
The effect of hericium polysaccharides combined with aspirin on the expression of CD133 and ABCG2 in lung cancer
Introduction
Lung cancer, a relatively common malignant tumor, is increasing its number of victims year after year, but its pathogenesis remains unclear. Many studies have suggested that lung tumors are caused by the presence of cancer stem cells (CSCs) in tumor tissues, and the number of these CSCs is extremely small, but their role in tumorigenesis is very significant. Given that CD133 and ABCG2 can be used as markers (1) for CSCs, an in-depth study on the characteristics of CD133 and ABCG2 and their role in lung tumors could provide new therapeutic targets for the prevention of lung tumors.
CD133, also known as Prominin-1, is distributed mainly in the cell membrane and is one of the well-studied CSC markers. Several studies have shown that CD133 is closely related to the formation of many tumors. For example, the expression of CD133 increases sequentially in cirrhotic tissues, adjacent tissues, and liver cancer tissues and is expressed in multiple tumor tissues, such as prostate cancer, bladder cancer, and glioma (2-4). Also, the level of CD133 in lung cancer is reportedly also significantly higher than that in normal tissues (5). ABCG2, also known as breast cancer resistance protein (BCRP), is a gene that belongs to the G subfamily of the ABC family and is a transmembrane transporter composed of 663 amino acids with an ATP-dependent drug efflux function (6,7). This gene can unselectively transport harmful substances out of the cells and plays a physiological protective role in normal organisms, but it exhibits multi-drug resistance (MDR) to tumor tissues. Existing studies have shown that both highly malignant tumor tissues and CSCs have strong drug resistance and high expression of ABCG2. According to the CSC theory, the MDR of tumors is derived from CSCs; for its resistance mechanism, in addition to stem cells’ better “self-renewal” ability and stationary phase characteristics, the most important factor is the high expression of the ABC transporter (8). Experiments have shown that there are multiple ABC transporters on the surface of CSCs, including ABCB1 (MDR1), ABCC1 (MRP1), and ABCG2 (BCRP), and per in vitro experiments, the efficacy of anti-tumor drugs (9) can be enhanced using ABC protein inhibitors.
Aspirin is a non-steroidal anti-inflammatory drug used widely in the treatment of cardiovascular diseases, cervical cancer, and pulmonary arterial hypertension (10,11). Its role in preventing tumors may be related to, among others, the non-selective inhibition of cyclooxygenase (COX-1 and COX-2), inhibition of tumor cell proliferation, promotion of tumor cell apoptosis, and inhibition of tumor angiogenesis. Hericium erinaceus is a type of edible fungus used for medical purposes and has as its main active substance hericium polysaccharide (HP). It has been reported that it has efficacies including anti-tumor, anti-bacteria, immune regulation, anti-oxidation, and lowering blood lipids (12,13). Our unpublished study also showed that HP inhibits PD-1 and enhances the activity of natural killer (NK) cells and cytotoxic T (CTL) cells.
In this experiment, we evaluated the inhibitory effect of HP combined with aspirin on lung CSCs in vitro and in vivo.
Methods
Cell proliferation assay
Cells were cultured in DMEM containing 10% fetal bovine serum with 5% CO2 at 37 °C. The A549 cells were inoculated into a 96-well plate at a cell density of 5,000 cells per well. The cells were then partitioned into: (I) the control group; (II) the HP low-dose group (HPL, 25 µg/mL); (III) the HP high-dose group (HPH, 100 µg/mL); (IV) the aspirin low-dose group (AL, 8 µg/mL); (V) the aspirin high-dose group (AH, 16 µg/mL); (VI) the HPL (25 µg/mL) + AL group (8 µg/mL); and (VII) the HPH(100 µg/mL) + AH group (16 µg/mL). HP was purchased from Nuoyuan Biotech Shanghai, China (the purity >98%), and aspirin (molecular weight: 180) was acquired from Sigma USA. Three parallel wells were set for each group and were cultured for 12, 24, 36, 48, 60, and 72 h. At the end of each culture, 10 µL of CCK8 solution was added to each well and cultured for another 4 h. The culture solution was carefully removed, and the absorbance value was measured at 490 nm using a microplate reader (14).
Colony formation assay
Cells were seeded at a density of 1×103 cells/well in a six-well plate and after incubation for 24 hours, were treated with aspirin alone, HP alone, or a combination of both drugs for 7 days. At the end of the 7 days, the cells were stained with 0.1% crystal violet, and the number of colonies was counted.
In vivo model
Forty-eight C57BL/6J male mice (6–8 weeks old) weighing 20±2 g were purchased from the Children Hospital Affiliated to Zhengzhou University and fed adaptively for one week and numbered with picric acid in a dry and clean animal room under 25 °C and 8 h/d light. The LL/2-luc-M38s lung cancer cell line (ATCC, USA) was cultured and adjusted to 106 cells/mL. Six mice were randomly selected for the control group, receiving standard feeding, and the remaining mice were inoculated with 0.2 mL of the Lewis lung cancer cell line subcutaneously in the dorsal area of the right axilla of the mice. At 24 h after the inoculation, the animals were weighed and randomly divided into 7 groups, with 6 mice in each group: (I) the model group; (II) the HPL group (mg/kg/d, i.p.); (III) the HPH group (100 mg/kg/d, i.p.); (IV) the AL group (8 mg/kg/d by gavage); (V) the AH group (16 mg/kg/d by gavage); (VI) the HPL (25 mg/kg/d) +AL group (8 mg/kg/d); (VII) the HPH (100 mg/kg/d) + AH group (16 mg/kg/d). The body weights of the mice were measured at the same time every 5 d. The size of the tumor was measured with a vernier caliper every 5 d after tumor formation, and the tumor volume was calculated according to the formula V = ab2/2; a represents the longest diameter of the tumor volume, b is the shortest diameter of the tumor volume; the length is measured in mm. The mice were weighed and sacrificed by cervical dislocation on the 30th day after the administration of our curative substances.
Western blot
The mice were left to fast for 24 h before sacrifice. After anesthetization by intraperitoneal injection with 10% chloral hydrate (0.35 mL/100 g), the mice were then placed supinely and fixed on an anatomical plate and the lungs were removed and weighed. Total protein was extracted from cells or lung cancer tissues using RIPA, and 75 µg of protein was applied to SDS-PAGE and then transferred to the PVDF membrane. Additionally, TBST (containing 5% skim milk) was used to block the non-specific binding for 2 h, and 1× TBST was washed 4 times (each wash lasted 5 min). Anti-CD133 (1:200), ABCG2 (1:250), and GAPDH (1:1,000) were added to incubate overnight at 4 °C. After washing the membrane the next day, the corresponding secondary antibody was added to incubate for 2 h at room temperature, followed by 1× TBST washing (3 times). Results were acquired using ECL luminescence in a dark room. All antibodies were procured from Santa Cruz Biotech, Dallas, Texas, USA. Grayscale analysis of bands was performed using the Image J software. Semi-quantitative analysis was achieved using GAPDH as an internal control, and the value was determined by the ratio of the target band to the gray value of the corresponding GAPDH.
Real-time fluorescent quantitative PCR
Total RNA was extracted from tumor tissues or cells using Trizol Reagent, and 2 µg of the RNA was used for reverse transcription. The resulting cDNA was amplified using a fluorescent quantitative PCR kit manufactured by Thermo Fisher Scientific, pre-denatured at 95 °C for 10 min and cycled (40 times) at 95 °C, 15 s and 60 °C, 60 s. Results were analyzed with 2−ΔΔCT. The relevant primer sequences were as follows: CD133, 5'-TGTGTGGAAAGATGGCTTCA-3' and 5'-GAGGGTCAATAATCCCAGCA-3'; ABCG2, 5'-TTACATCAGGGTTAAAAAAGCACAGG-3' and 5'-CCTGTGCTTTTTTAACCCTGATGTAA-3'; β-actin, TCCTTCCTGGGTATGGAATCCT and GCTCAGTAACAGTCCGCCTA.
Statistical analysis
The results were processed with ONE-WAY ANOVA of the statistical software SPSS16.0 for windows and were expressed as mean ± standard deviation (±s). P<0.05 showed that differences were statistically significant.
Results
Aspirin alone, HP alone, and the two drugs combined inhibited proliferation, colony formation, and the expression of CD133 and ABCG2 in A549 cells
Cell proliferation was assayed using the CCK-8 assay and colony formation assay. CCK-8 assay results showed that HPH reduced the proliferation of A549 cells significantly after 48 h of culture (P<0.05, Figure 1A). Both AL and AH also reduced the proliferation of A549 cells significantly after 48 h (Figure 1B). Furthermore, both HPL + AL and HPH + AH inhibited the proliferation of A549 cells, and the proliferation rates were significantly lower than those in the control group after 36 h of culture (Figure 1A). Cells in HPH + AH showed the lowest proliferation rate. Colony formation assay findings also suggested that HP alone, aspirin alone, and combined aspirin and HP markedly suppressed cell proliferation (Figure 1B), while combined aspirin and HP exhibited stronger inhibition when compared with aspirin alone or HP alone (Figure 1B).
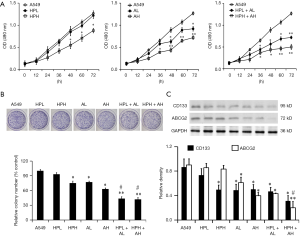
The expression levels of CD133 and ABCG2 were evaluated by the Western blot method after treatment of A549 cells with aspirin alone, HP alone, and the two drugs combined for 24 h. The results showed that the expression levels of CD133 decreased in the HPH, AL, AH, HPL + AL, and HPH + AH groups when compared with the control group. The expression of ABCG2 also decreased in the AL, AH, HPL + AL, and HPH + AH groups, and the expression levels of CD133 and ABCG2 was statistically lower in combined aspirin and HP when compared with aspirin alone or HP alone (Figure 1C).
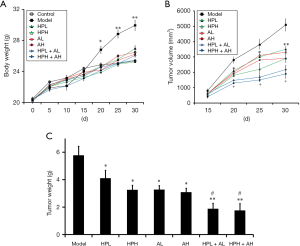
Aspirin alone, HP alone, and the two substances combined inhibited the growth of lung tumors
None tumor-bearing mice died during the procedure. Activities of tumor-bearing mice decreased significantly, and their mental states were poor in the model group, but the administration of aspirin alone or HP alone or the two combined increased the activities significantly and improved the mental states of mice. As seen in Figure 2A, most of the mice developed tumors around the 20th day, and the mice in the model group began to have a significant increase in body weight on the 20th day, with their body weights higher than those in other groups. Figure 2B shows that the tumor volume increased rapidly in the model group on the 20th day, which was significantly inhibited by HP alone, aspirin alone, or combined aspirin and HP. HPH showed significantly better inhibitory effects than HPL, but AL and AH showed no differences. Combined aspirin and HP displayed significantly better inhibitory effects than HP alone or aspirin alone (Figure 2B). Judging by the presentation in Figure 2C, the tumor weights of the mice in the model group were significantly higher than those of the mice in the other groups, which were inhibited significantly by HP alone, aspirin alone, and combined aspirin and HP. The combined application of aspirin and HP is synergistic.
Aspirin alone, HP alone, and the two substances combined inhibited the expression levels of CD133 and ABCG2
Western blot and real-time qPCR results showed that CD133 was upregulated in the model group but decreased significantly in the HP alone, aspirin alone, or combined HP and aspirin groups. The expression levels of CD133 in the combination group were lower than those in the HP alone or aspirin alone groups, and the difference was statistically significant, but there was no significant difference between the two doses (Figure 3A).
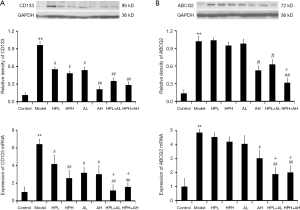
Western blot and real-time qPCR results showed ABCG2 was upregulated in the model group but decreased significantly in the AH or combined HP and aspirin groups. HP alone did not inhibit the expression of ABCG2. The expression levels of ABCG2 in the combination group were lower than those in the HP alone or aspirin alone groups (Figure 3B).
Discussion
The formation of lung tumors is the result of a combination of factors such as genes, environment, and body conditions (15,16). CD133 and ABCG2, as two of the markers of lung CSCs, are located mainly in the cell membrane, but some are situated in the cytoplasm. Many studies have shown that the biological functions of CD133 and ABCG2 are closely related to the formation of many tumors, but their role in the occurrence and development of tumors is still unclear. The results of this experiment demonstrate that there are self-renewing cell populations that express CD133 and ABCG2 in lung cancer. CD133 and ABCG2 are expressed primarily in the cell membrane of lung cancer cells, and a few of them are found in the cytoplasm, with the positive expressions of CD133 and ABCG2 related to the invasion and metastasis of tumor tissues.
At present, most studies believe that CSCs play an extremely crucial role in the formation, development, infiltration, and metastasis of tumors. Aspirin, a non-steroidal anti-inflammatory drug, has been revealed to reduce the incidence of colorectal tumors significantly in some studies (17). In vitro analyses have demonstrated that aspirin alone or in combination with 5-FU can inhibit the survival of colon cancer cells, the progression of the cell cycle, the expression of cyclooxygenase-2, and other processes (18). In accordance with the preceding claims, the results of our experiment showed that the growth of lung cancer and the expression levels of CD133 and ABCG2 in the aspirin groups were significantly lower than those in the control group in vitro and in vivo, indicating that aspirin has a certain inhibitory effect on the growth of lung cancer. However, the difference between the high-dose group and the low-dose group was not statistically significant, which could be due to the relatively small samples used.
HP is a traditional Chinese medicine ingredient that has an anti-cancer effect. HP inhibits gastric cancer growth via cell cycle arrest and apoptosis and enhances doxorubicin-induced apoptosis in human hepatocellular carcinoma cells (12,19). A previous study in our lab showed that HP could inhibit lung cancer growth by inhibiting PD-1 and enhancing the activity of NK and CTL cells. Our experimental results in the present study suggest that HP had significant inhibitory effects on the proliferation and colony formation of A549 cells and lung cancer growth in vivo. The ingredient also inhibited the expression of CD133, but not ABCG2. The effect of the combined use of HP and Aspirin showed better efficacies in inhibiting cancer cell proliferation and CD133 and ABCG2 expression than those of the single substance-treated groups, suggesting that the combined use had a more beneficial anti-cancer effect. However, further studies are needed to define specific combined dosage.
At present, no obvious side effects have been encountered using high doses of HP. The most common side effect of Aspirin is gastrointestinal dyspepsia. Although high-doses of Aspirin can cause a gastric ulcer, gastric bleeding, and other symptoms, no significant adverse reactions were registered in mice during our study using the combination of HP and Aspirin.
Conclusions
The results of this experiment confirm that Aspirin and HP play inhibitory roles in the expression of CD133 and ABCG2 and that the combined use of the two drugs has a synergistic effect. In-depth studies on non-steroidal, anti-inflammatory drugs and HP’s prevention and treatment of lung cancer may provide the theoretical and experimental bases for finding new therapeutic targets for lung cancer.
Acknowledgments
Funding: The research was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Animal Ethic Committee of People’s Hospital of Guizhou Province (GZPH-2016-116).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chang YW, Su YJ, Hsiao M, et al. Diverse targets of β-catenin during the epithelial-mesenchymal transition define cancer stem cells and predict disease relapse. Cancer Res 2015;75:3398-410. [Crossref] [PubMed]
- Sun YL, Yin SY, Xie HY, et al. Stem-like cells in hepatitis B virus-associated cirrhotic livers and adjacent tissue to hepatocellular carcinomas possess the capacity of tumorigenicity. J Gastroenterol Hepatol 2008;23:1280-6. [Crossref] [PubMed]
- Marcinkiewicz K, Scotland KB, Boorjian SA, et al. The androgen receptor and stem cell pathways in prostate and bladder cancers Int J Oncol 2012;40:5-12. (review). [PubMed]
- Haspels HN, Rahman MA, Joseph JV, et al. Glioblastoma stem-like cells are more susceptible than differentiated cells to natural killer cell lysis mediated through killer immunoglobulin-Like receptors-human leukocyte antigen ligand mismatch and activation receptor-ligand interactions. Front Immunol 2018;9:1345. [Crossref] [PubMed]
- Cao S, Wang Z, Gao X, et al. FOXC1 induces cancer stem cell-like properties through upregulation of beta-catenin in NSCLC. J Exp Clin Cancer Res 2018;37:220. [Crossref] [PubMed]
- de Gooijer MC, Zhang P, Weijer R, et al. The impact of P-glycoprotein and breast cancer resistance protein on the brain pharmacokinetics and pharmacodynamics of a panel of MEK inhibitors. Int J Cancer 2018;142:381-91. [Crossref] [PubMed]
- Rao DK, Liu H, Ambudkar SV, et al. A combination of curcumin with either gramicidin or ouabain selectively kills cells that express the multidrug resistance-linked ABCG2 transporter. J Biol Chem 2014;289:31397-410. [Crossref] [PubMed]
- Stavrovskaya AA, Rybalkina EY. Recent advances in the studies of molecular mechanisms regulating multidrug resistance in cancer cells. Biochemistry (Mosc) 2018;83:779-86. [Crossref] [PubMed]
- Ho MM, Hogge DE, Ling V. MDR1 and BCRP1 expression in leukemic progenitors correlates with chemotherapy response in acute myeloid leukemia. Exp Hematol 2008;36:433-42. [Crossref] [PubMed]
- Friel G, Liu CS, Kolomeyevskaya NV, et al. Aspirin and acetaminophen use and the risk of cervical cancer. J Low Genit Tract Dis 2015;19:189-93. [Crossref] [PubMed]
- Gao H, Cheng Y, Zong L, et al. Aspirin attenuates monocrotaline-induced pulmonary arterial hypertension in rats by suppressing the ERK/MAPK pathway. Clin Exp Hypertens 2017;39:34-41. [Crossref] [PubMed]
- Zan X, Cui F, Li Y, et al. Hericium erinaceus polysaccharide-protein HEG-5 inhibits SGC-7901 cell growth via cell cycle arrest and apoptosis. Int J Biol Macromol 2015;76:242-53. [Crossref] [PubMed]
- He X, Wang X, Fang J, et al. Structures, biological activities, and industrial applications of the polysaccharides from Hericiumerinaceus (Lion's Mane) mushroom: A review. Int J Biol Macromol 2017;97:228-37. [Crossref] [PubMed]
- Li L, Liu M, Kang L, et al. HHEX: A Crosstalker between HCMV Infection and Proliferation of VSMCs. Front Cell Infect Microbiol 2016;6:169. [Crossref] [PubMed]
- Qin L, Qiu K, Hu C, et al. Respiratory syncytial virus promoted the differentiation of Th17 cells in airway microenvironment through activation of Notch-1/Delta3. J Med Microbiol 2019;68:649-56. [Crossref] [PubMed]
- Antoniali G, Serra F, Lirussi L, et al. Mammalian APE1 controls miRNA processing and its interactome is linked to cancer RNA metabolism. Nat Commun 2017;8:797. [Crossref] [PubMed]
- Xu XR, Yousef GM, Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood 2018;131:1777-89. [Crossref] [PubMed]
- Ng K, Meyerhardt JA, Chan AT, et al. Aspirin and COX-2 inhibitor use in patients with stage III colon cancer. J Natl Cancer Inst 2014;107:345. [PubMed]
- Lee JS, Hong EK. Hericium erinaceus enhances doxorubicin-induced apoptosis in human hepatocellular carcinoma cells. Cancer Lett 2010;297:144-54. [Crossref] [PubMed]


