HCC (9). Although guidelines on the diagnosis and treatment of primary HCC have been published and up-dated (10,11), no guidelines have been developed for the management of recurrent HCC. Strategies for treating primary HCC, including surgical resection, split liver transplantation (SLT), transcatheter arterial chemoembolization (TACE) and radiofrequency ablation (RFA), have been used to treat recurrent HCC. However, the optimal treatment for recurrent HCC remains controversial (12). Although SLT may yield the best survival outcome because it can eliminate tumors and cirrhosis (13,14), it is often difficult to perform before the disease progression due to the lack of liver donors and strict patient selection criteria (15). TACE has also been widely used for the treatment of HCC recurrence and possesses a definite inhibitory effect on the residual tumors and tumor cell dissemination after tumor resection, but the 5-year survival rate following TACE is still low (0% to 50%) (16,17). Repeat surgical resection (RSR) is typically considered the first-line treatment for recurrent HCC (5), but it is complex and has many severe complications. In addition, only a small proportion (7–30%) of patients with recurrent HCC are eligible for RSR because the majority of patients have poor liver reserve function and/or diffuse intrahepatic recurrence (18,19). During the past two decades, RFA has been widely employed as a minimally invasive treatment for various solid tumors including recurrent HCC (20). Compared with surgical resection, RFA has many advantages, including less trauma, high repeatability, rapid recovery, less pain, and fewer complications. Thus, it has been considered the best alternative to surgical resection (21). Some studies have compared the clinical outcomes of RFA versus RSR for recurrent small HCC (≤3 cm in tumor diameter or ≤3 tumor nodules). The present study was designed to compare the efficacy of RFA versus RSR in the treatment of recurrent Barcelona Clinic Liver Cancer (BCLC) stage 0/A HCC after resection for primary HCC.
Methods
General data
This retrospective study involved 6,421 patients who underwent hepatectomy for HCC in the Affiliated Hospital of Nantong University and Third Affiliated Hospital of Second Military Medical University and developed recurrences between January 2008 and March 2018. Among them, 132 patients were diagnosed with BCLC stage 0/A HCC and underwent RSR or RFA, 24 of whom were lost to follow-up (9 received RSR and 15 received RFA). Finally, 108 patients were included in the final analysis. Of these, 57 patients underwent RSR and 51 received RFA. The general characteristics, clinicopathological features, and survival outcomes of these patients were retrospectively analyzed.
Follow-up and diagnosis
All patients underwent abdominal computed tomography (CT) or magnetic resonance imaging (MRI) within 1 month after hepatectomy to confirm complete removal of the tumor. Detection of serum alpha-fetoprotein (AFP), liver biochemistry, abdominal ultrasonography, and CT/MRI were performed once every 3 months. Intrahepatic recurrence was defined as new lesions on ultrasonography, CT and/or MRI with or without elevated serum AFP. The median follow-up time in the RSR group and RFA group was 41 months (range, 8–75 months) and 39 months (range, 8–78 months), respectively.
Treatment strategies
The inclusion criteria were as follows: (I) patients were diagnosed with recurrent BCLC stage 0/A HCC after resection of primary HCC; (II) a single tumor had the maximum diameter of ≤5 cm or multiple tumor nodules (≤3) had the largest diameter of ≤3 cm; (III) there were no macrovascular invasion and no lymph node or extrahepatic metastasis; (IV) the patients had good general condition, Child–Pugh class A/B liver function and no lesions in other organs; (V) the indocyanine green retention rate at 15 min (ICG R15) was ≤10%; (VI) there were no other interventions such as percutaneous intratumoral ethanol injection or transarterial chemoembolization (TACE); (VII) patients or their relatives signed the informed consent. The exclusion criteria were as follows: (I) there was any irreversible hepatic decompensation or severe portal hypertension such as massive refractory ascites, jaundice, hepatic encephalopathy, or moderate/severe esophageal varices; (II) there was severe coagulation dysfunction (prothrombin time of <50% and platelet count of <50×109/L); (III) there was evident vascular infiltration, cancer-associated thrombosis, or lymph node or extrahepatic metastasis.
Statistical analysis
Statistical analysis was performed using the SAS 9.4 software (SAS Institute, Cary, NC, USA). The normally distributed quantitative variables are expressed as mean ± standard deviation, and the intergroup comparisons were done with t-test. The abnormally distributed variables are presented as median ± interquartile range, and the intergroup comparisons were performed with rank sum test. The qualitative variables are presented with percentages, and the inter-group comparisons were done with Chi square test or Fisher’s exact test. The overall survival (OS) rate was analyzed with the Kaplan-Meier method, and the difference between two groups was compared with the log-rank test. Univariate and multivariate Cox proportional hazards models were employed to analyze the prognostic factors after recurrence. A value of two-tailed P<0.05 was considered statistically significant.
Results
Baseline characteristics of primary HCC
Table 1 shows the pathological features and treatments of primary HCC. The two groups had similar characteristics with respect to the tumor size, degree of tumor cell differentiation, number of tumor nodules, microvascular invasion, BCLC stage (10), and clinical stage of HCC [according to the Guidelines for the Diagnosis and Treatment of Primary Liver Cancer in China (2017 edition) (11)]. The serum AFP level was similar between two groups. Most patients underwent open surgery and 13 patients underwent laparoscopic surgery for primary HCC. The number of patients who underwent minor hepatectomy (removal of one liver segment) or major hepatectomy (removal of two or more segments) was also comparable between two groups.
Table 1
| Characteristic | RSR (n=57) | RFA (n=51) | P value |
|---|---|---|---|
| BCLC stage | 0.307 | ||
| 0 | 7 (12.28) | 10 (19.61) | |
| A | 49 (85.96) | 38 (74.51) | |
| B | 1 (1.75) | 3 (5.88) | |
| Tumor size (cm) | 3.3 (2.3) | 3.4 (2.1) | 0.442 |
| Minor/major hepatectomy | 0.318 | ||
| Minor | 45 (78.95) | 44 (86.27) | |
| Major | 12 (21.05) | 7 (13.73) | |
| Tumor cell differentiation | 0.314 | ||
| Well | 28 (49.12) | 25 (49.02) | |
| Moderate | 25 (43.86) | 18 (35.29) | |
| Poor | 4 (7.02) | 8 (15.69) | |
| AFP level (ng/mL) | 641.82 (386.19) | 584.80 (533.20) | 0.212 |
| Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition) | 0.532 | ||
| I | 56 (98.25) | 48 (94.12) | |
| II | 1 (1.75) | 3 (5.88) | |
| No. of tumor nodules | 1 | ||
| 1 | 53 (92.98) | 48 (94.12) | |
| 2–3 | 3 (5.26) | 3 (5.88) | |
| >3 | 1 (1.76) | 0 | |
| Microvascular invasion | 0.355 | ||
| − | 48 (84.21) | 46 (90.20) | |
| + | 9 (15.79) | 5 (9.80) |
Data are presented as n (%). RSR, repeat surgical resection; RFA, radiofrequency ablation; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein.
Characteristics of recurrent HCC and patients at recurrence
Table 2 shows the characteristics and liver/kidney functions of the patients at the time of intrahepatic recurrence. The age, sex, American Society of Anesthesiologists grade, body mass index, ICG R15, Child-Pugh class, cirrhosis, portal hypertension, incidence of complications, positive rate of viral hepatitis, and other laboratory data were similar between two groups. In the RSR group, 55 patients (96.49%) had Child-Pugh class A liver function and 2 (3.51%) had class B. In the RFA group, 46 patients (90.20%) had Child-Pugh class A liver function and 5 (9.80%) had class B (P=0.19). The ICG R15 was 5.0 (1.9) in the RSR group and 4.8 (1.8) in the RFA group. The liver biochemical markers and renal function were comparable between two groups before and after primary treatment. Table 3 shows the general features of recurrent HCC after primary operation. The time to recurrence, size, location, and number of recurrent tumors and serum AFP level were similar between two groups. The recurrent tumors were also assessed by the Chinese clinical staging system for HCC [Guidelines for the Diagnosis and Treatment of Primary Hepatocellular Carcinoma (2017 edition)] and BCLC staging system, and results showed no significant differences between two groups. No extrahepatic metastasis was found. Notably, biopsy was not performed in the RFA group to prevent tumor metastasis via the puncture access.
Table 2
| Characteristic | RSR (n=57) | RFA (n=51) | P value |
|---|---|---|---|
| Age (year) | 57.05±12.04 | 60.26±9.50 | 0.125 |
| Sex | 0.220 | ||
| F | 16 (28.07) | 20 (39.22) | |
| M | 41 (71.93) | 31 (60.78) | |
| BMI | 24.34±3.30 | 25.26±4.37 | 0.227 |
| ASA | 0.417 | ||
| 1 | 35 (61.40) | 27 (52.94) | |
| 2 | 21 (36.84) | 21 (41.18) | |
| 3 | 1 (1.75) | 3 (5.88) | |
| ICG R15 | 5.0 (1.90) | 4.8 (1.80) | 0.153 |
| Liver cirrhosis (−, +) | 0.300 | ||
| − | 18 (31.58) | 21 (41.18) | |
| + | 39 (68.42) | 30 (58.82) | |
| Portal hypertension (−, +) | 0.485 | ||
| − | 39 (68.42) | 38 (74.51) | |
| + | 18 (31.58) | 13 (25.49) | |
| HBsAg (−, +) | 0.811 | ||
| − | 4 (7.02) | 3 (5.88) | |
| + | 53 (92.98) | 48 (94.12) | |
| Co-morbid illness (−, +) | 0.100 | ||
| − | 39 (68.42) | 27 (52.94) | |
| + | 18 (31.58) | 24 (47.06) | |
| Child-Pugh class | 0.185 | ||
| A | 55 (96.49) | 46 (90.20) | |
| B | 2 (3.51) | 5 (9.80) | |
| Total bilirubin (μmol/L) | 15.2±3.4 | 14.3±4.9 | 0.308 |
| ALT (U/L) | 33 (42.0) | 29 (21.0) | 0.113 |
| AST (U/L) | 35 (12.0) | 38 (21.0) | 0.399 |
| Albumin (g/L) | 34.55±6.93 | 33.30±4.45 | 0.259 |
| Platelet count (×109/L) | 138.60±54.02 | 122.40±52.86 | 0.119 |
| Prothrombin time (s) | 12.43±1.44 | 12.86±1.22 | 0.100 |
| Creatinine (μmoI/L) | 70 (10.0) | 68 (8.0) | 0.124 |
Data are presented as mean ± standard deviation or n (%). RSR, repeat surgical resection; RFA, radiofrequency ablation; F, female; M, male; BMI, body mass index; ASA, American Society of Anesthesiologists; ICG R15, indocyanine green retention rate at 15 min; HBsAg, hepatitis B virus surface antigen; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Table 3
| Characteristic | RSR (n=57) | RFA (n=51) | P value |
|---|---|---|---|
| Time to recurrence (months) | 29 (21.0) | 24 (32.0) | 0.383 |
| Tumors size (cm) | 3.2 (2.5) | 2.6 (0.9) | 0.153 |
| Recurrence sites | 0.8303 | ||
| Local | 28 (49.12) | 24 (47.06) | |
| Intrahepatic | 29 (50.88) | 27 (52.94) | |
| Extrahepatic | – | – | |
| AFP level (ng/mL) | 167.97 (357.23) | 266.32 (420.28) | 0.3623 |
| No. of tumor nodules | 0.838 | ||
| 1 | 52 (91.23) | 48 (94.12) | |
| 2–3 | 5 (8.77) | 3 (5.88) | |
| BCLC stage | 0.1305 | ||
| 0 | 23 (40.35) | 28 (54.90) | |
| A | 34 (59.65) | 23 (45.10) | |
| Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition) stage | 0.838 | ||
| Ia | 52 (91.23) | 48 (94.12) | |
| Ib | 5 (8.77) | 3 (5.88) |
Data are presented as n (%). RSR, repeat surgical resection; RFA, radiofrequency ablation; AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer.
Clinical outcomes of patients with recurrent HCC
Table 4 shows the intraoperative and postoperative complications in two groups. Intraoperative death, postoperative hospital death, and biliary leakage were not observed in both groups. Notably, four patients in the RFA group had incomplete ablation of tumors, among whom three underwent one session of RFA again and one underwent two sessions. In the RSR group, two patients underwent a second open surgery because of heavy postoperative bleeding. The treatment time in the RFA group was much shorter than that in the RSR group {60 [27] vs. 152 [47] min, P<0.001}, and no intraoperative blood transfusion was needed. In contrast, nine patients (15.8%) in the RSR group received intraoperative blood transfusion. The incidence of treatment-related complications was significantly higher in the RSR group than in the RFA group (42.11% vs. 11.76%, P<0.001). The median hospital stay was significantly shorter in the RFA group than in the RSR group (3 vs. 9 days, P<0.001). The postoperative complications were as follows: in the RFA group, only one patient developed atrial fibrillation, which was alleviated by symptomatic treatment; one patient developed pulmonary infection, which was complicated with chronic bronchitis and managed by antimicrobial therapy in a local hospital after discharge; three patients had the hemoglobin level of <70 g/L, which was improved after infusion of concentrated red blood cells; six patients had high fever (body temperature >38.5 °C); three patients had mild pleural effusion, which was improved after symptomatic treatment. Compared with RFA group, more patients in the RSR group had major complications (e.g., cardiopulmonary insufficiency, pulmonary infection, and massive abdominal hemorrhage) or minor complications (e.g., fever, pleural and peritoneal effusion, and incision infection).
Table 4
| Characteristic | RSR (n=57) | RFA (n=51) | P value |
|---|---|---|---|
| Operation time (min) | 156 (47.0) | 60 (27.0) | <0.001 |
| Intraoperative blood transfusion (+/−) | 0.0031 | ||
| − | 48 (84.21) | 51 (100.00) | |
| + | 9 (15.79) | 0 (0) | |
| Postoperative hospital stay (day) | 9 (4.0) | 3 (3.0) | <0.001 |
| Cardiac disorder | 0.4296 | ||
| − | 53 (92.98) | 50 (98.04) | |
| + | 4 (7.02) | 1 (1.96) | |
| Lung infection | 0.008 | ||
| − | 47 (82.46) | 50 (98.04) | |
| + | 10 (17.54) | 1 (1.96) | |
| Intra-abdominal hemorrhage | 0.4969 | ||
| − | 55 (96.49) | 51 (100.00) | |
| + | 2 (3.51) | 0 (0) | |
| Hb <70 g/L | 0.008 | ||
| − | 43 (75.44) | 48 (94.12) | |
| + | 14 (24.56) | 3 (5.88) | |
| Fever (temperature >38.5 °C) | 0.3987 | ||
| − | 47 (82.46) | 45 (88.24) | |
| + | 10 (17.54) | 6 (11.76) | |
| Ascites | <0.001 | ||
| − | 43 (75.44) | 51 (100.00) | |
| + | 14 (24.56) | 0 (0.00) | |
| Pleural effusion | <0.001 | ||
| − | 35 (61.40) | 48 (94.12) | |
| + | 22 (38.60) | 3 (5.88) | |
| Wound infection | 0.059 | ||
| − | 52 (91.23) | 51 (100.00) | |
| + | 5 (8.77) | 0 (0.00) |
Data are presented as n (%). RSR, repeat surgical resection; RFA, radiofrequency ablation; Hb, hemoglobin.
Long-term survival outcomes of patients in two groups
The median follow-up time after the first recurrence in the RSR group and RFA group was 35 months (range, 6–60 months) and 37 months (range, 7–60 months), respectively. The 1-, 3-, and 5-year OS rates were 96.5%, 80.9%, and 60.6% in the RSR group and 96.1%, 76.8%, and 59.4% in the RFA group (P=0.48) (Figure 1). The 1-, 3-, and 5-year OS rates after the treatment for HCC recurrence were 78.9%, 50.5%, and 29.7% in the RSR group and 80.3%, 50.9%, and 26.0% in the RFA group (P=0.67) (Figure 2). The 1-, 3-, and 5-year DFS rates were 68.4%, 39.4%, and 26.6% in the RSR group and 62.8%, 32.8%, and 20.4% in the RFA group (P=0.55) (Figure 3).
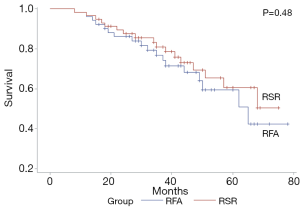
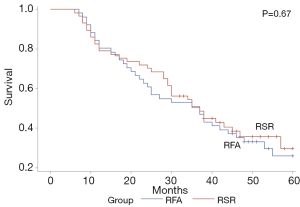
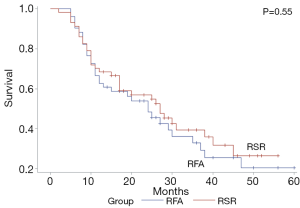
Table 5 shows the patients with second recurrent HCC, including 42 in the RSR group and 40 in the RFA group. The AFP level was not significantly different between the RSR group and the RFA group. The patients with second recurrent HCC mainly received multidisciplinary treatment including TACE with or without target therapy, traditional Chinese medicine treatment, and nutritional support. Cox regression analysis showed the time to the first recurrence and the interval between treatment for the first recurrence and development of second recurrence were prognostic factors for poor OS after resection of primary HCC; they were also significant prognostic factors in the multivariate analysis (Table 6).
Table 5
| Characteristic | RSR | RFA | P value |
|---|---|---|---|
| AFP (ng/mL) | 129.60 (150.32) | 163.19 (145.79) | 0.362 |
| Second recurrence | 57 | 51 | |
| No recurrence | 25 (43.9) | 11 (21.6) | |
| Intrahepatic | 28 (49.1) | 34 (66.7) | |
| Extrahepatic | 4 (7.0) | 6 (11.7) | |
| Treatment of second | 42* | 40 | |
| PEIT | 0 | 4 (10.0) | |
| TACE | 27 (64.3) | 18 (45.0) | |
| Re-resection | 3 (7.1) | 0 | |
| RFA | 5 (12.0) | 12 (30.0) | |
| Conservative | 7 (16.6) | 6 (15.0) |
Data are presented as n (%). *, re-resection. RSR, repeat surgical resection; RFA, radiofrequency ablation; AFP, alpha-fetoprotein; PEIT, percutaneous intratumoral ethanol injection; TACE, transcatheter arterial chemoembolization.
Table 6
| Characteristic | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | 95% CI | 95% CI | P value | ||
| Age (year) | 1.001 | 0.514–1.948 | 0.9986 | ||||
| Sex (M/F) | 0.910 | 0.379–2.187 | 0.8337 | ||||
| Liver cirrhosis (–/+) | 0.670 | 0.304–1.478 | 0.3209 | ||||
| HBsAg (–/+) | 1.656 | 0.223–12.287 | 0.6217 | ||||
| Co-morbid illness (–/+) | 2.186 | 0.765–6.244 | 0.1442 | ||||
| Child-Pugh class (A/B) | 1.370 | 0.680–2.760 | 0.3782 | ||||
| Tumor size (cm) | 1.882 | 0.966–3.664 | 0.0630 | ||||
| Time from initial resection to 1st recurrence (months) | 0.950 | 0.924–0.976 | 0.0002 | 0.970 | 0.949–0.992 | 0.0068 | |
| Time from treatment of 1st recurrent HCC to 2nd recurrence (months) | 0.969 | 0.954–0.985 | 0.0001 | 0.957 | 0.938–0.976 | <.0001 | |
| No. of tumor nodules (1/≥2) | 1.087 | 0.559–2.116 | 0.8057 | ||||
| Total bilirubin (μmol/L) | 1.890 | 0.956–3.738 | 0.0673 | ||||
| ALT (U/L) | 1.418 | 0.617–3.260 | 0.4107 | ||||
| AST (U/L) | 1.051 | 0.504–2.190 | 0.8949 | ||||
| Albumin (g/L) | 1.583 | 0.690–3.631 | 0.2785 | ||||
| Platelet count (×109/L) | 0.855 | 0.438–1.671 | 0.6476 | ||||
| AFP | 1.466 | 0.749–2.871 | 0.2643 | ||||
HR, hazard ratio; CI, confidence interval; M, male; F, female; HBsAg, hepatitis B surface antigen; HCC, hepatocellular carcinoma; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, alpha-fetoprotein.
Discussion
Patterns of HCC recurrence
The 5-year recurrence rate following primary hepatectomy ranges from 60% to 80% (6). Postoperative recurrence is the main cause of failed HCC resection (7,8). In particular, intrahepatic recurrence is the most common form of recurrent HCC and has been reported in up to 68% to 96% of patients (8). Postoperative recurrence of HCC can be divided into two types: intrahepatic metastasis (IM) and multicentric occurrence (MO) (22). Some studies propose an alternative criterion that refers to early and late recurrences as IM (≤1 year) and MO (>1 year), respectively (23). Early recurrence mainly occurs in patients with intrahepatic metastasis. The main causes are the spread of primary cancer due to minimal residual disease after tumor resection and the development of microvascular invasion (24). In contrast, late recurrence is mainly multicentric HCC, which is related to cirrhosis; that is, new cancer foci arise on the background of cirrhosis (25). The vascular invasion, degree of tumor differentiation, and size and number of tumor nodules are reported as risk factors for recurrence (25,26). Therefore, rational clinical treatment should be selected according to the characteristics of recurrent cancer, reserved liver function, and general condition of patients (27).
Comparison between RSR and RFA
Application of RSR and RFA
RSR is the preferred treatment for patients with intrahepatic recurrence and good liver function (28). However, surgical removal of recurrent HCC remains challenging due to the abdominal adhesion after initial surgery and the high-risk location of tumors adjacent to major vessels or bile duct structures (29). RSR is not feasible for most patients with intrahepatic recurrence because the patient are intolerable to anesthesia or surgery due to poor general condition (including old age, poor physical performance and poor cardiopulmonary function), small residual liver volume, and poor reserved liver function. The re-resection rate is reported to be only 7% to 30% in these patients (30). During the past two decades, the emergence of RFA has revolutionized the treatments for HCC (31). RFA can increase the focal temperature by utilizing a high-frequency alternating electrical current via electrodes placed in tissues, which may cause coagulative necrosis of target lesions. As a minimally invasive treatment, it can be performed percutaneously, laparoscopically, or via an open incision (32). It has become a popular treatment due to its advantages, including high repeatability, minimal invasion, mild postoperative pain, quick recovery, few complications, short hospital stay, and low cost (33). For tumors smaller than 2 cm in diameter, the complete ablation rate is reported to be higher than 90%; however, the complete ablation rate decreases as the tumor size increases. In addition, RFA is not recommended for tumors larger than 5 cm in diameter or those in high-risk areas (34). RFA is safe and effective for a single tumor with the maximum diameter of ≤5 cm or multiple tumor nodules (≤3) with the largest diameter of ≤3 cm (35). According to the BCLC staging criteria and the Guidelines for the Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition), patients in the present study were diagnosed with HCC at BCLC stage 0/A or Chinese stage Ia/Ib. In other countries, only BCLC stage 0 and A1 HCC is eligible for surgical removal; in China, however, the indications to surgery have been expanded, and surgery is the preferred treatment for stage Ia and Ib HCC. Studies have compared RFA with RSR in the treatment of recurrent small HCC (≤3 cm in diameter or ≤ 3 tumor nodules), but there were limitations in these studies. For example, they did not include tumors sized 3 to 5 cm. In the present study, the efficacy was compared between RSR and RFA in treating recurrent HCC at BCLC stage 0/A.
Comparisons of perioperative outcomes
There is evidence showing that RSR is an effective and important treatment for recurrent HCC (28); however, severe treatment-associated complications are not rare and should not be ignored (12). In the present study, the incidence of postoperative complications in RSR group and RFA group was 42.11% and 11.76%, respectively (P<0.001) (Table 4); these were consistent with those reported in a meta-analysis (36), in which the RSR was associated with more complications (especially severe complications) than RFA. In our study, however, no severe complications (such as bile leakage, acute liver failure, septic shock, and perioperative death) were noted in either group. Only intraperitoneal hemorrhage due to incomplete hemostasis was found in the RSR group and was successfully treated with a second operation. Before the surgery, three-dimensional reconstruction and printing were employed to measure the residual liver volume, and the relationships between surrounding tissues and the tumor were determined in the present study, which improved surgical management, shortened the operation time, and reduced intraoperative bleeding. During the operation, ultrasonography was performed to determine the tumor size and to accurately locate and identify the surrounding blood vessels, which were helpful to determine the cutting plane, thus ensure the safety of surgical margin and minimize unnecessary exposure to the tumor. For deeply located tumors, RFA is usually used if the residual liver volume is <40% during the preoperative simulation of anatomical hepatectomy. RSR is typically applied if the tumors locate near the hollow viscera (<5 mm) or near the bile duct, gallbladder, diaphragm, hepatic capsule, hepatic portal, heart, portal vein or hepatic vein. In the RFA group, percutaneous ablation under ultrasound guidance was performed in most cases, although two patients underwent open ablation and two underwent laparoscopic ablation. As a minimally invasive and relatively simple procedure, RFA is less affected by the general conditions of the patients and the location and size of the tumors (37). This may explain the low incidence of postoperative complications in the RFA group. In addition, RFA can protect the non-neoplastic liver parenchyma, minimize surgical injury, and shorten the hospital stay, making it more cost-effective than RSR (35). Sun et al. (22) found that the median hospital stay of patients receiving RSR was significantly longer than that of patients undergoing RFA (13 vs. 5 days, P<0.05). Similarly, in the present study, the median hospital stay in RSR group and RFA group was 9 and 3 days, respectively (P<0.001) (Table 4). In addition, the high repeatability of RFA renders it a preferred treatment for recurrent HCC (38). In the present study, multisite ablation was used to ensure the margin ≥5 mm away from the tumor, and CT or MRI was performed within 1 month after the procedure to determine whether the ablation was complete. Incomplete ablation was found in four patients, among whom three underwent a second RFA and one received two additional RFA sessions; thus, the final complete ablation rate was 100%. These indicate that RFA a safer and more feasible treatment for recurrent HCC.
Comparison of long-term survival
The long-term survival of patients after RFA versus RSR for recurrent HCC is still controversial (38,39). Ina meta-analysis (40), there were no significant differences in the 1-, 3-, and 5-year OS rates between the RFA group and the RSR group (three or fewer nodules; ≤6 cm in diameter), whereas RSR was associated with higher 3- and 5-year DFS rates. Sun et al. (22) reported 100 cases of recurrent small HCC (three or fewer nodules; ≤3 cm in diameter) and found that the OS and DFS rates were similar between the RFA group and the RSR group. The present study showed that RFA achieved similar long-term survival to RSR in patients with recurrent BCLC stage 0/A HCC after primary tumor resection (Figures 1 and 2). This might be explained by the fact that this study was a non-randomized controlled study. In addition, the tumor diameter in the RFA group was smaller than in the RSR group. This suggests that there might be selective between two groups. Therefore, a prospective randomized controlled trial is needed to further confirm our findings.
Comparison of the second recurrence
In the present study, both RSR and RFA completely eliminated recurrent HCC. However, the second recurrence of HCC was still common. Up to 73.7% of patients in RSR group and 78.4% of patients in RFA group developed a second recurrence. This was consistent with the findings reported by Chan et al. (3), which showed that 72.4% of RSR treated patients and 84.4% of RFA treated patients had a second recurrence. It has been reported that RSR can remove tiny and hidden lesions more completely than RFA; consistent with this, patients in the RFA group had a higher recurrence rate than those in the RSR group (36). However, this difference was negligible in our study. In addition, the high repeatability of RFA, as an inherent advantage of this procedure in treating recurrent HCC, was demonstrated again (41). In the present study, 12.0% and 84.4% of patients who developed a second recurrence in the RSR group and the RFA group, respectively underwent a second RFA session. In contrast, only 7.1% of patients who developed a second recurrence in RSR group underwent a third surgical resection.
Survival predictors
Studies have shown that the interval between the first hepatectomy and the first recurrence, the interval between the first recurrence and the second recurrence, the size of recurrent tumor, and the serum albumin level are predictors of OS (42). Our study showed that the interval between the first hepatectomy and the first recurrence and the interval between the first recurrence and the second recurrence significantly affected the OS; however, the serum albumin level and tumor size had no significant correlation with OS (Table 6). There might two possible explanations for these differences. First, the albumin was supplemented in perioperative period, which achieved a positive nitrogen balance. Second, the tumors were relatively small in our cases and could be completely eliminated by multiple sessions of multisite ablation; moreover, R0 resection could be achieved with a resection margin of >1 cm under the premise of an adequate residual liver volume.
Conclusions
In this study, our results show that RFA and RSR can achieve long-term survival benefits for recurrent BCLC stage 0/A HCC. RSR is still considered the preferred treatment for recurrent BCLC stage 0/A HCC. RFA has the advantages of minimal invasion, few operative complications, short hospital stay, and high repeatability and is the best alternative treatment for patients with unresectable tumors. However, our study was a retrospective study and had a small sample size, and about 18.2% of patients were lost to follow up, which limited the extension of our findings. Thus, our findings are needed to be further confirmed in multicenter, high-quality, prospective randomized controlled trials.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the retrospective nature of the study. This study had been approved by the Ethics Committee of Affiliated Hospital of Nantong University (No: 2018-L006).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-73.e1. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Chan AC, Poon RT, Cheung TT, et al. Survival analysis of re-resection versus radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg 2012;36:151-6. [Crossref] [PubMed]
- Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 2013;59:300-7. [Crossref] [PubMed]
- Fan ST, Mau Lo C, Poon RT, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg 2011;253:745-58. [Crossref] [PubMed]
- Goh BK, Teo JY, Chan CY, et al. Importance of tumor size as a prognostic factor after partial liver resection for solitary hepatocellular carcinoma: Implications on the current AJCC staging system. J Surg Oncol 2016;113:89-93. [Crossref] [PubMed]
- Choo SP, Tan WL, Goh BKP, et al. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer 2016;122:3430-46. [Crossref] [PubMed]
- Shrager B, Jibara G, Schwartz M, et al. Resection of hepatocellular carcinoma without cirrhosis. Ann Surg 2012;255:1135-43. [Crossref] [PubMed]
- Roayaie S, Obeidat K, Sposito C, et al. Resection of hepatocellular cancer </=2 cm: results from two Western centers. Hepatology 2013;57:1426-35. [Crossref] [PubMed]
- Saraswat VA, Gaurav P, Sachin S. Treatment algorithms for managing hepatocellular carcinoma. J Clin Exp Hepatol 2014;4:S80-9. [Crossref] [PubMed]
- Zhou J, Sun HC, Wang Z, et al. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer 2018;7:235-60. [Crossref] [PubMed]
- Dai WC, Cheung TT. Strategic overview on the best treatment option for intrahepaitc hepatocellular carcinoma recurrence. Expert Rev Anticancer Ther 2016;16:1063-72. [Crossref] [PubMed]
- Krenzien F, Schmelzle M, Struecker B, et al. Liver Transplantation and Liver Resection for Cirrhotic Patients with Hepatocellular Carcinoma: Comparison of Long-Term Survivals. J Gastrointest Surg 2018;22:840-8. [Crossref] [PubMed]
- Lacaze L, Scotte M. Surgical treatment of intra hepatic recurrence of hepatocellular carcinoma. World J Hepatol 2015;7:1755-60. [Crossref] [PubMed]
- Lim C, Shinkawa H, Hasegawa K, et al. Salvage liver transplantation or repeat hepatectomy for recurrent hepatocellular carcinoma: An intent-to-treat analysis. Liver Transpl 2017;23:1553-63. [Crossref] [PubMed]
- Arai T, Kobayashi A, Ohya A, et al. Assessment of treatment outcomes based on tumor marker trends in patients with recurrent hepatocellular carcinoma undergoing trans-catheter arterial chemo-embolization. Int J Clin Oncol 2014;19:871-9. [Crossref] [PubMed]
- Jianyong L, Jinjing Z, Wentao W, et al. Preoperative transcatheter arterial chemoembolization for resectable hepatocellular carcinoma: a single center analysis. Ann Hepatol 2014;13:394-402. [Crossref] [PubMed]
- Chan DL, Morris DL, Chua TC. Clinical efficacy and predictors of outcomes of repeat hepatectomy for recurrent hepatocellular carcinoma - a systematic review. Surg Oncol 2013;22:e23-30. [Crossref] [PubMed]
- Zhou Y, Sui C, Li B, et al. Repeat hepatectomy for recurrent hepatocellular carcinoma: a local experience and a systematic review. World J Surg Oncol 2010;8:55. [Crossref] [PubMed]
- Rhim H, Lim HK, Choi D. Current status of radiofrequency ablation of hepatocellular carcinoma. World J Gastrointest Surg 2010;2:128-36. [Crossref] [PubMed]
- Gao HJ, Chen MS. Radiofrequency ablation therapy and its strategies in multidisciplinary treatment of hepatocellular carcinoma. Chinese Journal of Hepatology 2012;20:245. [PubMed]
- Sun WC, Chen IS, Liang HL, et al. Comparison of repeated surgical resection and radiofrequency ablation for small recurrent hepatocellular carcinoma after primary resection. Oncotarget 2017;8:104571-81. [Crossref] [PubMed]
- Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015;261:947-55. [Crossref] [PubMed]
- Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 2011;254:108-13. [Crossref] [PubMed]
- Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 2010;89:500-7. [Crossref] [PubMed]
- Yang SL, Luo YY, Chen M, et al. A systemic review and meta-analysis comparing the prognosis of multicentric occurrence and vs. intrahepatic metastasis in patients with recurrent hepatocellular carcinoma after hepatectomy. HPB (Oxford) 2017;19:835-42. [Crossref] [PubMed]
- Umeda Y, Matsuda H, Sadamori H, et al. A prognostic model and treatment strategy for intrahepatic recurrence of hepatocellular carcinoma after curative resection. World J Surg 2011;35:170-7. [Crossref] [PubMed]
- Shimada M, Matsumata T, Taketomi A, et al. Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery 2010;83:127-31. [PubMed]
- Roayaie S, Bassi D, Tarchi P, et al. Second hepatic resection for recurrent hepatocellular cancer: a Western experience. J Hepatol 2011;55:346-50. [Crossref] [PubMed]
- Chok KS, Chan SC, Poon RT, et al. Re-resection for metachronous primary hepatocellular carcinoma: is it justified? ANZ J Surg 2012;82:63-7. [Crossref] [PubMed]
- Lee S, Jeong WK, Rhim H. Repeated percutaneous radiofrequency ablation for hepatocellular carcinoma in patients with cirrhosis: assessment of safety based on liver function and portal hypertension parameters. J Vasc Interv Radiol 2014;25:1573-9. [Crossref] [PubMed]
- Machairas N, Papaconstantinou D, Stamopoulos P, et al. The Emerging Role of Laparoscopic Liver Resection in the Treatment of Recurrent Hepatocellular Carcinoma: A Systematic Review. Anticancer Res 2018;38:3181-6. [PubMed]
- Joliat GR, Allemann P, Labgaa I, et al. Treatment and outcomes of recurrent hepatocellular carcinomas. Langenbecks Arch Surg 2017;402:737-44. [Crossref] [PubMed]
- Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2010;138:400-1. [Crossref] [PubMed]
- Guo WX, Sun JX, Cheng YQ, et al. Percutaneous radiofrequency ablation versus partial hepatectomy for small centrally located hepatocellular carcinoma. World J Surg 2013;37:602-7. [Crossref] [PubMed]
- Chen X, Chen Y, Li Q, et al. Radiofrequency ablation versus surgical resection for intrahepatic hepatocellular carcinoma recurrence: a meta-analysis. J Surg Res 2015;195:166-74. [Crossref] [PubMed]
- Chen M. Radiofrequency ablation and multidisciplinary treatment for liver cancer. Zhonghua Yi Xue Za Zhi 2015;95:2174. [PubMed]
- Wang K, Liu G, Li J, et al. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol 2015;41:236-42. [Crossref] [PubMed]
- Song KD, Lim HK, Rhim H, et al. Repeated Hepatic Resection versus Radiofrequency Ablation for Recurrent Hepatocellular Carcinoma after Hepatic Resection: A Propensity Score Matching Study. Radiology 2015;275:599-608. [Crossref] [PubMed]
- Cai H, Kong W, Zhou T, et al. Radiofrequency ablation versus reresection in treating recurrent hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 2014;93:e122. [Crossref] [PubMed]
- Lee DH, Lee JM, Lee JY, et al. Radiofrequency ablation for intrahepatic recurrent hepatocellular carcinoma: long-term results and prognostic factors in 168 patients with cirrhosis. Cardiovasc Intervent Radiol 2014;37:705-15. [Crossref] [PubMed]
- Liang HH, Chen MS, Peng ZW, et al. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: a retrospective study. Ann Surg Oncol 2008;15:3484-93. [Crossref] [PubMed]


