Patterns of local failure in patients with high-grade glioma after postoperative radiotherapy with or without chemotherapy
Introduction
The addition of concurrent and adjuvant temozolomide (TMZ) to postoperative radiotherapy occupied a key role in improving prognosis of patients in HGG. It extended the median survival time of glioblastoma multiforme (GBM) from 12.1 to 14.6 months, and has been considered the gold standard treatment for newly diagnosed GBM due to significant survival benefit with minimal additional toxicity (1). The target volume design after postoperative adjuvant radiotherapy (RT) was inconsistent between EORTC and RTOG guidelines (2,3). However, from the existing data, there was no significant difference in the prognosis and recurrence patterns between the two radiotherapy strategies (4-7).
Since 1994, with the discovery of important molecular markers (the co-deletion of chromosomal arms 1p and 19q, the recurrent mutations in the isocitrate dehydrogenase genes as IDH1/2, etc.) affecting the prognosis of glioma (8-11). In 2016, the World Health Organization Classification of Tumors of the Central Nervous System for the first time integrated histological and molecular parameters, published the revision of WHO classification, which provided new tips and challenges in clinical practice (12), which not only enabled us to integrate them into the diagnostic process and to implement treatment strategies in patients by directing them to appropriate clinical practice (13), but also provided superior prognostic effect and more specific failure patterns. The relationship between molecular subtypes and on local failure patterns after standard adjuvant therapy has not been reported.
The aim of this study retrospectively analyzes the failure patterns of postoperative adjuvant chemoradiotherapy for HGG under the new WHO classification, particularly focusing on relationship of recurrence pattern and molecular subtypes.
Methods
Study design and eligibility
This retrospective study was conducted in Ruijin Hospital of Shanghai Jiaotong University. Ethical approval was obtained from hospital’s review board. Data were collected from the hospital’s medical records and the online data-management system. Patient information was anonymized and de-identified before analysis. Patients were deemed eligible when they started curative intent radiotherapy, with or without chemotherapy. There were no medical interventions in the current study.
Patients and pretreatment evaluation
Clinical data: the patient’s clinical data from Ruijin Hospital affiliated to Shanghai Jiaotong University School of Medicine from April 2014 to April 2018 were included. A total of 56 patients diagnosed as WHO III–IV were included. Patients, who underwent pathological typing according to the 2016 WHO Classification of Tumors of the Central Nervous (CNCS), including tumor grade and IDH status.
We collected the basic data such as patient’s age, gender, preoperative magnetic resonance image (MRI), preradiotherapy KPS status, surgical procedure, degree of resection, pathological grade, target delineation method (RTOG or EORTC criteria), treatment schedule, progress pattern, progress time, survival status and date of death (Table 1).
Table 1
| Characteristics | No. (%) |
|---|---|
| Gender | |
| Male | 34 (60.7) |
| Female | 22 (39.3) |
| Age (years) | |
| Median age [range] | 58.5 [28–82] |
| <55 | 22 (39.3) |
| ≥55 | 34 (60.7) |
| KPS (pre-radiotherapy) | |
| <70 | 12 (21.4) |
| ≥70 | 44 (78.5) |
| Type of surgery | |
| Biopsy | 7 (12.5) |
| Subtotal resection | 33 (58.9) |
| Total resection | 16 (28.6) |
| Concurrent TMZ | |
| Yes | 50 (89.3) |
| No | 6 (10.7) |
| Adjuvant TMZ | |
| No | 13 (23.2) |
| <6 courses | 12 (21.4) |
| 6 courses | 17 (30.4) |
| >6 courses | 14 (25.0) |
| Histological classification | |
| III | 16 (28.6) |
| IV | 40 (71.4) |
| IDH type | |
| IDHwt | 30 (53.6) |
| IDHmt | 13 (23.2) |
| NOS | 13 (23.2) |
| Delineation guidelines | |
| RTOG | 40 (71.4) |
| EORTC | 16 (28.6) |
IDHwt, isocitrate dehydrogenase wide type; IDHmt, IDHmutant.
All patients were classified by pathological grade and IDH type according to the 2016 WHO Central Nervous System Classification (12). Complete examinations required IDH mutation assessment by immunohistochemistry, using antibodies of IDH1 R132H mutant protein and/or mutation hotspots that could identify alternatives sequencing IDH1 mutations such as R132C or IDH2 mutations (12,14,15). In the end, there were16 cases of WHO III, 40 cases of WHO IV, 13 cases of IDH mutation, 30 cases of wild type and 13 cases of IDH NOS (Table 2).
Table 2
| Patients in 2016 WHO classification | No. (%) |
|---|---|
| AA, IDHwt | 6 (10.7) |
| AA, IDHmt | 5 (8.9) |
| AA, NOS | 3 (5.4) |
| AO, IDHmt and 1p19q-codeleted | 2 (3.6) |
| GBM, IDHwt | 24 (42.9) |
| GBM, IDHmt | 6 (10.7) |
| GBM, NOS | 10 (17.9) |
| SUM | 56 (100.0) |
Treatment planning and delivery
All patients received radiation therapy and were immobilized in the supine position with a tailored head-thermoplastic mask, followed by a CT simulation. CT simulation (Brilliance Big Bore, Phillips, Amsterdam, The Netherlands) was performed at a slice thickness of 3–5 mm from the head to below the fourth cervical vertebra. CT scans were fused with enhancement MRI imaging within two weeks before radiotherapy, and delineated slice-by-slice according to EORTC or RTOG guidelines. The target volume delineation was in accordance with RTOG guidelines in 43 patients and 13 cases of EORTC guidelines (2,3). The standard prescription dose is 60 Gy (2 Gy per day from Monday to Friday). A 6-MV photon beam is provided by a Varian TrueBeam or Elekta linear accelerator using a multi-leaf collimator.
Combined chemotherapy
Based on the treatment guidelines for HGG at our hospital, concurrent chemoradiotherapy or radiotherapy alone was recommended to patients with HGG. Fifty patients with concurrent TMZ, 43 patients with adjuvant chemotherapy. The median course of adjuvant chemotherapy were 6 [2–23] cycles. The concurrent TMZ regimen was continuous daily TMZ at a dose of 75 mg/m2/day, 7 days a week during the whole radiotherapy period, and was followed by six cycles of adjuvant TMZ (150 to 200 mg/m2 for 5 days every 28 days). The duration of adjuvant cycles varied. Six patients did not receive any chemotherapy due to economic or other reasons.
Recurrence definition
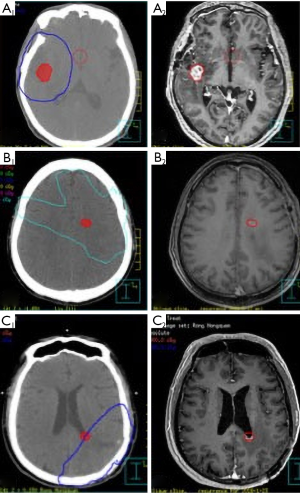
According to the RTOG or EORTC guidelines, the ECLIPSE treatment system or the Pinnacle version 8.0 planning system was used to delineate the target volume. The disease progression was determined by the physicians in the multi-disciplinary discussion of the central nervous system (CNS) of Ruijin Hospital based on the change of clinical symptoms and radiological MRI images. Once MRI indicated an abnormally enlarged lesion in the intracranial irradiation field, MRS examination or PET-MRI was recommended, or short-term routine MRI follow-up within 1–2 months to exclude from pseudoprogression. The initial relapsed T1 enhancement MRI imaging was transferred and fused to the original treatment planning CT. In order to reduce the bias, the tumor volume of the recurrent tumor on the MRI was first outlined and then fused with the treatment plan. The recurrent tumors were analyzed to determine the volume of recurrent tumor present within the 95% isodose line of the boost plan of the completed treatment. If ≥80% of T1 enhanced tumor volume was within 95% isodose line, recurrence was defined as “in-field”; “marginal” if >20 but ≤80% of the tumor volume was within the 95% isodose cure. They were both local recurrence. If <20% of the tumor volume is in the 95% isodose line, it was “out-field” or distant failure. Examples of the three failure patterns are shown in Figure 1 respectively.
Follow-up
All patients were evaluated weekly during treatment. After the completion of radiation therapy, the patients were followed up every 3 months during the first and second year, and every 3–6 months during the next 2–3 years, then every 6 months after that. Routine MRI to the head was performed during follow-up session. The last follow-up was followed by telephone and/or hospital outpatient visits.
Statistical analysis
Estimated overall survival (OS), defined as the first surgery or biopsy time to death. And progression-free survival (PFS), defined as the first surgery or biopsy time to any recurrence were calculated using the Kaplan-Meier method. All statistical tests were two-sided and P values of 0.05 or less were considered significant Chi-square (v2) analysis was used to investigate differences in tumor recurrence distribution among all patients. The Statistical Package for the Social Sciences, version 22.0 (SPSS Inc.), was used for all statistical analysis.
Results
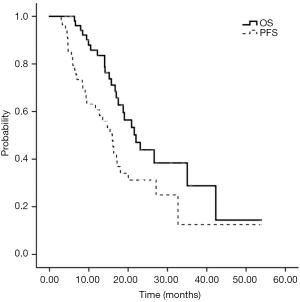
The last follow-up time was December 2018, and the follow-up rate was 100%. The follow-up time ranged from 3.9 to 53.9 (median 15.5) months. At the time of this analysis, twenty-five patients (44.6%) died due to intracranial tumor progression and 31 (55.4%) survived. Survival analysis showed that the median OS was (22.0±2.7) (95% CI: 16.7–27.2) months. The 1-, 2-, and 3-year survival rates were 85.8%, 43.9%, and 28.8%, respectively. Thirty-three (58.9%) patients progressed with a median PFS of (15.9±1.6) (95% CI: 12.9–19.0) months (Figure 2).
As shown in Table 3, of 33 patients experienced recurrence, 22 (66.7%) had in-field recurrence within a 9.3-month median period (range, 3–32.7 months), and 3 patients (9.1%) were respectively marginal recurrence occurred at 5.9, 6.4, and 15 months, and 6 patients (18.2%) developed out-field progression at a median of 16.1 (4.5–27.1) months, and 2 patients developed both in- and out-field recurrence (6.1%) at 3.2, 4.7 months, respectively.
Table 3
| Failure pattern | n (%) | PFS (median) (months) | OS (median) (months) |
|---|---|---|---|
| In-field | 22 (66.7) | 9.3 | 17.5 |
| Out-field | 6 (18.2) | 16.1 | 22 |
| In- and out-field | 2 (6.1) | 4.7 | 17 |
| Marginal | 3 (9.1) | 6.4 | 14 |
OS was 42.3 months in IDHmt patients, 15.2 months in IDHwt patients, and 21.6 months in IDH NOS patients (P=0.001) (Figure 3), with respective progression-free survival rates of 32.6, 9.5 and 16.1 months (P=0.004).
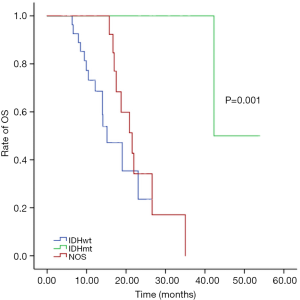
The incidence of recurrence of IDHmt (4 cases, 30%) was significantly lower than that of IDHwt (19 cases, 63.3%) and IDH NOS (10 cases, 76.9%) (P=0.001). Local recurrence (in the field and margin) of radiotherapy was 16 cases (84.2%) of IDH wild type and 2 cases (50%) of IDH mutant type (P=0.05).
Discussion
All most all HGG patients experienced local recurrence after curative treatment options. The WHO classification of CNS tumors provided new insights into HGG clinical practice. Improvements in tumor profiling drastically altered understanding of recurrent pattern, which could lead to changes of target delineation (16). With the discovery of molecular pathology closely related to OS, researchers attempted to integrate different molecular and histologic parameters to develop a new tumor classification based on prognosis (10,17). And new subtypes integrated with molecular data provided superior prognostic information (14). Therefore, when considering diagnosis and treatment scheme, the focus should be on pathological grade as well as molecular alterations (13).
To the best of our knowledge, this was the first study to explore the relationship of patterns of local failure and molecular classifications in patients with high grade glioma after postoperative radiotherapy with or without chemotherapy. We demonstrated that recurrent pattern after adjuvant to radiotherapy with or without chemotherapy in newly diagnosed patients with HGG was correlated with molecular subtypes. In this study, altogether 33 patients who relapsed, 22 (66.7%) had in-field recurrence within a 9.3-month median period (range, 3–32.7 months), and 3 patients (9.1%) marginal progression occurred at 5.9, 6.4, and 15 months, respectively, and 6 patients (18.2%) developed distant progression at a median of 16.1 (4.5–27.1) months, with 2 patients (6.1%) at 3.2 and 4.7 months occur simultaneously in- and out-field.
The incidence of recurrence of IDH mutants (4 cases, 30%) was significantly lower than that of IDH wild type (19 cases, 63.3%) and IDH NOS (10 cases, 76.9%) (P=0.001). Local recurrence (in the field and margin) of radiotherapy was 16 cases (84.2%) of IDH wild type and 2 cases (50%) of IDH mutant type (P=0.05). IDH widetype HGG had a higher probability of in-field recurrence after radiotherapy, and RTOG target delineation guide could be appropriate, while EORTC guidelines were recommended to IDHmt HGG.
The available data showed that patterns of failure in HGG following surgery, radiotherapy, and chemotherapy had consistently shown that 80–90% of recurrences are within 2 cm from the original site (18,19), as the main cause of treatment failure. Our results were consistent with these published data (18,19), and the most common recurrence developed in 2 cm from primary tumor, accounting for 75.8%. The local failure rate was insignificant between group RTOG and EORTC guideline, 82.6%, and 60.0% (P=0.17). According to the RTOG protocol, the irradiated brain volume is relatively large. However, clinical target volume delineation using postoperative residual tumor and cavity plus a 2-cm margin, rather than intentionally including peritumoral edema, did not appear to alter the central pattern of failure for patients with HGG. Brain irradiation was associated with neurotoxic side effects including radionecrosis and cognitive decline (20,21). The volume of irradiated brain was considered the one of main reasons of these complications. So it was obviously that RTOG guideline was inappropriate for the above patient with IDHmt HGG, in view of their prolonged survival and the RT related risk of cognitive impairment. At the same time, the reduced irradiation volume of normal brain was subjected to high-dose irradiation, so as to further improve local control (6).
In the future, however, the definition of GTV and CTV may be achieved with the use of metabolic MRS, PET/CT and PET/MR fusion-imaging, which will further reduce volume to be irradiated for this subgroup patients (22-24).
There were several limitation must be addressed in the current study. The retrospective nature of this analysis and single institute design with the relatively small sample certainly serve pitfalls of this study. Given the aggressive nature of HGG and the complexity of molecular alterations, effective treatment methods are a major unmet medical need Identification and validation of new biomarkers and incorporation them into target delineation could reduce the local failure and improve long term survival of this devastating disease (25).
Conclusions
In this study, we highlighted the importance of genomic research on identifying local failure patterns and providing recommendations of target delineation for HGG treatment. IDHwt HGG had a higher probability of in-field recurrence after radiotherapy, and RTOG target delineation guide was appropriate, while EORTC guidelines were recommended to IDHmt HGG. Further investigations in prospective randomized clinical trials were warranted to confirm our results.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.05.33). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the retrospective nature of the study. Ethical approval was obtained from hospital’s review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Niyazi M, Brada M, Chalmers AJ, et al. ESTRO-ACROP guideline "target delineation of glioblastomas". Radiother Oncol 2016;118:35-42. [Crossref] [PubMed]
- Cabrera AR, Kirkpatrick JP, Fiveash JB, et al. Radiation therapy for glioblastoma: Executive summary of an American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. Pract Radiat Oncol 2016;6:217-25. [Crossref] [PubMed]
- Paulsson AK, McMullen KP, Peiffer AM, et al. Limited margins using modern radiotherapy techniques does not increase marginal failure rate of glioblastoma. Am J Clin Oncol 2014;37:177-81. [Crossref] [PubMed]
- Chang EL, Akyurek S, Avalos T, et al. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int J Radiat Oncol Biol Phys 2007;68:144-50. [Crossref] [PubMed]
- Minniti G, Amelio D, Amichetti M, et al. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol 2010;97:377-81. [Crossref] [PubMed]
- Rapp M, Baernreuther J, Turowski B, et al. Recurrence Pattern Analysis of Primary Glioblastoma. World Neurosurg 2017;103:733-40. [Crossref] [PubMed]
- Reifenberger J, Reifenberger G, Liu L, et al. Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol 1994;145:1175-90. [PubMed]
- Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009;360:765-73. [Crossref] [PubMed]
- Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med 2015;372:2499-508. [Crossref] [PubMed]
- Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008;321:1807-12. [Crossref] [PubMed]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Ippen FM, Colman H, van den Bent MJ, et al. Precision Medicine for Primary Central Nervous System Tumors: Are We There Yet? Am Soc Clin Oncol Educ Book 2018;158-67. [Crossref] [PubMed]
- Galanis E, Nassiri F, Coy S, et al. Integrating Genomics Into Neuro-Oncology Clinical Trials and Practice. Am Soc Clin Oncol Educ Book 2018;38:148-57. [Crossref] [PubMed]
- Mellai M, Piazzi A, Caldera V, et al. IDH1 and IDH2 mutations, immunohistochemistry and associations in a series of brain tumors. J Neurooncol 2011;105:345-57. [Crossref] [PubMed]
- van den Bent MJ, Dubbink HJ, Sanson M, et al. MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: a report from EORTC Brain Tumor Group Study 26951. J Clin Oncol 2009;27:5881-6. [Crossref] [PubMed]
- Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006;9:157-73. [Crossref] [PubMed]
- Chan JL, Lee SW, Fraass BA, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol 2002;20:1635-42. [Crossref] [PubMed]
- Wallner KE, Galicich JH, Krol G, et al. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys 1989;16:1405-9. [Crossref] [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [Crossref] [PubMed]
- Torres IJ, Mundt AJ, Sweeney PJ, et al. A longitudinal neuropsychological study of partial brain radiation in adults with brain tumors. Neurology 2003;60:1113-8. [Crossref] [PubMed]
- Navarria P, Reggiori G, Pessina F, et al. Investigation on the role of integrated PET/MRI for target volume definition and radiotherapy planning in patients with high grade glioma. Radiother Oncol 2014;112:425-9. [Crossref] [PubMed]
- Pafundi DH, Laack NN, Youland RS, et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol 2013;15:1058-67. [Crossref] [PubMed]
- Dhermain F. Radiotherapy of high-grade gliomas: current standards and new concepts, innovations in imaging and radiotherapy, and new therapeutic approaches. Chin J Cancer 2014;33:16-24. [Crossref] [PubMed]
- Kebir S, Weber M, Lazaridis L, et al. Hybrid 11C-MET PET/MRI Combined With "Machine Learning" in Glioma Diagnosis According to the Revised Glioma WHO Classification 2016. Clin Nucl Med 2019;44:214-20. [Crossref] [PubMed]