
Antitumor activity of integrin αVβ3 antibody conjugated-cationic microbubbles in liver cancer
Introduction
Integrins are cell-surface glycoproteins that trigger a diversity of signaling pathways, including cell adhesion and angiogenesis (1,2), and are involved in various human cancers. Integrin expression is hypothesized to render cancer cells to proliferate and migrate (3). Thus, integrins represent attractive targets for the prevention of cancer spread and tumor progression (4). Integrin αVβ3 is constitutively expressed in quiescent endothelial cells at low level (5), but it is highly expressed in tumors, such as prostate cancer (6), breast cancer (7), and melanoma (4). Integrin αVβ3 overexpression in hepatocarcinoma (HCC) is associated PI3K/Akt and TGF-β/ERK signaling pathways and promotes tumor progression, metastasis, and clinical staging (8-10).
The inhibition of integrin αVβ3 might suppress tumor proliferation. A series of integrin αVβ3 inhibitors have been developed to target tumors. RGD (Arg-Gly-Asp) peptides, a group of canonical antagonist of integrin αVβ3, show inhibitory activity in cell mobility and cell attachment in breast cancer (11). By enhancing internalization rate of these micelles in melanoma, the integrin αVβ3 targeting peptide (RGDfK) show synergistic cytotoxicity with docetaxel/cisplatin-coloaded micelles (12). Besides RGD peptides, by binding to NC1 domain of collagen XIX, integrin αVβ3 could inhibit FAK/PI3K/Akt/mTOR pathway (13).
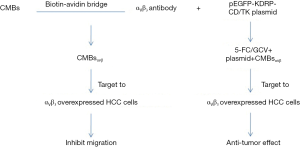
Our previous study also has demonstrated that by conjugating with αVβ3 integrin antibody, non-targeting cationic microbubbles (CMBs) can specifically target to HepG2 cells (14), and substantially increase pEGFP-KDRP-CD/TK plasmid transfection efficiency. The development of ultrasound contrast agent opens up a new idea of carrying target-delivery genes or drugs for chemotherapy in liver tumor patients (15). Ultrasound-targeted microbubble destruction (UTMD) provides a non-invasive, safe, and repeatable method for gene delivery (16-18). To further improve gene delivery efficiency, researchers design conjugated MBs to specifically bind to the membrane proteins on the surface of tumor cells. In the present study, CMBsαvβ3 could significantly suppress tumor growth in HepG2 xenografts mice model. In vitro, CMBsαvβ3 significantly impaired the wound healing and inhibited cell proliferation of HepG2 cells. CMBsαvβ3 also facilitated 5-FC/GCV + CD/TK double suicide gene-induced anti-tumor activity. These findings suggested CMBsαvβ3 as a promising gene delivery agent with potential anti-tumor activity itself.
Methods
Cell line and preparation of non-targeting CMBs
As previously described (14), human liver cancer HepG2 cells were purchased from Xiangya Cell Bank, Central South University (Changsha, China). 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), dipalmitoylphosphatidylcholine (DPPC), biotinylated dipalmitoylphosphatidyl-ethanolamine (DSPE-PEG2000-Biotin) and 1,2-dis-tearoyl-3-trimethyl-ammonium-propane (DTAP; Avanti, Alabaster, AL, USA) were mixed in a 5 mL plastic tube to form a suspension at a molar ratio of 46:36:8:2 (19). Perfluorinated propane (C3F8) was purchased from the Special gas Co., Ltd. Factory (Nanjing, China). Following lyophilization, 1 mL of phosphate-buffered saline (PBS) was added to the samples to rehydrate them and then C3F8 gas was slowly injected into the container to replace the air. Samples were then agitated using an ultrasonic mechanical vibrator with high speed shearing method for 90 s to form a milky white solution.
Preparation of CMBsαvβ3
Integrin αVβ3 antibody was conjugated to the distal end of the DSPE-PEG2000-Biotin molecules through biotin-streptavidin coupling chemical method (20). Briefly, 500 µL CMBs (1×109/mL) were mixed with 100 µg biotinylated anti-αVβ3 antibody in an ultrasonic agitating reaction for 30 min. Then, after centrifugation at 50 g for 5 min, the upper layer (CMBsαvβ3) was washed with PBS three times and then collected. The morphology and particle distribution of CMBsαvβ3 was observed by optical microscopy. The particle size and surface potential were measured by a Zetasizer 3000HS (Malvern, Worcestershire, UK). All experiments were performed for five times.
Plasmids
As described in our previous study (14), the restructured plasmid pEGFP-KDRP-CD/TK coding for green fluorescent protein (GFP) contained CD/TK double suicide gene and was driven by KDR promoter (21). The molecular weight of this plasmid is 2,300 kDa with 3,486 bp. The plasmid was amplified and then isolated and purified using QIAGEN plasmid giga kit (Qiagen, Valencia, CA, USA) following the manufacturer’s protocol.
Fluorescent assay
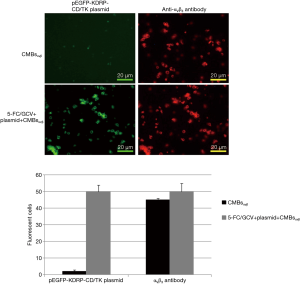
To confirm binding of CMBsαvβ3 to HepG2 cells, rhodamine mouse anti-human immunoglobulin (Ig) G was adopted to detected αVβ3 antibody. After treated with CMBsαvβ3 alone or plus pEGFP-KDRP-CD/TK plasmid, HepG2 cells were incubated with rhodamine mouse anti-human Ig G for 1 h at room temperature. Unbound (Ig) G was removed by two rounds of centrifugal washing. The binding was observed under a fluorescence microscope (CKX41; Olympus, Tokyo, Japan). EGFP expression levels were also evaluated by fluorescence microscope.
Animal experiments
As described previously (14), nude mice bearing HepG2 liver cancer were randomly divided into 3 groups: normal saline (NS) group, CMBs treatment group and CMBsαvβ3 treatment group. All treatments were terminated at 10 days. The tumor growth was observed and tumor volumes were calculated. Five days after treatment, HepG2 xenografts were removed and weighted. Tumor tissues RNA were extracted for quantitative RT-PCR (qRT-PCR) and caspase-3 activity assay.
qRT-PCR screening
Tumor RNA was isolated and converted into cDNA. qRT-PCR was performed using ABI-7500 (AppliedBiosystems, Fostercity, CA, USA) by mixing equal amounts of cDNAs, iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) and specific primers. All real-time data were normalized to β-actin.
Caspase-3 activity assay
Tumor tissue protein was extracted as previously described (22). Caspase-3 activity was tested instantly using the colorimetric substrate Ac-DEVD-pNA (Beyotime, Nanjing, China) according to the manufacturer’s instructions. After incubation overnight at 37 °C, caspase-3 activity was measured on an enzyme-linked immunosorbent assay instrument (Bio-Rad, USA) at 490 nm.
Scratch closure test
The migration of HepG2 cells exposed to CMBsαvβ3 was evaluated by scratch closure test. We took images of the scratched area at 0, 12, and 24 h after scratch and measured widths of the scratched by the Image-Pro Plus 6.0 software.
Anti-tumor effect evaluation
To determine the effects of CMBsαvβ3 on the cell cycle, propidium iodide (PI) staining after 75% alcohol fixation was used, followed by flow cytometry analysis. MTT assay was performed to evaluate the cell proliferation inhibition after CMBsαvβ3 treatment.
Statistical analysis
All data are expressed as mean ± standard deviation (SD), unless otherwise noted. Differences among groups were analyzed by one-way ANOVA and LSD method for multiple comparisons among groups using the SPSS software package (Version 19.0 for windows, SPSS, Chicago, Illinois, USA) with P<0.05 considered statistically significant.
Results
CMBsαvβ3 treatment suppresses HepG2 tumor growth
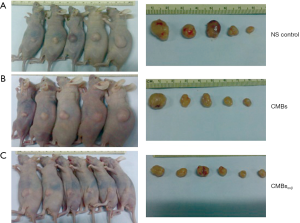
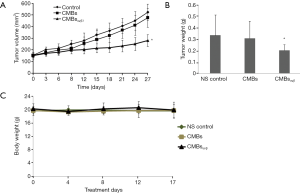
To investigate the anti-tumor effect of CMBsαvβ3in vivo, HepG2 xenograft mice models were used. The nude mice bearing HepG2 liver cancers were randomly divided into 3 groups: NS group, CMBs treatment group and CMBsαvβ3 treatment group. The whole treatment period was 10 days. Tumor volumes were evaluated at day 0 to day 17 from the treatment initial day. As shown in Figure 1, mice in NS (Figure 1A), CMBs (Figure 1B), CMBsαvβ3 (Figure 1C) groups were sacrificed and tumors were removed and weighed at 17 days after treatment. Compared to control (NS) group, CMBsαvβ3 treatment significantly suppressed tumor growth (P<0.05; Figure 2A). But CMBs treatment had no such effect. The xenograft tumors were weighted at the experiment endpoint. Compared to control group and CMBs group, CMBsαvβ3 treatment significantly suppressed tumor weight (P<0.05, Figure 2B). Mice body weights of all three treatment groups did not change significantly during the experiment period (Figure 2C). These findings implied an anti-tumor effect of CMBsαvβ3.
CMBsαvβ3 binds to tumor cells in xenografts and suppresses Bcl-2 and p53 mRNA level
CMBsαvβ3 is supposed to specifically bind to integrin αVβ3 on HepG2 cell surface through the conjugated integrin αVβ3 antibody. To confirm this binding, we adopted rhodamine mouse anti-human immunoglobulin (Ig) G and fluorescence microscope to detect the localization of CMBsαvβ3 on HepG2 cells. As demonstrated in Figure S1, the binding of CMBsαvβ3 to the surface of HepG2 cells was detected under fluorescence microscope. The red fluorescent signals suggested a tight binding of CMBsαvβ3 to HepG2 xenograft cells.
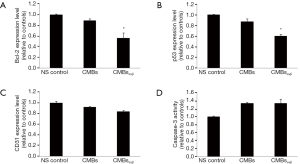
In previous reports, integrin αVβ3 inhibitor has shown effect to inhibit Bcl-2 expression (23) and modify p53 level (24). Consistent with these reports, qRT-PCR results in Figure 3A,B showed that CMBsαvβ3 treatment significantly suppressed the Bcl-2 and p53 mRNA levels, compared to control. CD31 mRNA level was not affected by CMBsαvβ3 treatment (Figure 3C). No obvious caspase-3 activation was detected in CMBsαvβ3 treatment group (Figure 3D). These data implied that the suppression of Bcl-2 and p53 might be responsible for CMBsαvβ3-mediated anti-tumor effect. But caspase-3-mediated apoptosis pathway was not involved in CMBsαvβ3-mediated anti-tumor effect.
CMBsαvβ3 inhibits HepG2 cells proliferation and migration in vitro
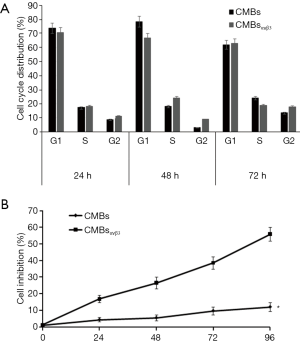
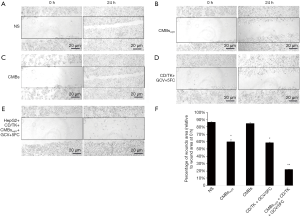
The anti-tumor CMBsαvβ3 effect of was also evaluated by cell cycle and MTT assays. As shown in Figure 4A, cell cycle distribution was not modified by CMBsαvβ3 treatment. MTT assay showed that HepG2 cell proliferation was obviously inhibited by CMBsαvβ3 treatment (Figure 4B). Integrin αVβ3 triggers signaling pathway to promote cell adhesion and migration (3). To examine the anti-migrant effect of CMBsαvβ3, HepG2 cells were exposed to CMBsαvβ3 for 24 h, and then evaluated by scratch closure test. At 24 h after scratch, areas of the scratched wounds were measured by Image J software (Figure 5), Compared to NS treatment (Figure 5A), at 24 h after scratch, CMBsαvβ3 treatment significantly inhibited the healing of scratched wounds (Figure 5B).
CMBsαvβ3 facilitates CD/TK transfection and promotes antitumor activity
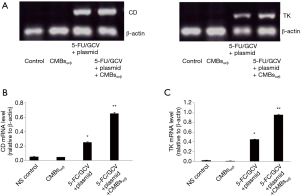
In our previous publication (14), we had demonstrated that CMBsαvβ3 combined with CD/TK transfection + 5-FC/GCV had a higher suppressing effect in HepG2 xenograft mice model than CD/TK transfection + 5-FC/GCV alone. Moreover, CMBsαvβ3 plus CD/TK + GCV/5-FC treatment induced more TUNEL-positive cells than CD/TK transfection + 5-FC/GCV treatment alone (14). Here, as demonstrated in Figure S1, the green fluorescence signals indicated a successful CD/TK transfection in HepG2 cells. qRT-PCR assay further confirmed the expression of CD and TK mRNA in HepG2 cells (Figure S2). When coupled with CMBsαvβ3, CD/TK gene expression levels were higher than CD/TK plasmid alone in HepG2 cells (P<0.05; Figure S2B,C).
The effect of CMBsαvβ3 in GCV/5-FC + CD/TK activity was also evaluated. As demonstrated in Figure 5C, CD/TK suicide gene transfection alone did not prevent the wound healing. When combined with CMBsαvβ3, CD/TK + GCV/5-FC could significantly suppress the healing of the scratched wounds (Figure 5E). Figure 5F compared the percentages of wound area relative to the initial wound area among treatment groups. Compared to NS control, both CMBsαvβ3 treatment alone and CD/TK + GCV/5-FC treatment alone could significantly suppress the wounds healing (P<0.05). Moreover, compared to CD/TK + GCV/5-FC treatment alone group, CMBsαvβ3 plus CD/TK + GCV/5-FC treatment had even lower wound healing rate (P<0.05). These results implied that CMBsαvβ3 not only inhibited HCC cell migration itself, but also increased the anti-migrant effect of CD/TK + GCV/5-FC treatment.
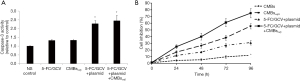
While no obvious caspase-3 activation was detected in CMBsαvβ3 treatment group, CD/TK + 5-FC/GCV treatment and CD/TK + 5-FC/GCV plus CMBsαvβ3 treatment could induce significant caspase-3 activation in HepG2 cells (Figure 6A). These data implied that caspase-3-mediated apoptosis pathway was involved in CD/TK + 5-FC/GCV-mediated anti-tumor effect, but not in CMBsαvβ3 treatment. MTT assay showed a significant higher suppression in cell proliferation in CD/TK + 5-FC/GCV plus CMBsαvβ3 treatment group, compared to CMBsαvβ3 alone or CD/TK + 5-FC/GCV treatment alone group (Figure 6B). Based on these findings, we suggested that CMBsαvβ3 not only had potential anti-tumor activity itself, but also facilitated CD/TK gene transfection and promoted the anti-tumor effects (Figure 7).
Discussion
In the era of precision medicine, targeting and accuracy become more and more important and practicable. Application of CMBs to treat cancers (25), brain disease (26,27), hepatic fibrosis (21), and heart diseases (28) are non-invasive, targeted and safe. Our designed CMBsαvβ3 carries CD/TK double suicide gene, and focused UTMD helps local gene delivery.
In this study, we reported a novel system CMBsαvβ3, with high affinity to integrin αVβ3 on HepG2 surface, and showed potent anti-tumor activity in HepG2 mice models. This integrin αVβ3 targeting system not only suppressed tumor growth alone, but also promoted CD/TK gene expression and 5-FC/GCV killing effect. Unlike other integrin ligands/analogues/peptides or integrin-based drug-load systems, CMBsαvβ3 initiated a completely new research field for the combination of multiple anti-tumor effects in one system. We believe the major anti-tumor actions of CMBsαvβ3 includes following two aspects: (I) inhibiting migration of integrin αVβ3 -overexpressed tumor cells; (II) facilitating CD/TK suicide gene transfection and promoting 5-FC/GCV-induced apoptotic cell death. This proposed model was described in Figure 7.
Biotinylated specific antibodies coupled to streptavidin-containing microbubbles through the biotin-streptavidin linkage have been used for molecular ultrasound imaging to monitor the receptor expression (29), such as VEGF receptor (30,31). Like Cyclic RGD peptides, biotin-streptavidin linkage is commonly used to specifically couple quantum dots to integrins (32). Cilengitide, a cyclic pentapeptide, is efficient to treat glioblastoma by targeting integrin αVβ3 and αVβ5 (33,34). Combination of UTMD with cilengitide-nanotherapy increases the tumor Cilengitide level over 3-fold and significantly reduce renal clearance, which help reduce Cilengitide dose level and increase killing effect (35). From our animal experiment results, focused UTMD with CMBsαvβ3 showed significantly anti-tumor effect in HepG2 xenograft tumors (Figure 1).
Integrins αVβ3 and αVβ5 ligands contain the RGD sequence. Cyclic RGD peptides are canonical integrin inhibitors, such as cilengitide (36). Humanized monoclonal integrin antibodies abituzumab and vedolizumab have demonstrated anti-tumor activity preclinically (37). Other inhibitors including salvicine and borrelidin are non-specific (38). CMBsαvβ3 specifically targets to integrin αVβ3 and showed high affinity to HepG2 cells in our models (Figure S1).
Integrin inhibitors always demonstrate anti-angiogenic (39) and anti-metastasis (40) functions. By targeting to integrin αVβ3 on neovasculature, cilengitide increased systemic radio-immunotherapy efficacy of therapy in p53 mutant and bcl-2 overexpressing breast cancer cells (41). In this study, CMBsαvβ3 significantly suppressed Bcl-2 and p53 levels, while CD31 level was moderately suppressed in mice models (Figure 3). In vitro experiment and inhibited HepG2 cell migration (Figure 5). Caspase-3 activity was not influenced by CMBsαvβ3, which is consistent with our previous study that CMBsαvβ3 alone did not induce apoptotic death in HepG2 animal model (14).
Conclusions
CMBsαvβ3 exerted the anti-tumor activities by inhibiting migration of integrin αVβ3-overexpressed tumor cells and facilitating CD/TK suicide gene transfection and promoting 5-FC/GCV-induced apoptotic cell death. Application of CMBsαvβ3 could initiate an accurate-targeting field for the combination of multiple anti-tumor effects in one system.
Acknowledgments
We thank Dr. Yu Sun from Nanjing Origin Bioscienses Incorporation for animal model and CMBs construction.
Funding: This work was funded by a grant from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.05.29). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The animal experiment in this study complied with the Third Xiangya Hospital guidelines and approved by the Third Xiangya Hospital Ethics Committee (2012-S119).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kampen KR. Membrane proteins: the key players of a cancer cell. J Membr Biol 2011;242:69-74. [Crossref] [PubMed]
- Sayedyahossein S, Dagnino L. Integrins and small GTPases as modulators of phagocytosis. Int Rev Cell Mol Biol 2013;302:321-54. [Crossref] [PubMed]
- Weber MR, Zuka M, Lorger M, et al. Activated tumor cell integrin alphavbeta3 cooperates with platelets to promote extravasation and metastasis from the blood stream. Thromb Res 2016;140:S27-36. [Crossref] [PubMed]
- Yacobovich S, Tuchinsky L, Kirby M, et al. Novel synthetic cyclic integrin alphavbeta3 binding peptide ALOS4: Antitumor activity in mouse melanoma models. Oncotarget 2016;7:63549-60. [Crossref] [PubMed]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994;264:569-71. [Crossref] [PubMed]
- McCabe NP, De S, Vasanji A, et al. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene 2007;26:6238-43. [Crossref] [PubMed]
- Chakravarty R, Chakraborty S, Dash A. Molecular Imaging of Breast Cancer: Role of RGD Peptides. Mini Rev Med Chem 2015;15:1073-94. [Crossref] [PubMed]
- Jin Y, Chen JN, Feng ZY, et al. OPN and alphavbeta3 expression are predictors of disease severity and worse prognosis in hepatocellular carcinoma. PLoS One 2014;9:e87930. [Crossref] [PubMed]
- Xia H, Chen J, Shi M, et al. EDIL3 is a novel regulator of epithelial-mesenchymal transition controlling early recurrence of hepatocellular carcinoma. J Hepatol 2015;63:863-73. [Crossref] [PubMed]
- Xu ZZ, Xiu P, Lv JW, et al. Integrin alphavbeta3 is required for cathepsin B-induced hepatocellular carcinoma progression. Mol Med Rep 2015;11:3499-504. [Crossref] [PubMed]
- Georgoulis A, Havaki S, Drosos Y, et al. RGD binding to integrin alphavbeta3 affects cell motility and adhesion in primary human breast cancer cultures. Ultrastruct Pathol 2012;36:387-99. [Crossref] [PubMed]
- Song W, Tang Z, Zhang D, et al. Anti-tumor efficacy of c(RGDfK)-decorated polypeptide-based micelles co-loaded with docetaxel and cisplatin. Biomaterials 2014;35:3005-14. [Crossref] [PubMed]
- Oudart JB, Doue M, Vautrin A, et al. The anti-tumor NC1 domain of collagen XIX inhibits the FAK/ PI3K/Akt/mTOR signaling pathway through alphavbeta3 integrin interaction. Oncotarget 2016;7:1516-28. [Crossref] [PubMed]
- Xue Q, Liu Y, He R, et al. Lyophilized Powder of Catalpol and Puerarin Protects Neurovascular Unit from Stroke. Int J Biol Sci 2016;12:367-80. [Crossref] [PubMed]
- Jing H, Cheng W, Zhang JW, et al. Galactosylated poly-L-lysine targeted microbubbles for ultrasound mediated antisense c-myc gene transfection in hepatocellular carcinoma cells. Arch Med Sci 2015;11:292-300. [Crossref] [PubMed]
- Cui K, Yan T, Luo Q, et al. Ultrasound microbubble-mediated delivery of integrin-linked kinase gene improves endothelial progenitor cells dysfunction in pre-eclampsia. DNA Cell Biol 2014;33:301-10. [Crossref] [PubMed]
- Fujii H, Li SH, Wu J, et al. Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair. Eur Heart J 2011;32:2075-84. [Crossref] [PubMed]
- Suzuki J, Ogawa M, Takayama K, et al. Ultrasound-microbubble-mediated intercellular adhesion molecule-1 small interfering ribonucleic acid transfection attenuates neointimal formation after arterial injury in mice. J Am Coll Cardiol 2010;55:904-13. [Crossref] [PubMed]
- Hou L, Zhao X, Wang P, et al. Antitumor activity of antimicrobial peptides containing CisoDGRC in CD13 negative breast cancer cells. PLoS One 2013;8:e53491. [Crossref] [PubMed]
- Kaur S, Kenny HA, Jagadeeswaran S, et al. {beta}3-integrin expression on tumor cells inhibits tumor progression, reduces metastasis, and is associated with a favorable prognosis in patients with ovarian cancer. Am J Pathol 2009;175:2184-96. [Crossref] [PubMed]
- Zhao YZ, Lin Q, Wong HL, et al. Glioma-targeted therapy using Cilengitide nanoparticles combined with UTMD enhanced delivery. J Control Release 2016;224:112-25. [Crossref] [PubMed]
- Saini R, Sorace AG, Warram JM, et al. An animal model allowing controlled receptor expression for molecular ultrasound imaging. Ultrasound Med Biol 2013;39:172-80. [Crossref] [PubMed]
- Pochon S, Tardy I, Bussat P, et al. BR55: a lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging of angiogenesis. Invest Radiol 2010;45:89-95. [Crossref] [PubMed]
- Nunn A, Pochon S, Tardy I, et al. Microbubble-conjugated vascular endothelial growth factor receptor 2 binding peptide. In: Bethesda: National Center for Biotechnology Information. Molecular Imaging and Contrast Agent Database (MICAD), 2004.
- Lieleg O, Lopez-Garcia M, Semmrich C, et al. Specific integrin labeling in living cells using functionalized nanocrystals. Small 2007;3:1560-5. [Crossref] [PubMed]
- Ishida J, Onishi M, Kurozumi K, et al. Integrin inhibitor suppresses bevacizumab-induced glioma invasion. Transl Oncol 2014;7:292-302.e1. [Crossref] [PubMed]
- Kurozumi K, Ichikawa T, Onishi M, et al. Cilengitide treatment for malignant glioma: current status and future direction. Neurol Med Chir (Tokyo) 2012;52:539-47. [Crossref] [PubMed]
- Ruffini F, Graziani G, Levati L, et al. Cilengitide downmodulates invasiveness and vasculogenic mimicry of neuropilin 1 expressing melanoma cells through the inhibition of alphavbeta5 integrin. Int J Cancer 2015;136:E545-58. [Crossref] [PubMed]
- Élez E, Kocakova I, Hohler T, et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial. Ann Oncol 2015;26:132-40. [Crossref] [PubMed]
- Hariharan S, Gustafson D, Holden S, et al. Assessment of the biological and pharmacological effects of the alpha nu beta3 and alpha nu beta5 integrin receptor antagonist, cilengitide (EMD 121974), in patients with advanced solid tumors. Ann Oncol 2007;18:1400-7. [Crossref] [PubMed]
- Harisi R, Jeney A. Extracellular matrix as target for antitumor therapy. Onco Targets Ther 2015;8:1387-98. [PubMed]
- Oliveira-Ferrer L, Hauschild J, Fiedler W, et al. Cilengitide induces cellular detachment and apoptosis in endothelial and glioma cells mediated by inhibition of FAK/src/AKT pathway. J Exp Clin Cancer Res 2008;27:86. [Crossref] [PubMed]
- Burke PA, DeNardo SJ, Miers LA, et al. Cilengitide targeting of alpha(v)beta(3) integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res 2002;62:4263-72. [PubMed]
- Xu J, Zeng X, Liu Y, et al. A novel dual-targeted ultrasound contrast agent provides improvement of gene delivery efficiency in vitro. Tumour Biol 2016;37:8609-19. [Crossref] [PubMed]
- Fan CH, Ting CY, Lin CY, et al. Noninvasive, Targeted, and Non-Viral Ultrasound-Mediated GDNF-Plasmid Delivery for Treatment of Parkinson's Disease. Sci Rep 2016;6:19579. [Crossref] [PubMed]
- Tan JK, Pham B, Zong Y, et al. Microbubbles and ultrasound increase intraventricular polyplex gene transfer to the brain. J Control Release 2016;231:86-93. [Crossref] [PubMed]
- Zhang SH, Wen KM, Wu W, et al. Efficacy of HGF carried by ultrasound microbubble-cationic nano-liposomes complex for treating hepatic fibrosis in a bile duct ligation rat model, and its relationship with the diffusion-weighted MRI parameters. Clin Res Hepatol Gastroenterol 2013;37:602-7. [Crossref] [PubMed]
- Sun L, Huang CW, Wu J, et al. The use of cationic microbubbles to improve ultrasound-targeted gene delivery to the ischemic myocardium. Biomaterials 2013;34:2107-16. [Crossref] [PubMed]
- Christiansen JP, French BA, Klibanov AL, et al. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound Med Biol 2003;29:1759-67. [Crossref] [PubMed]
- Lindner JR, Song J, Xu F, et al. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation 2000;102:2745-50. [Crossref] [PubMed]
- Martenson RE, Deibler GE, Kies MW. Extraction of rat myelin basic protein free of other basic proteins of whole central nervous system tissue. An analysis of its electrophoretic heterogeneity. J Biol Chem 1969;244:4268-72. [PubMed]


