Dual drive coexistence of ALK rearrangement and KRAS mutation advanced lung adenocarcinoma and response to crizotinib
Introduction
Lung cancer is known as the leading cause of cancer-related death. Histologically, the adenocarcinoma is considered to be one of the most common forms of lung cancer (1). Anaplastic lymphoma kinase (ALK) rearrangements are identified in 4% of patients with non-small cell lung cancer (NSCLC), mostly in the form of echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) gene fusion (2). Both in vitro and in vivo studies have shown that EML4-ALK fusion genes act as strong oncogenic drivers and therapeutic targets in lung cancers (3,4). V-KI-RAS2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations occur in approximately 20–30% of Caucasian patients with lung adenocarcinoma. Studies have shown that ALK gene arrangements are mutually exclusive with epidermal growth factor receptor (EGFR) and KRAS mutations (5,6).
Herein, we report a rare case of a patient with a lung adenocarcinoma co-existing EML4-ALK fusion gene, as well as a KRAS p.G13D mutation. We also report that the patient was sensitive to treatment with crizotinib, a tyrosine kinase inhibitor (TKI).
Case presentation
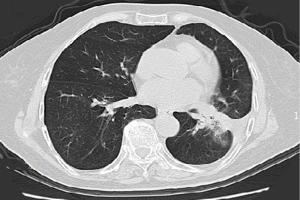
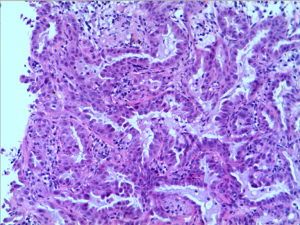
A 47-year-old, non-smoking female was referred to our hospital in January 2018 with a 1-month history of cough and phlegm. A chest computed tomography (CT) scan revealed a 5.0 cm mass in the left lower lung associated with multiple nodules in both lungs (Figure 1). Tumor biopsy pathology conducted on January 31, 2016, revealed that the patient had a stage IV (cT4N3M1) adenocarcinoma. Hematoxylin and eosin (H&E) staining revealed a typical morphology for adenocarcinoma cells (Figure 2).
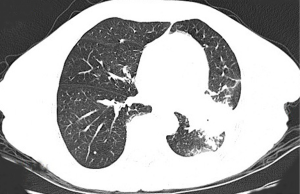
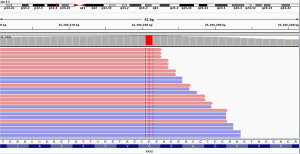
Thoracic radiotherapy followed by three cycles of chemotherapy with pemetrexed (750 mg) and cisplatin (40 mg) were administered between February 2018 and May 2018. However, emission computed tomography (ECT) revealed tumor progression after one month and gemcitabine (1.5 g) and nedaplatin (120 mg) were prescribed. Unfortunately, the patient relapsed in August 2018 and a tumor progression was observed. An next-generation sequencing (NGS) analysis of the hydrothorax revealed a variant of EML4-ALK fusion (the allele frequency: 16.6%) (Figure 3) accompanied by a point mutation of KRAS (p.G13D, the allele frequency: 15.7%) (Figure 4). Based on molecular findings, treatment was initiated with crizotinib in September, 2018. After 2 months of therapy, the patient achieved a partial response (Figure 5). Afterwards, the patient was further administrated with crizotinib for 6.0 months with a stable disease before tumor progression. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.

Discussion
Non-smoking lung cancer patients represent a distinct population with their unique epidemiological, clinicopathological and molecular features (7). ALK rearrangements and mutations in KRAS and EGFR are considered to be the main oncogenic alterations in non-smoking lung cancer (8). In this case, the patient was diagnosed with concurrent KRAS mutation and EML4-ALK translocation.
According to previous studies, ALK gene arrangements appear to be mutually exclusive with KRAS mutations (9,10). Nevertheless, subsequent studies identified several lung cancer patients with concurrent KRAS mutation and ALK translocation (11). Subpopulation of NSCLC with EML4-ALK rearrangement present distinguished clinical and pathological characteristics (5). In general, most of these patients occur in non-smokers and younger adenocarcinoma cases, with advanced clinical stage (12).
Patients who harbor ALK gene rearrangement can benefit from treatment with TKIs. Crizotinib, a selective inhibitor of ALK was approved for the treatment of NSCLC patients with ALK gene rearrangement (13). Clinical data demonstrate a significant improvement in objective response rate (ORR) and progression-free survival (PFS) in ALK translocated NSCLC (14).
Kirsten rat sarcoma viral oncogene (KRAS) is a GTP-binding protein, involved in G-protein-coupled receptor signaling (5). KRAS is one of the most frequently mutated oncogenes in NSCLC, which is usually associated with smoking history (15). Although the patients with the wild-type KRAS gene may benefit from the TKI therapy, debate over the prognostic role of KRAS mutation status in NSCLC continues (16). In this case, the patient presented with clinicopathological characteristics of EML4-ALK rearranged NSCLCs, such as young age, non-smoking history, and advanced adenocarcinoma condition. However, a KRAS p.G13D mutation was identified. Indeed, several KRAS genetic mutations including p.G13D concurrent with ALK and ROS1 have been characterized in human malignancies, such as colorectal cancer and lung adenocarcinoma (11,17,18). Previously, KRAS mutations have been reported to indicate primary resistance to crizotinib in ALK-rearranged lung adenocarcinomas (19). Similarly, another study reported that point mutations of the KRAS oncogene may interfere with ROS1 signaling and lead to unresponsiveness to crizotinib in ROS1-targeted therapies in lung cancer (18). Nevertheless, a favorable response to erlotinib was reported in a lung adenocarcinoma with both EGFR exon 19 deletion and KRAS G13D mutations (20). Moreover, excellent response to crizotinib has been previously reported in a lung adenocarcinoma with concurrent KRAS mutation and ALK rearrangement (11). Based on the patient’s results, we believe that the presence of KRAS mutation does not affect the receipt of crizotinib in ALK fusion patients. Thus, the precise role and mechanisms of KRAS mutation in ALK- or ROS1-translocated NSCLC remains indecisive and needs further investigation.
In summary, the present case indicated that the patient with concurrent EML4-ALK rearrangement and KRAS mutation experienced an excellent disease control with crizotinib for a PFS of 11 months. Although oncogenic drivers are mutually exclusive in the majority of NSCLC, 2 or more oncogenic events may be present in a minority of cases. Moreover, a further understanding of the pathogenesis of lung cancer with multiple oncogenic drivers will promote the optimal treatment for NSCLC.
Acknowledgments
Funding:
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.06.23). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Woo CG, Seo S, Kim SW, et al. Differential protein stability and clinical responses of EML4-ALK fusion variants to various ALK inhibitors in advanced ALK-rearranged non-small cell lung cancer. Ann Oncol 2017;28:791-7. [PubMed]
- Guo F, Liu X, Qing Q, et al. EML4-ALK induces epithelial-mesenchymal transition consistent with cancer stem cell properties in H1299 non-small cell lung cancer cells. Biochem Biophys Res Commun 2015;459:398-404. [Crossref] [PubMed]
- Christopoulos P, Endris V, Bozorgmehr F, et al. EML4-ALK fusion variant V3 is a high-risk feature conferring accelerated metastatic spread, early treatment failure and worse overall survival in ALK(+) non-small cell lung cancer. Int J Cancer 2018;142:2589-98. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Yoshida T, Oya Y, Tanaka K, et al. Differential Crizotinib Response Duration Among ALK Fusion Variants in ALK-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3383-9. [Crossref] [PubMed]
- Dias M, Linhas R, Campainha S, et al. Lung cancer in never-smokers - what are the differences? Acta Oncol 2017;56:931-5. [Crossref] [PubMed]
- Martín Martorell P, Huerta M, Compañ Quilis A, et al. Coexistence of EGFR, KRAS, BRAF, and PIK3CA Mutations and ALK Rearrangement in a Comprehensive Cohort of 326 Consecutive Spanish Nonsquamous NSCLC Patients. Clin Lung Cancer 2017;18:e395-402. [Crossref] [PubMed]
- Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res 2013;19:4273-81. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Campos-Gomez S, Lara-Guerra H, Routbort MJ, et al. Lung adenocarcinoma with concurrent KRAS mutation and ALK rearrangement responding to crizotinib: case report. Int J Biol Markers 2015;30:e254-7. [Crossref] [PubMed]
- Tan PS, Bilger M, de Lima Lopes G, et al. Meta-analysis of first-line therapies with maintenance regimens for advanced non-small-cell lung cancer (NSCLC) in molecularly and clinically selected populations. Cancer Med 2017;6:1847-60. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 2011;12:1004-12. [Crossref] [PubMed]
- Porta M, Crous-Bou M, Wark PA, et al. Cigarette smoking and K-ras mutations in pancreas, lung and colorectal adenocarcinomas: etiopathogenic similarities, differences and paradoxes. Mutat Res 2009;682:83-93. [Crossref] [PubMed]
- De Roock W, Jonker DJ, Di Nicolantonio F, et al. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA 2010;304:1812-20. [Crossref] [PubMed]
- Homa I, Sawicki M, Wojas-Krawczyk K, et al. Rare co-existence of mutation in KRAS and ALK gene re-arrangement in an adenocarcinoma patient--a case report. Anticancer Res 2014;34:3701-5. [PubMed]
- Zhu YC, Lin XP, Li XF, et al. Concurrent ROS1 gene rearrangement and KRAS mutation in lung adenocarcinoma: A case report and literature review. Thorac Cancer 2018;9:159-63. [Crossref] [PubMed]
- Mengoli MC, Barbieri F, Bertolini F, et al. K-RAS mutations indicating primary resistance to crizotinib in ALK-rearranged adenocarcinomas of the lung: Report of two cases and review of the literature. Lung Cancer 2016;93:55-8. [Crossref] [PubMed]
- Lee CN, Chen HY, Liu HE. Favorable response to erlotinib in a lung adenocarcinoma with both epidermal growth factor receptor exon 19 deletion and K-ras G13D mutations. J Clin Oncol 2010;28:e111-2. [Crossref] [PubMed]