
Synchronous primary carcinomas of the prostate, thyroid and rectum: case report and review of the literature
Introduction
Individual primary thyroid, prostate, and rectal cancers are not uncommon. In the following case, a patient diagnosed with moderately differentiated adenocarcinoma of the rectum was found to also have primary tumors in the prostate and thyroid. The treatment approach followed and its outcomes are described below.
Case presentation
In December 2016, a 62-year-old Chinese male was admitted to our institution after one year of irregular rectal bleeding.
In addition, the patient also reported a history of narrow stools without pus or mucus, tenesmus, and abdominal pain. A colonoscopy was performed at a regional hospital, and a moderately differentiated adenocarcinoma was confirmed by a pathologist there, after which the patient was referred to our hospital for rectal radical resection. Pelvic magnetic resonance imaging (MRI) indicated a clinical stage for the rectal cancer of T2N1 (UICC TMN staging of colorectal cancer, 8th edition), and identified an isolated abnormal signal in a nodule in the prostatic transitional zone. The patient’s serum carcinoembryonic antigen (CEA) was 1.69 ng/mL, and serum prostate-specific antigen (PSA) was 86.59 ng/mL. A jugular ultrasound disclosed an 8 mm × 6 mm soft-tissue mass in the thyroid gland, classified as 4A. Both rectal and prostate tumors were confirmed histologically by biopsy. To identify the primary tumor and to improve the results of immunohistochemistry, a secondary biopsy via colonoscopy and a fine-needle aspiration of the thyroid nodule were carried out with the patient’s consent. A bone scan was negative for systemic bone metastasis.
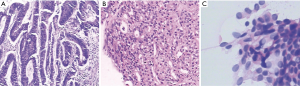
Rectal tumor immunohistochemical staining was positive for CDX-2, villin, and CK20, but negative for PSA and PSAP (prostate-specific acid phosphatase). The pathologic diagnosis was moderately differentiated adenocarcinoma of the rectum (Figure 1A). In the prostate mass immunohistochemistry, androgen receptor (AR), PSA, P504S, and PSAP were positive, while P63, 34βE12, ERG, CK7, CK20, CDX-2, and villin were negative. Ki67 was positive in 5% of cells. The pathologic findings indicated adenocarcinoma of the prostate (Figure 1B), with a Gleason score 4+3=7, grade group 3. The fine-needle aspiration cytology of the thyroid nodule showed papillary thyroid carcinoma (Figure 1C). The clinical stage of the rectal cancer was T2N1M0 stage IIIA (UICC, 8th edition). At the same time, the prostatic carcinoma was T2bN0M0 stage IIB (2010 AJCC TNM staging of prostate cancer).
After a multidisciplinary team discussion, concurrent chemoradiotherapy and hormonal therapy for the rectal and prostate cancers was chosen. The patient’s history of myocardial infarction and poor cardiac function did not exceed the risk assessment threshold, and surgery was not recommended. During the first phase of planned therapy, treatment of the papillary thyroid carcinoma was limited to clinical observation.
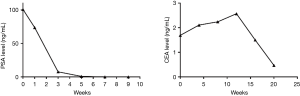
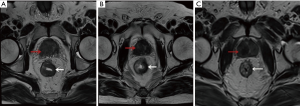
Pelvic external beam radiotherapy (RT) for rectal cancer consisted of a total of 60 Gy delivered (as 6-MV photons) in 30 fractions of 2 Gy, five times weekly. During radiotherapy, capecitabine (Zhengdatianqing Medicine China, Ltd.) was given at a dose of 1,600 mg/m2/day. Radiotherapy treatment for prostate cancer was provided once a day, 5 days a week, for a total dose of 76 Gy in 38 fractions. The target volume included the prostate and seminal vesicles. For the first 13 days of radiotherapy, bicalutamide tablets (AstraZeneca China, Ltd.) were given at a dose of 150 mg/day. On the fourteenth day of the course, this was adjusted to 50 mg/day, and goserelin (AstraZeneca UK, Ltd.) was subcutaneously injected at a dose of 3.6 mg, concurrently. This protocol involved sequential treatment, starting with determining the planned target volume (PTV) and planned gross tumor volume (PGTV) of the rectal lesions and the PTV-prostate (Figure 2). PGTV-rectum lesions included the rectal lesions with a 5-mm margin, while PTV-prostate included the prostate with a 1-cm margin; the doses were as noted above. The clinical target volume (CTV) enclosed all the above volumes plus the seminal vesicles, the mesorectum, and an additional volume encompassing pelvic nodes at risk of harboring occult rectal cancer. The PTV included the CTV with a 5-mm margin, for a total of 45 Gy in 25 fractions. At the end of radiotherapy, the patient’s serum CEA level was 2.24 ng/mL and his PSA level dropped from 100.7 to 0.036 ng/mL (Figure 3); pelvic MRI showed stable disease.

After the completion of radiotherapy, the patient continued to undergo a fourth cycle of chemotherapy for three months, with oxaliplatin (Hengrui Medicine China, Ltd.) and raltitrexed (Zhengdatianqing Medicine China, Ltd.) once every 3 weeks, 50 mg/day bicalutamide, and goserelin once every 4 weeks. Pelvic MRI indicated that the rectal mass was reduced to a thickness of 8mm, the prostatic lesion was reduced to 11 mm × 16 mm, and the thyroid soft-tissue mass to 8 mm × 6 mm (Figure 4). At this time, no local recurrence or distant metastasis was found.
Discussion
Adenocarcinomas of the rectum and prostate, and papillary thyroid carcinomas are the most common cancers in each of their respective tissues. Synchronous primary carcinomas in rectum, prostate, and thyroid are extremely rare, as indicated by a review of the published literature.
Presenting symptoms for rectal cancer are rectal bleeding, incomplete obstruction, tenesmus, and sacral or perineal pain. Voiding difficulty and frequency, pelvic or perineal pain, hematuria, and hematospermia are common clinical symptoms of prostate cancer. Papillary thyroid tumors are often clinically silent until they reach a large size, at which point they can cause dyspnea, dysphagia, hoarseness, and symptoms of sympathetic nerve compression. Digital rectal examination often reveals no obvious lesions in patients with prostate adenocarcinoma. PSA levels stay within the normal range in the majority of cases. If imaging findings and PSA indicate abnormal growth, ultrasonography or computed tomography-guided prostate biopsy can assist to confirm the diagnosis. However, preoperative presacral mass biopsy is not recommended, as transurethral or transperineal biopsy can potentially cause tumor spreading or rupture, abscess formation, fecal fistula, and meningitis. In addition, such biopsies may incompletely sample the lesion, leading to a false diagnosis. Consequently, if the tumors are potentially resectable, those procedures should be avoided (1).
Except for unresectable and metastatic tumors, surgical resection is the primary treatment for prostate and rectal carcinomas. These carcinomas are also sensitive to chemotherapy and radiotherapy. In the present case, standardized radiotherapy for rectal cancer was delivered in 28 fractions of 1.8 Gy, once a day, five days a week, for a total dose of 50.4 Gy. The target volume for prostate cancer included the prostate and partial seminal vesicles, for a total dose of 74 Gy, once a day, five times per week (2). The dose was specified at the intersection of the beam axes, according to the guidelines of the International Commission on Radiation Units (3,4). As synchronous rectal and prostate carcinomas were present, a modified three-dimensional conformal radiotherapy plan was devised to involve both tumors. Although few published data exist pertaining to coincident treatment and to outcomes with synchronous tumors, the role of neoadjuvant chemoradiotherapy in the treatment of locally advanced cancers is well established, with reductions in local recurrence compared to surgery alone (2,5-8). Two treatment protocols are commonly used. One is short-course neoadjuvant chemoradiotherapy, in which 25 Gy is delivered in five fractions over one week, followed by surgery one to two weeks later. The other is long-course neoadjuvant chemoradiotherapy, delivering 50 Gy in 25 fractions over five weeks, with surgery to be performed six to eight weeks afterwards. Neither regimen is considered appropriate treatment for non-metastatic prostate cancer, where treatment options include radical prostatectomy, external beam radiotherapy (EBRT, 74 Gy), brachytherapy, chemotherapy, hormonal therapy, watchful waiting, or a combination of the above.
The treatment of prostate cancer is determined based on its clinical stage, PSA level, Gleason score, and on the patient’s life expectancy. While rectal cancer treatment is primarily directed toward potentially curative surgical resection, patients with low-risk prostate cancer (Gleason grade ≤6, PSA 4–10 ng/mL, clinical stage ≤ T2a) may choose watchful waiting, or may postpone treatment. Meanwhile patients with high-risk prostate cancer (Gleason grade ≥8, PSA >20 ng/mL) may undergo EBRT in combination with three years of adjuvant hormonal therapy (9). Selected patients with T3 tumors, PSA <20 ng/mL, Gleason score <8 and a life expectancy of >10 years are suitable candidates for radical prostatectomy (3,10).
The differing dosage regimens and irradiation fields used in the treatment of rectal and prostate cancer pose a challenge, as the lower dose used in the rectal cancer would be considered subtherapeutic for prostate cancer, while the higher dose used in the definitive treatment of prostate cancer could increase the technical difficulty of low anterior resection and the risk of anastomotic failure.
Approximately 20% of patients who receive long-course neoadjuvant chemoradiotherapy for rectal cancer have a complete pathological response (11). In our case, the patient was diagnosed by pathology with two synchronous pelvic malignancies: a T2N1M0 adenocarcinoma of the rectum and a T2bN0M0 adenocarcinoma of the prostate, Gleason score 4+3=7; serum PSA was 86.59 ng/mL. The fine-needle aspiration of the thyroid showed papillary thyroid carcinoma. This is a patient with low-risk rectal cancer and high-risk prostate cancer; his PSA level had increased to 100.7 ng/mL before radiotherapy. Preoperative neoadjuvant chemoradiotherapy based on 5-fluorouracil (5-FU), combined with total mesorectal excision has become the standard treatment for locally advanced (defined as cT3-4b; cTxN1-2) rectal cancer. Brachytherapy and external beam radiotherapy with androgen suppression are well established in the management of high-risk prostate cancer. Lavan et al. conducted a retrospective review of ten cases of synchronous locally advanced rectal cancer and non-metastatic prostate cancer. Pelvic RT 45–50.4 Gy with 5-Fu was delivered for rectal cancer, and a total dose ranging from 70 to 79.2 Gy was delivered for prostate cancer. There were no acute toxicities and two significant late toxicities, with a median follow-up of 2.2 y (range, 1.2–6.3 y) (12). In the present case, the patient was unwilling to undergo a non-anal-preserving surgery, due to concerns about the impact of home nursing colostomy care on his quality of life; additionally, the patient’s prior myocardial infarction and poor cardiac function did not exceed the surgical risk assessment threshold. The chosen route of radical long-course radiochemotherapy and androgen deprivation therapy for the rectal and prostate cancer, with clinical observation for the thyroid carcinoma, may have been optimal for this patient. Various factors will affect survival rate, including tumor histologic type, grade, stage, and surgical margin status. In our case, it has been 17 months since the last chemotherapy. No local recurrence or distant metastasis has been found. The patient is asymptomatic, with stable disease and improved quality of life. We will carry out long-term follow-up of the patient to monitor his curative status.
MRI and systemic examination are necessary for patients with rectal cancer, as the results allow us to define the stage and determine the best treatment. For synchronous primary carcinomas of the prostate, thyroid, and rectum, we recommend surgery and/or chemoradiation therapy. Many thyroid and prostate cancers will not be clinically relevant in a patient’s lifetime. A change in chemotherapy regimen and/or in the timing of surgery and/or radiotherapy should be considered when poor cardiac function suggests that the patient may not tolerate surgery. When faced with a patient with synchronous multiple primary tumors, therapeutic decision-making should depend on the clinical disease extent, and chemoradiation therapy and/or surgery plans must be drafted to suit the individual patient, to improve quality of life and overall prognosis.
Additional information
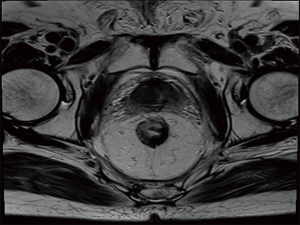
On April 10, 2019, the patient reexamined the pelvic MRI to show the rectal masses were reduced to 6 mm and the size of prostatic lesions is 11 mm × 16 mm and CT showed same 8 mm × 6 mm soft-tissue mass for thyroid. His serum CEA level was 0.52 ng/mL and PSA level 0.04 ng/mL. It's been 23 months since the patient was completed treatment, no local recurrent or distant metastasis was found (Figure S1).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jao SW, Beart RW Jr, Spencer RJ, et al. Retrorectal tumors: Mayo Clinic experience, 1960-1979. Dis Colon Rectum 1985;28:644-52. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger WGerman Rectal Cancer Study Group, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Bolla M, de Reijke TM, van Tienhoven GEortc Radiation Oncology Group and Genito-Urinary Tract Cancer Group, et al. Duration of androgen suppression in the Treatment of prostate cancer N Engl J Med 2009;360:2516-27. [Crossref] [PubMed]
- International Commission on Radiation Units and Measurements. Prescribing, recording and reporting photon beam therapy Report 50. ICRU, Bethesda 1993.
- Enola P, Adam HO, Glimelius B, et al. The risk of subsequent malignant diseases after cancers of the colon and rectum. A nationwide cohort studies. Cancer 1990;65:2091-100. [Crossref] [PubMed]
- Swedish rectal cancer trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997;336:980-7. [Crossref] [PubMed]
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [Crossref] [PubMed]
- Van Gijn W, Marijnen CA, Nagtegaal IDDutch Colorectal Cancer Group, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12 - year follow-up of the multicentre, randomised con - trolled TME trial. Lancet Oncol 2011;12:575-82. [Crossref] [PubMed]
- Terris MK, Wren SM. Results of a screening program for prostate cancer in patients scheduled for abdominoperineal resection for colorectal pathologic findings. Urology 2001;57:943-5. [Crossref] [PubMed]
- Heidenrich A, Aus G, Bolla MEuropean Association of Urology, et al. EAU guidelines on prostate cancer . Eur Urol 2008;53:68-80. [Crossref] [PubMed]
- Beddy D, Hyland JM, Winter DC, et al. A simplified tumor regression grade correlates with survival in locally advanced rectal carcinoma treated with neoadjuvant chemoradiotherapy. Ann Surg Oncol 2008;15:3471-7. [Crossref] [PubMed]
- Lavan NA, Kavanagh DO, Martin J, et al. The curative management of synchronous rectal and prostate cancer. Br J Radiol 2016;89:20150292. [Crossref] [PubMed]


