
Stereotactic body radiotherapy for organ-confined prostate cancer
Background
Prostate cancer is the most common malignancy in men. An estimated 233,000 cases will be diagnosed in the United States in 2014 (1). PSA screening has led to earlier stage diagnoses; in 1998, 92% of prostate cancers were diagnosed with clinically organ-confined disease (2). The 7th edition of the AJCC Staging Manual (3), adopted in 2010, added Gleason score and PSA to the TNM staging system. Nearly 50% of patients (4) diagnosed with prostate cancer fall in prognostic Group 1, which includes patients with a clinical stage of T1-T2a, PSA <10, and Gleason 6. Active surveillance has become a suitable alternative for AJCC stage I, also referred to as “low-risk”, patients (5). The PIVOT trial randomized PSA-era diagnosed patients between radical prostatectomy and observation; in the low risk group, treatment afforded no cancer-specific or overall survival benefit, bolstering the argument against definitive treatment in this subgroup. In intermediate- and high-risk patients, the PIVOT trial showed surgery afforded, respectively, 50% and 60% reductions in prostate cancer deaths. This clear benefit justifies treatment in these subgroups.
According to the NCI Consensus Conference (6) and the Prostate Cancer Panel of the American Urological Association in 1995 (6), treatment options that should be discussed include radical prostatectomy, external beam radiation therapy (RT), interstitial brachytherapy and watchful waiting.
Historical evolution of radiotherapy for prostate cancer
Radiotherapy was first used to treat prostate cancer in the first half of the 20th century; the application of radium or kilovoltage therapy yielded disappointing results (7,8). The development of megavoltage external beam platforms in the 1950’s (9-11) allowed higher doses to be delivered, with encouraging outcomes. The next important development was CT imaging and computerized treatment planning, which facilitated 3-dimension conformal external beam planning and intensity modulated radiotherapy (IMRT). These more sophisticated treatment plans yielded better dose conformity to the target, allowing further dose escalation. Conformal, dose-escalated techniques have yielded varying disease-free outcomes, approximately similar to those seen with radical prostatectomy (see Table 1), although not without toxicity.
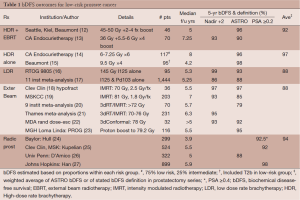
Full table
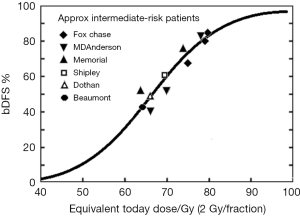
Several randomized trials (28-30) have confirmed that dose escalation yields improved relapse-free survival rates. Fowler’s dose-response analysis in intermediate-risk patients (31) (see Figure 1) indicate doses exceeding 90 Gy are necessary to minimize recurrence rates. A meta-analysis of seven randomized dose-escalation trials yielded the same conclusion (32). A variety of strategies have been employed to further escalate dose and/or reduce toxicity to surround normal tissues.
Modern radiotherapy plans still had to account for variations in patient positioning, inaccuracies in treatment delivery, and internal organ motion. Radiation oncologists account for these uncertainties by adding a radial margin around the intended target, creating a “planning target volume (PTV)”. This expanded target extends the high-dose treatment region into the surrounding normal structures. A PTV expansion of about 1 cm in required when skin marks are used for positioning. Set-up uncertainty can be reduced by placing gold fiducials in the prostate and imaging prior to treatment delivery. This does not account for movement within a given treatment session, or “intrafractional” motion. Kupelian (33) demonstrated that in 15% of treatment sessions, the prostate moved more than 5 mm. A study from the Mayo Clinic (34) recommended a 5-mm margin to account for intrafractional motion. The expanded PTV required in IMRT employing pre-treatment image guidance has limited the maximum safe dose around 82 Gy, if delivered at 2-Gy per fraction.
Proton therapy offers the prospect of prostate dose escalation while limiting exposure to normal tissues. Proton beams deposit radiation until after passing beyond the target, where the dose then falls off rapidly. This reduces the radiation dose to normal tissues, potentially yielding fewer side effects. However, like IMRT, proton beam plans must account for prostate motion, thus the same large PTVs must be targeted. Also, since most proton beam plans employ only two beams, conformal dose sculpting around the prostate is not possible. While proton therapy reduces the volume of normal tissues receiving low dose radiation, large volumes of the rectum still receive high-dose radiation. In one study (35), protons yielded a 50% greater incidence of rectal toxicity compared to IMRT. The American College of Radiology Study 03-12 demonstrated (36) significant (8%) late grade 3+ rectal toxicity when proton dose was escalated to 82 Gy. Proton dose escalation beyond 82 Gy is thus not possible with current technology, and long-term toxicity GI toxicity appears to be no better, and perhaps inferior to IMRT.
Transperineal ultrasound-guided brachytherapy allows the delivery of conformal, high-dose radiotherapy to the prostate, with a rapid dose fall-off outside of the implanted region. In low dose rate (LDR) implants, 70-100 iodine-125 (I-125) or palladium-103 (Pd-103) sources are permanently placed within the prostate; these “seeds” slowly deliver radiation over the ensuing 2-6 months. For patients with low-risk prostate cancer, a single LDR implant (monotherapy) yields favorable long-term outcomes (37-39). Patients with intermediate- or high-risk disease usually require a five-week course of external beam radiotherapy plus the LDR implant (40,41). When post-implant dosimetry demonstrates the prostate received a biologically equivalent dose (BED) of around 200 Gy, LDR brachytherapy yields exceptionally high relapse-free survival rates (42). This is equivalent to about 110 Gy at 2 Gy/fx, assuming α/β=1.5. Unfortunately toxicity following LDR brachytherapy appears to be greater than IMRT. Fox-Chase (43) reported 3-year grade 2+ GI and GU toxicities rates were three- and five-fold greater following seed implants. Sanda’s patient-reported quality of life (QOL) study (44) did not directly compare treatments, however greater declines in urinary and bowel scores were observed following brachytherapy than after external beam radiotherapy.
Hypofractionation
High-dose rate (HDR) brachytherapy has been used in the treatment of prostate cancer since the 1980’s (45-52). Catheters are placed temporarily in the prostate, and then loaded with a high-dose Iridium-192 source, delivering a few fractions of very high-dose RT. Initial protocols employing HDR combined conventionally fractionated external beam RT with an HDR boost. More recent reports have employed HDR as monotherapy (14,15,53-56). Adjusting for pre-treatment risk factors, these studies yield biochemical disease-free survival (bDFS) outcomes at least as favorable to those seen with LDR brachytherapy or conformal dose-escalated RT or IMRT (see Table 1). A prospective study from William Beaumont Hospital (15) comparing HDR monotherapy versus LDR brachytherapy (Pd-103) showed a superior 5-year event-free survival (98% vs. 85%, P=0.01) and a trend towards improved freedom from cancer failure (98% vs. 92%, P=0.1) in the HDR cohort. The same group showed toxicity and QOL following HDR brachytherapy was more favorable than either LDR brachytherapy or conformal external beam RT (54,57). These results suggest prostate cancer favorably responds to hypofractionated regimens.
Radiation oncologists fractionate RT dose to reduce toxicity to surrounding normal tissues. For most cancers, by delivering dose over several weeks, equivalent cancer-killing effect is achieved with reduced long-term toxicity. The effect of dose fractionation on both cancer and normal tissues can be estimated using the “linear-quadratic model”. In this model, the alpha-beta ratio reflects the response of normal tissues or cancers to changes in RT dose per fraction. Most cancers respond to RT as do rapidly-dividing normal tissues (e.g., skin or mucous membranes), and thus have high α/β ratios, in the 8-12 Gy range (58). Tissues with lower α/β ratios are more sensitive to large dose per fraction (also known as hypofractionated) RT.
The results of HDR and other hypofractionated regimens led radiobiologists to reconsider α/β ratio of prostate carcinoma. Numerous studies have concluded that prostate cancer has an unusually low/ratio of about 1.5 Gy (31,59-62). A pooled analysis (63) of 5,093 patients yielded a α/β ratio of 1.55 Gy. A low α/β ratio is consistent with other biologic properties of prostate cancer: an unusually long tumor doubling times (64), and a very low proportion of proliferating cells (65). If the α/β ratio for prostate cancer is smaller than the α/β ratios for late effects in the surrounding normal tissues (3-5 Gy), then a therapeutic gain could be achieved by hypofractionation. In this setting, larger doses per fraction should result in equivalent or improved cancer control with reduced toxicity (66-68).
Several prospective clinical trials have evaluated the efficacy of hypofractionated radiotherapy in organ-confined prostate cancer. A large prospective study from the Cleveland Clinic (69) demonstrated favorable relapse-free survival and low toxicity with 70 Gy given in 2.5 Gy fractions. A trial from Royal Adelaide Hospital in Australia (70) randomized 217 patients between 64 Gy in 2 Gy/fx versus 55 Gy in 2.75 Gy/fx; these schedules are isoeffective if prostate α/β=2.5. The hypofractionated arm showed a significantly better bDFS (53% vs. 43%), with equal toxicity in the two arms. In an Italian trial (71), 168 high-risk patients were randomized between 62 Gy in 3.1 Gy/fx versus 80 Gy in 2 Gy/fx (isoeffective if prostate α/β=1.8; both arms received 9 months of androgen ablation). Toxicities were equal. Overall relapse rates were equivalent, although improved cancer control was suggested if presenting PSA was 20 or less. Thus the radiobiologic assertion that the α/β ratio for prostate cancer is low (1.5-1.8) has been confirmed by class 1 evidence.
Stereotactic body radiotherapy (SBRT) is the precise external delivery of very high-dose radiotherapy to targets in the body, with treatment completed in one to five fractions. Dose conformality is achieved using cross-firing ionizing radiation beams and image-guidance. By concentrating dose in the targeted cancer, SBRT maximizes cell-killing. Rapid dose fall-off minimizes radiation-related injury to adjacent normal tissues. Organ-confined prostate cancer should be ideally suited for SBRT as (I) dose escalation should yield better outcomes; (II) the toxicity from treatment is due to high-dose radiation exposure to the organs immediately adjacent to the prostate; and (III) the unique radiobiology of prostate cancer favors hypofractionation.
SBRT platforms
Several external beam platforms can theoretically deliver stereotactic radiotherapy for prostate cancer. Table 2 summaries the capability of these devices. At a minimum, target localization prior to daily treatments is required. This can be performed using x-ray imaging of implanted fiducials, or on-board CT imaging. If intra-fractional image guidance is not employed, then at least 5 mm PTV expansions are required to account for target motion. If the target can be localized during treatment, then smaller PTV expansions can be employed, potentially reducing dose to surrounding organs. The accuracy of different real-time localization systems can vary considerably. For example, with the Novalis or Varian TrueBeam systems, the operator may opt to perform intrafractional localization and correction multiple times during treatment, or only once prior to treatment. With the Calypso system, the operator sets a threshold (typically 3-5 mm) beyond which treatment is interrupted and the patient positioning corrected. With the CyberKnife, continuous image acquisition and target correction occurs routinely; the Stanford group showed that when intrafractional correction is done every 40 seconds, this device achieves sub-millimeter accuracy (72).
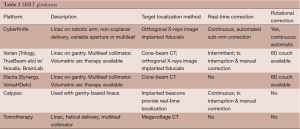
Full table
Correction for target motion must account for translational (i.e., anterior/posterior, right/left, and superior/inferior) motion. Since rotational motion, particularly pitch, can be substantial, correction for rotations may be beneficial, although this potential benefit has not been quantified. The use of multiple non-coplanar beams should yield better dose conformality than single-plane treatments. While non-coplanar delivery is possible for any platform, in practice centers employing gantry-based linacs treat in a coplanar fashion, as non-coplanar delivery adds complexity and time. The intrinsically non-coplanar CyberKnife platform is reported (73) to yield more conformal treatment plans than IMRT.
Clinical SBRT outcomes
The first report (74) of hypofractionated stereotactic radiotherapy treated 40 low-risk patients using a conventional linear accelerator with daily localization of implanted fiducials. 33.5 Gy was delivered in 5 fractions to the prostate plus a 4-5 mm margin. Toxicities were acceptable. Four-year nadir +2 bDFS was 90%, suggesting further dose escalation would be beneficial.
The feasibility of SBRT employing further dose escalation was first reported by King at Stanford University (75) using the CyberKnife platform. 36.25 Gy in 5 fractions of 7.25 Gy was delivered to the prostate plus a 3-5 mm margin. In the most recent update (76) of long-term outcomes in 67 patients, there were no grade 4+ toxicities. Two patients had a grade 3 urinary toxicity, and there were no grade 3 GI toxicities. Toxicities compared favorably to other radiation modalities. Five-year Kaplan-Meier PSA relapse-free survival was 94%. The majority of subsequent reports of prostate SBRT have employed the same platform. In a series of 304 patients treated with CyberKnife at Winthrop hospital, five-year bDFS was 97%, 90.7%, and 74.1% in low-, intermediate- and high-risk groups, respectively. Five grade 3 complications were reported, all GU, for an incidence rate of 2%. In a pooled analysis of eight institutions (77), 1,100 patients were treated with CyberKnife SBRT and followed a median of 36 months. Five-year bDFS rates were 95%, 84%, and 81% in low-, intermediate- and high-risk groups, respectively. In a multi-center study (78) Cyberknife treated 129 intermediate-risk prostate cancers 40 Gy in 5 fractions of 8 Gy each, with only one grade 3 toxicity reported (GU: bladder injury). More recent reports (79,80) have shown similar favorable outcomes with gantry-based platforms.
The mature series evaluating dose-escalated SBRT are summarized in Table 3. In low-risk patients treated to 35-36.25 Gy in 5 fractions, 5-year bDFS ranges from 94-97%. In the low-risk patients treated in the 8-institution pooled analysis (77) and in Katz’ series (84), no difference in 5-year bDFS was seen when dose was escalated from 35 to 40 Gy. Sunnybrook (79) demonstrated 97% 5-year bDFS in 84 low-risk patients treated to 35 Gy in 5 fractions with a gantry-based system. In a series (80) of 98 low-risk patients treated to 40 Gy in 5 fractions with real-time tracking on a gantry-based linac, only one biochemical failure was reported at 5 years. Current data shows no evidence of a dose response beyond 7 Gy ×5 in low risk patients. These SBRT outcomes compare favorably to the 92-94% 5-year bDFS typically reported with conventionally fractionated external beam radiotherapy (see Table 1).
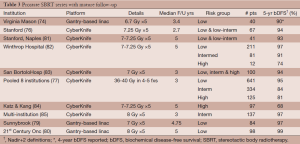
Full table
In intermediate-risk patients treated with SBRT, bDFS outcomes vary. In a multi-center study (85) of 137 intermediate-risk patients given 8 Gy ×5 fractions on the CyberKnife platform, 5-year bDFS was 97%. In a pooled analysis of eight institutions (77), 5-year bDFS in intermediate-risk patients was only 84%. However, those patients that received biologically higher doses (38 Gy in 4 fractions or 40 Gy in 5 fractions) had 5-yr bDFS of 96.7%. The apparent improvement in bDFS in the higher-dose cohort was not statistically significant. Longer follow-up and comparisons of larger populations will be necessary to confirm trends suggesting dose escalation beyond 7.25 Gy ×5 yields better relapse-free survival in intermediate risk patients. These 5-year relapse-free survival rates compare favorably to fractionated EBRT (23,86) outcomes, which are typically around 85%.
Mature data evaluating SBRT in high-risk prostate cancer are limited. The largest series is a pooled analysis of 8 institutions (77), in which 125 high-risk patients received Cyberknife with or without androgen deprivation therapy (ADT). 5-year bDFS was favorable at 81%. Katz (84) reported on a series of 97 high risk patient treated with either 5 fractions CyberKnife (35-36.25 Gy) or CyberKnife boost (19-21 Gy in 3 fractions following 45 Gy pelvic RT). 46 of the 97 patients received ADT. 5-year bDFS was 68%. The addition of pelvic radiotherapy or ADT had no impact on relapse free survival, although pelvic RT was associated with greater GI toxicity.
SBRT toxicity
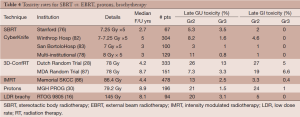
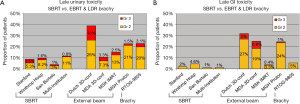
Rates of late physician-reported GI and GU toxicities from mature SBRT series and from 3D conformal, IMRT, proton and LDR brachytherapy series are summarized in Table 4. Since median follow-up on the SBRT series is the 3-5 year range, these rates may underestimate the true rates of toxicities, as more toxicities may develop with longer follow-up. Nevertheless, Figure 2A, which illustrates the rates of grade 2+ toxicities for various modalities, suggests SBRT late urinary toxicity rates compare favorably to external beam. Late rectal toxicity rates appear to be consistently less than those seen with external beam radiotherapy (Figure 2B). These series employed a robotic non-coplanar delivery platform which corrected for target motion in real-time (Cyberknife), although recent reports of SBRT employing conventional gantry-based platforms (79,80) also suggest favorable toxicity. A recent study (88) comparing Medicare claims found SBRT was associated with 38% more diagnoses of urethritis, incontinence and obstruction, compared to IMRT. This study did not evaluate patients treated with G0039 and G0040 codes (used with CyberKnife delivery) so the increased toxicity may be related to the differences in treatment technique and/or platforms. Finally, most SBRT series limited PTV doses to 35-40 Gy in 5 fractions. In a multi-center dose-escalation SBRT study (89), 5 of 91 patients treated to 50 Gy in 5 fractions required colostomy for rectal injury. This emphasizes the need to respect dose constraints for critical structures surrounding the prostate.
Full table
Patient-reported toxicity
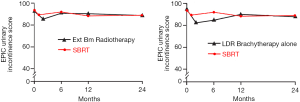
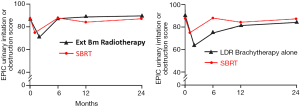
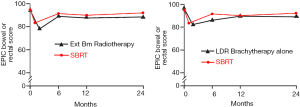
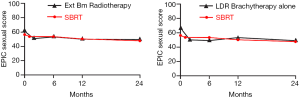
Following definitive therapy for prostate cancer, prospective patient-completed QOL questionnaires more accurately estimate treatment-related toxicity, compared to physician reports (90,91). In Katz’ report of 304 patients treated with CyberKnife SBRT, urinary and bowel QOL decreased immediately following treatment, and then returned to baseline. Patient-reported QOL outcomes from a prospective multi-institutional study (85) of 309 patients treated with Cyberknife are illustrated in Figures 3,4,5,6 below. QOL outcomes of various organ domains from the validated EPIC instrument are superimposed on the benchmark external beam and brachytherapy outcomes reported in Sanda’s (92) study. Long-term changes in urinary incontinence scores following SBRT were similar to those observed in external beam and in brachytherapy (Figure 3). Urinary irritation/obstruction scores following SBRT appeared to be less adversely affected than after brachytherapy (Figure 4). While there were small changes in bowel QOL following SBRT (Figure 5), these declines appeared less prominent than following EBRT and brachytherapy. EPIC sexual score declined progressively during the four years after treatment (Figure 6). Because this methodology does not account for potential differences between SBRT and EBRT/LDR patient populations, no firm conclusions can be drawn. Nonetheless, these patient-reported SBRT QOL outcomes are encouraging.
Cost effectiveness
Although delivery of SBRT is technically more involved that IMRT, treatment is completed in only 5 fractions, rather than the 39-48 fractions required for IMRT. A Markov decision analysis model (93) showed the mean cost of $22,152 for SBRT versus $35,431 for IMRT. Another study of Medicare claims (88) reported mean costs of $13,645 and $21,023 for SBRT and IMRT, respectively. Furthermore, the substantial time-cost to patients (94) for conventional prostate treatment can be mitigated with SBRT.
Conclusions
SBRT offers a cost-effective alternative to external beam radiotherapy which is much more convenient for the patient. The radiobiology of prostate cancer would predict that this approach should yield superior outcomes compared to conventional protracted courses. For low- and intermediate-risk prostate cancer patients treated on a robotic, non-coplanar RT platform, five-year relapse-free survival rates are at least equivalent, or possibly superior to conventionally fractionated RT. Physician-reported late urinary toxicity appears to be similar to external beam RT, and late GI toxicity appears to be less than with external beam and LDR brachytherapy. Patient-reported QOL outcomes show urinary and bowel function return to near baseline levels two years following robotic SBRT. Long-term changes in rectal QOL appear to be superior to those reported after IMRT or LDR brachytherapy. For high-risk prostate cancer, initial CyberKnife series suggest favorable outcomes. Emerging outcomes in low-risk patients treated on gantry-based platforms are similarly encouraging. A prospective randomized trial would be required to confirm these favorable SBRT outcomes relative to other modalities. But given these excellent cancer control rates and toxicity profiles, SBRT delivered on platforms which have real-time image guidance appears to be an acceptable approach for stage I-II prostate cancer. Further studies are also required to determine if similar favorable outcomes are possible with SBRT delivered on other platforms, and in patients with high-risk disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sandra Vermuelen, Kevin T. Murphy, Huan Giap) for the series “SBRT/SRS in Radiation Research” published in Translational Cancer Research. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.3978/j.issn.2218-676X.2014.08.06). The series “SBRT/SRS in Radiation Research” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Ma J, Zou Z, et al. Cancer Statistics, 2014. CA Cancer J Clin 2014;64:9-29. [PubMed]
- Paquette EL, Sun L, Paquette LR, et al. Improved prostate cancer-specific survival and other disease parameters: Impact of prostate specific antigen testing. Urology 2002;60:756-9. [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. eds. AJCC cancer staging manual (7th ed). New York, NY: Springer, 2010.
- Shao YH, Demissiek K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst 2009;101:1280-3. [PubMed]
- Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol 2010;28:126-31. [PubMed]
- Middleton RG, Thompson IM, Austenfield MS, et al. Prostate cancer clinical guidelines panel summary report on the management of clinically localized prostate cancer. J Urol 1995;154:2144-8. [PubMed]
- Deming CL. Results in one hundred cases of cancer of prostate and seminal vesicles, treated with radium. Surg Gynaec & Obstet 1922;34:99-118.
- Hultberg S. Results of treatment with radiotherapy in carcinoma of the prostate. Acta radiol 1946;27:339-50. [PubMed]
- Bagshaw MA, Kaplan HS, Sagerman RH. Linear Accelerator Supervoltage Radiotherapy. VII. carcinoma of the prostate. Radiology 1965;85:121-9. [PubMed]
- Budhraja SN, Anderson JC. An assessment of the value of radiotherapy in the management of carcinoma of the prostate. Br J Urol 1964;36:535-540. [PubMed]
- George FW, Carlton CE Jr, Dykhuizen RF, et al. Cobalt-60 telecurietherapy in the definitive treatment of carcinoma of the prostate: a preliminary report. J Urol 1965;93:102-9. [PubMed]
- Galalae RM, Martinez A, Mate T, et al. Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys 2004;58:1048-55. [PubMed]
- Demanes DJ, Rodriguez RR, Schour L, et al. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy’s 10-year results. Int J Radiat Oncol Biol Phys 2005;61:1306-16. [PubMed]
- Schour L, Demanes DJ, Altieri GA, et al. High Dose Rate Monotherapy for Prostate Cancer. Int J Radiat Oncol Biol Phys 2005;63:S315.
- Ghilezan M, Vargas C, Gustafson G, et al. Similar 5-year Clinical Outcome for High Dose Rate (HDR) and Low Dose Rate (LDR) Brachytherapy (BT) for Early Prostate Cancer Patients. Int J Radiat Oncol Biol Phys 2005;63:S37.
- Lawton CA, DeSilvio M, Lee WR, et al. Results of a phase II trial of transrectal ultrasound-guided permanent radioactive inmplantation of the prostate for definitive management of localized adenocarcinoma of the prostate (RTOG 98-05). Int J Radiat Oncol Biol Phys 2007;67:39-47. [PubMed]
- Zelefsky MJ, Kuban DA, Levy LB, et al. Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys 2007;67:327-33. [PubMed]
- Kupelian PA, Thakkar VV, Khuntia D, et al. Hypofractionedintensite-modulated radiotherapy (70Gy at 2.5 Gy per fraction) for localized prostate cancer: long-term outcomes. Int J Radiat Oncol Biol Phys 2005;63:1463-8. [PubMed]
- Zelefsky MJ, Chan H, Hunt M, et al. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol 2006;176:1415-9. [PubMed]
- Kupelian P, Kuban D, Thames H, et al. Improved biochemical relapse-free survival with increased external radiation doses in patients with localized prostate cancer: the combined experience of nine institutions in patients treated in 1994 and 1995. Int J Radiat Oncol Biol Phys 2005;61:415-9. [PubMed]
- Thames HD, Kuban DA, DeSilvio ML, et al. Increasing external beam dose for T1-T2 prostate cancer: effect on risk groups. Int J Radiat Oncol Biol Phys 2006;65:975-81. [PubMed]
- Cheung R, Tucker SL, Lee AK, et al. Dose-response characteristics of low- and intermediate-risk prostate cancer treated with external beam radiotherapy. Int J Radiat Oncol Biol Phys 2005;61:993-1002. [PubMed]
- Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol 2010;28:1106-11. [PubMed]
- Hull GW, Rabbani F, Abbas F, et al. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol 2002;167:528-34. [PubMed]
- Kupelian PA, Potters L, Khuntia D, et al. Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy > or =72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1-T2 prostate cancer. Int J Radiat Oncol Biol Phys 2004;58:25-33. [PubMed]
- D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969-74. [PubMed]
- Han M, Partin AW, Zahurak M, et al. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol 2003;169:517-23. [PubMed]
- Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 2006;24:1990-6. [PubMed]
- Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 2002;53:1097-105. [PubMed]
- Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/american college of radiology 95-09. J Clin Oncol 2010;28:1106-11. [PubMed]
- Fowler JF, Ritter MA, Chappell RJ, et al. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys 2003;56:1093-104. [PubMed]
- Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys 2009;74:1405-18. [PubMed]
- Kupelian P, Willoughby T, Mahadevan A, et al. Multi-institutional clinical experience with the Calypso System in localization and continuous, real-time monitoring of the prostate gland during external radiotherapy. Int J Radiat Oncol Biol Phys 2007;67:1088-98. [PubMed]
- Beltran C, Herman MG, Davis BJ. Planning target margin calculations for prostate radiotherapy based on intrafraction and interfraction motion using four localization methods. Int J Radiat Oncol Biol Phys 2008;70:289-95. [PubMed]
- Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 2012;307:1611-20. [PubMed]
- Coen JJ, Bae K, Zietman AL, et al. Acute and late toxicity after dose escalation to 82 GyE using conformal proton radiation for localized prostate cancer: initial report of American College of Radiology Phase II study 03-12. Int J Radiat Oncol Biol Phys 2011;81:1005-9. [PubMed]
- Blasko JC, Grimm PD, Sylvester JE, et al. Palladium-103 brachytherapy for prostate carcinoma. Int J Radiat Oncol Biol Phys 2000;46:839-50. [PubMed]
- Grimm PD, Blasko JC, Sylvester JE, et al. 10-year biochemical (prostate-specific antigen) control of prostate cancer with (125)I brachytherapy. Int J Radiat Oncol Biol Phys 2001;51:31-40. [PubMed]
- Stone NN, Stock RG, Unger P. Intermediate term biochemical-free progression and local control following 125iodine brachytherapy for prostate cancer. J Urol 2005;173:803-7. [PubMed]
- Potters L, Morgenstern C, Calugaru E, et al. 12-year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J Urol 2005;173:1562-6. [PubMed]
- Merrick GS, Butler WM, Wallner KE, et al. Impact of supplemental external beam radiotherapy and/or androgen deprivation therapy on biochemical outcome after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 2005;61:32-43. [PubMed]
- Stock RG, Stone NN, Cesaretti JA, et al. Biologically effective dose values for prostate brachytherapy: effects on PSA failure and posttreatment biopsy results. Int J Radiat Oncol Biol Phys 2006;64:527-33. [PubMed]
- Eade TN, Horwitz EM, Ruth K, et al. A comparison of acute and chronic toxicity for men with low-risk prostate cancer treated with intensity-modulated radiation therapy or (125)I permanent implant. Int J Radiat Oncol Biol Phys 2008;71:338-45. [PubMed]
- Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250-61. [PubMed]
- Borghede G, Hedelin H, Holmäng S, et al. Irradiation of localized prostatic carcinoma with a combination of high dose rate iridium-192 brachytherapy and external beam radiotherapy with three target definitions and dose levels inside the prostate gland. Radiother Oncol 1997;44:245-50. [PubMed]
- Martinez A, Gonzalez J, Stromberg J, et al. Conformal prostate brachytherapy: initial experience of a phase I/II dose-escalating trial. Int J Radiat Oncol Biol Phys 1995;33:1019-27. [PubMed]
- Martinez AA, Pataki I, Edmundson G, et al. Phase II prospective study of the use of conformal high-dose-rate brachytherapy as monotherapy for the treatment of favorable stage prostate cancer: a feasibility report. Int J Radiat Oncol Biol Phys 2001;49:61-9. [PubMed]
- Mate TP, Gottesman JE, Hatton J, et al. High dose-rate afterloading 192Iridium prostate brachytherapy: feasibility report. Int J Radiat Oncol Biol Phys 1998;41:525-33. [PubMed]
- Kestin LL, Martinez AA, Stromberg JS, et al. Matched-pair analysis of conformal high-dose-rate brachytherapy boost versus external-beam radiation therapy alone for locally advanced prostate cancer. J Clin Oncol 2000;18:2869-80. [PubMed]
- Kovács G, Galalae R, Loch T, et al. Prostate preservation by combined external beam and HDR brachytherapy in nodal negative prostate cancer. Strahlenther Onkol 1999;175:87-8. [PubMed]
- Galalae RM, Kovács G, Schultze J, et al. Long-term outcome after elective irradiation of the pelvic lymphatics and local dose escalation using high-dose-rate brachytherapy for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys 2002;52:81-90. [PubMed]
- Stromberg J, Martinez A, Gonzalez J, et al. Ultrasound-guided high dose rate conformal brachytherapy boost in prostate cancer: treatment description and preliminary results of a phase I/II clinical trial. Int J Radiat Oncol Biol Phys 1995;33:161-71. [PubMed]
- Martinez AA, Pataki I, Edmundson G, et al. Phase II prospective study of the use of conformal high-dose-rate brachytherapy as monotherapy for the treatment of favorable stage prostate cancer: a feasibility report. Int J Radiat Oncol Biol Phys 2001;49:61-9. [PubMed]
- Grills IS, Martinez AA, Hollander M, et al. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. J Urol 2004;171:1098-104. [PubMed]
- Martin T, Baltas D, Kurek R, et al. 3-D conformal HDR brachytherapy as monotherapy for localized prostate cancer. A pilot study. Strahlenther Onkol 2004;180:225-32. [PubMed]
- Mark R, Vallabhan G, Akins A, et al. Interstitial High Dose Rate (HDR) Brachytherapy for Early Stage Prostate Cancer. Int J Radiat Oncol Biol Phys 2005;63:S304.
- Saputo K, Isler K, Wallace M, et al. A Quality of Life (QOL) Assessment of Prostate Cancer Patients Undergoing Different Radiation Treatment modalities. Int J Radiat Oncol Biol Phys 2005;63:S446.
- Williams MV, Denekamp J, Fowler JF. A review of alpha/beta ratios for experimental tumors: implications for clinical studies of altered fractionation. Int J Radiat Oncol Biol Phys 1985;11:87-96. [PubMed]
- Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999;43:1095-101. [PubMed]
- King CR, Fowler JF. A simple analytic derivation suggests that prostate cancer alpha/beta ratio is low. Int J Radiat Oncol Biol Phys 2001;51:213-4. [PubMed]
- Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys 2001;50:1021-31. [PubMed]
- Brenner DJ, Martinez AA, Edmundson GK, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002;52:6-13. [PubMed]
- Proust-Lima C, Taylor JM, Sécher S, et al. Confirmation of a low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys 2011;79:195-201. [PubMed]
- Lee WR, Hanks GE, Corn BW, et al. Observations of pretreatment prostate-specific antigen doubling time in 107 patients referred for definitive radiotherapy. Int J Radiat Oncol Biol Phys 1995;31:21-4. [PubMed]
- Haustermans KM, Hofland I, Van Poppel H, et al. Cell kinetic measurements in prostate cancer. Int J Radiat Oncol Biol Phys 1997;37:1067-70. [PubMed]
- D’Souza WD, Thames HD. Is the alpha/beta ratio for prostate cancer low? Int J Radiat Oncol Biol Phys 2001;51:1-3. [PubMed]
- Duchesne GM, Peters LJ. What is the alpha/beta ratio for prostate cancer? Rationale for hypofractionated high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 1999;44:747-8. [PubMed]
- Fowler JF, Chappell RJ, Ritter MA. The prospects for new treatments for prostate cancer. Int J Radiat Oncol Biol Phys 2002;52:3-5. [PubMed]
- Kupelian PA, Willoughby TR, Reddy CA, et al. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys 2007;68:1424-30. [PubMed]
- Yeoh EE, Botten RJ, Butters J, et al. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys 2011;81:1271-8. [PubMed]
- Arcangeli S, Strigari L, Gomellini S, et al. Updated results and patterns of failure in a randomized hypofractionation trial for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012;84:1172-8. [PubMed]
- Xie Y, Djajaputra D, King CR, et al. Intrafractional motion of the prostate during hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:236-46. [PubMed]
- Hossain S, Xia P, Huang K, et al. Dose gradient near target-normal structure interface for nonisocentric CyberKnife and isocentric intensity-modulated body radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2010;78:58-63. [PubMed]
- Madsen BL, Hsi RA, Pham HT, et al. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: first clinical trial results. Int J Radiat Oncol Biol Phys 2007;67:1099-105. [PubMed]
- King CR, Lehmann J, Adler JR, et al. CyberKnife radiotherapy for localized prostate cancer: rationale and technical feasibility. Technol Cancer Res Treat 2003;2:25-30. [PubMed]
- King CR, Brooks JD, Gill H, et al. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:877-82. [PubMed]
- King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol 2013;109:217-21. [PubMed]
- Meier R, Kaplan I, Beckman A, et al. Stereotactic Body Radiation Therapy for Intermediate-risk Organ-confined Prostate Cancer: Interim Toxicity and Quality of Life Outcomes from a Multi-institutional Study. Int J Radiat Oncol Biol Phys 2012;84:S148.
- Loblaw D, Sethukavalan P, Cheung P, et al. Comparison of Biochemical and Toxicity Outcomes From a Contemporaneous Cohort Study of Low-Risk Prostate Cancer Treated With Different Radiation Techniques. Int J Radiat Oncol Biol Phys 2013;87:S26.
- Mantz CA, Fernandez E. Real-Time Target Tracking Prostate SBRT and the Real-Time Tracking System 4D Localization System: 5-Year Quality of Life and Disease Outcomes. Int J Radiat Oncol Biol Phys 2013;87:S393.
- Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: five-year outcomes. Radiat Oncol 2011;6:3. [PubMed]
- Katz AJ, Santoro M, Diblasio F, et al. Stereotactic body radiotherapy for localized prostate cancer: disease control and quality of life at 6 years. Radiat Oncol 2013;8:118. [PubMed]
- Bolzicco G, Favretto MS, Satariano N, et al. A single-center study of 100 consecutive patients with localized prostate cancer treated with stereotactic body radiotherapy. BMC Urol 2013;13:49. [PubMed]
- Katz A, Kang J. Stereotactic body radiotherapy with or without external beam radiation as treatment for organ confined high-risk prostate carcinoma: a six year study. Radiat Oncol 2014;9:1. [PubMed]
- Meier R, Kaplan I. Quality of life outcomes from a multicenter study of SBRT for low- and intermediate-risk prostate cancer. Presented at the 33rd Annual Meeting of ESTRO. Vienna, Austria, 2014.
- Cahlon O, Zelefsky MJ, Shippy A, et al. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys 2008;71:330-7. [PubMed]
- Kuban DA, Tucker SL, Dong L, et al. Long-term Results of the M.D. Anderson Randomized Dose-Escalation Trial for Prostate Cancer. Int J Radiat Oncol Biol Phys 2008;70:67-74. [PubMed]
- Yu JB, Cramer LD, Herrin J, et al. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: comparison of toxicity. J Clin Oncol 2014;32:1195-201. [PubMed]
- Kim DW, Cho LC, Straka C, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1-2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014;89:509-17. [PubMed]
- Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA 1995;273:129-35. [PubMed]
- Talcott JA, Rieker P, Clark JA, et al. Patient-reported symptoms after primary therapy for early prostate cancer: results of a prospective cohort study. J Clin Oncol 1998;16:275-83. [PubMed]
- Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250-61. [PubMed]
- Hodges JC, Lotan Y, Boike TP, et al. Cost-effectiveness analysis of stereotactic body radiation therapy versus intensity-modulated radiation therapy: an emerging initial radiation treatment option for organ-confined prostate cancer. J Oncol Pract 2012;8:e31s-7s. [PubMed]
- Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst 2007;99:14-23. [PubMed]


