Accelerated partial breast irradiation in elderly breast cancer patients
Introduction
Early breast cancer (EBC) is a frequent disease in women with a constantly increasing incidence in all age categories (1). Due to the increase in life expectancy, EBC has a higher prevalence in women aged 65 and older (2). Management of the treatment of EBC has become an important concern in developed countries. Breast-conserving surgery (BCS) with adjuvant whole-breast irradiation (WBI) and adjuvant hormonal treatment has been the standard treatment of EBC for decades. The use of WBI following BCS significantly decreases 10-year locoregional recurrence rates in all patients and 15-year mortality in some of them (3).
Despite the undoubted advantages of WBI in terms of improved relapse rates and eventually survival, this approach presents several important drawbacks. First, WBI requires an extended treatment time (3 to 7 weeks), which places a heavy burden on patients and also increases staff and radiation technique workloads. Secondly, WBI may be overtreatment given that the vast majority of local relapses (>85%) that occur after BCS (with or without WBI) occur in or close to the primary tumour bed (4). As a result, irradiating the entire breast may needlessly put patients at risk of developing clinically-significant side effects. For these reasons, partial breast irradiation (PBI), which targets only the postoperative cavity, has been established as an alternative to WBI in selected patients (5-7).
To date, the largest prospective randomized trial of external beam PBI is the multicentric IMPORT-LOW trial, which has demonstrated that PBI of 40 Gy in 15 daily fractions is not inferior to the same schedule of WBI in terms of local control and toxicity (8). Results of this trial favour this treatment as an easy technique of simple field reduction implementable in all radiotherapy centres that already provide breast radiotherapy. However, compared to standard 3-week WBI, there is no reduction of the treatment duration with this approach.
In accelerated partial breast irradiation (APBI), the total dose of radiation is given over 1 week or a shorter period of time to the part of the breast with the highest risk of microscopic disease. Accelerated radiotherapy uses fewer fractions of radiation to a smaller target volume, with a higher dose per single fraction, and with usually more than one fraction per day. Altogether, APBI aims to improve treatment tolerability and toxicity by limiting the treated volume and overall treatment time, without compromising clinical outcomes.
This paper is a review concerning evidence, process, techniques, and results of ABPI in elderly EBC patients.
Radiation therapy in the elderly
The treatment of elderly breast cancer patients differs from the therapeutic approach in younger ones. Radiation therapy can be highly effective and well tolerated in elderly patients (9), and age alone is not a limiting factor for adjuvant radiotherapy in breast carcinoma. However, elderly patients are prone to geriatric frailty and comorbid conditions, the incidence and severity of which increases with age. Weak underlying functional reserve and limited life expectancy of older patients can both limit the expected long-term benefits of standard adjuvant treatment (10). The radiation oncologist must be mindful of the potential to overestimate the functional reserve of the elderly and overtreat such patients, with the risk of unnecessary treatment morbidity and non-cancer related death. Moreover, compliance to a long course of WBI is often suboptimal, especially for patients living further away from a radiation centre (11).
In the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis, the absolute reduction rate of local recurrence (LR) with WBI in patients aged 60 years or older with low/intermediate-grade, hormone receptor-positive cancer was very small (5–10% reduction in 10-year risk of recurrence) (3). Consequently, several trials have demonstrated that omission of adjuvant WBI in elderly patients does not result in survival deterioration, albeit with statistically significant increase of local recurrence (12-14). To date, there has been no absolute age limit beyond which adjuvant irradiation does not improve local control in EBC. Therefore, there may also be a potential risk of undertreating older women because of an underestimation of life expectancy in patients of advanced age but with few significant comorbid conditions (15).
APBI
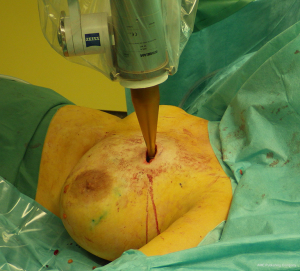
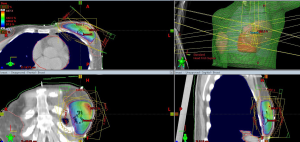
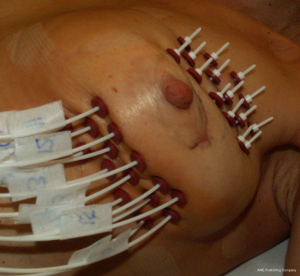
APBI allows the delivery of adjuvant radiotherapy after BCS in 1 week or less with fewer radiation-related side effects due to the more precisely-targeted dose delivery. To date, APBI is considered a standard postoperative treatment in low-risk EBC patients with suitable pathological characteristics (Table 1). There are currently multiple techniques to deliver APBI. Intraoperative PBI delivers a single fraction of radiotherapy in the perioperative period, using linear accelerator (Linac) electron beam (16) or intraoperative kilovoltage photon therapy (Figure 1) (17,18). High-precision external beam radiotherapy (EBRT) using 3D-conformal radiotherapy (3D-CRT, Figure 2) (19) or intensity-modulated radiotherapy (IMRT) (20). The most evidence-based APBI technique is brachytherapy, either multicatheter interstitial brachytherapy (MIB, Figure 3) (21,22), or industrial catheters-based brachytherapy (e.g., MammoSite, Contura or double-balloon applicator, ClearPath or SAVI implants).
Table 1
| Variables | ASTRO, Smith 2009 (5) | GEC-ESTRO, Polgár 2010 (6) | ABS, Shah 2013 (7) |
|---|---|---|---|
| Age (years) | ≥60 | ≥50 | ≥50 |
| BRCA mutation | Not present | – | – |
| Tumor size | ≤2 cm | ≤3 cm | ≤3 cm |
| Nodal status | pN0 (SN or ALND) | pN0 (SN or ALND) | pN0 (SN or ALND) |
| Resection margin | ≥2 mm | ≥2 mm | Negative |
| Tumor grade | Any | Any | – |
| Lymphovascular space invasion | Not present | Not present | Not present |
| Estrogen receptors | Positive | Positive | Positive/negative |
| Multicentricity | Unicentric | Unicentric | – |
| Multifokality | Unifocal | Unifocal | – |
| Histology | Invasive ductal | Invasive ductal | Any invasive, ductal in situ |
| Extensive intraducal component | Not present | Not present | – |
| Neoadjuvant therapy | Not allowed | Not allowed | – |
A systematic review with meta-analysis of 8653 women treated by APBI in eight randomized trials found that patients treated with APBI had a higher rate of local recurrence versus WBI, but without any differences in survival or other clinical outcomes (23). Later, a Cochrane review of partial breast irradiation for early breast cancer (24) found no clear evidence of a difference between PBI/APBI and WBI in terms of cause-specific survival, distant metastasis-free survival, relapse-free survival, loco-regional recurrence-free survival, or mastectomy rates. Finally, recent meta-analysis of 9 randomized trials and 8,720 patients showed lower 5-year non-breast cancer and overall mortality in patients treated with (A)PBI compared to whole breast irradiation. These findings suggest that APBI may avoid deaths from other causes in EBC patients. Since APBI trials included EBC patients with advanced age (Table 2), the need to avoid any harmful effects of treatment may be vital especially in the elderly and should be discussed with patients appropriately.
Table 2
| Trial | Randomization | No. of patients | Age eligible (years) | Age real (years) |
|---|---|---|---|---|
| Milan ELIOT (Veronesi 2013) (16) | WBI: 50 Gy/25 fr. | 654 | Any | Mean 60 (range 48–75) |
| APBI: IORT single fraction 21 Gy | 651 | |||
| TARGIT-A (Vaidya 2014) (17) | WBI: 56 Gy/28 fr. | 1,730 | ≥45 | 62±7.4 |
| APBI: IORT single fraction 20 Gy | 1,721 | 63±8.2 | ||
| RAPID 3D-CRT (Olivotto 2013) (19) | WBI: 42.5 Gy/16 fr, 50 Gy/25 fr. | 1,065 | ≥40 | 88% ≥60 |
| APBI: 38.5 Gy/10 fr./5 days | 1,070 | 88% ≥60 | ||
| Stanford 3D-CRT (Horst 2016) (18) | Single arm: 34–38.5 Gy/10 fr./5 days | 141 | Any | Median 60 (range 37–87) |
| Florence IMRT (Livi 2015) (20) | WBI: 50 Gy/25 fr.; IMRT | 260 | ≥40 | 53.5% ≥60 |
| APBI: 30 Gy/5 fr. | 260 | 60.7% ≥60 | ||
| Budapest MIB (Polgár 2013) (21) | WBI: 50 Gy/25 fr. | 130 | Any | Mean 58 (range 30–84) |
| APBI: 7×5.2 Gy HDR MIB | 128 | Mean 59 (range 31–80) | ||
| GEC-ESTRO MIB (Strnad 2016) (22) | WBI: 50 Gy/25 fr. + boost 10 Gy | 551 | ≥40 | Median 62 (range 54–67) |
| APBI: HDR or PDR MIB | 633 | Median 62 (range 54–68) |
APBI, accelerated partial breast irradiation; WBI, whole breast irradiation; IORT, intraoperative radiotherapy; IMRT, intensity modulated radiation therapy; MIB, multicatheter interstitial brachytherapy; 3D-CRT, 3D conformal radiotherapy; HDR, high dose rate; PDR, pulse dose rate; SD, standard deviation.
Several medical societies, notably the American Society for Radiation Oncology (ASTRO), the Groupe Européen de Curiethérapie - European Society for Radiotherapy and Oncology (GEC-ESTRO), and the American Brachytherapy Society (ABS), have provided recommendations aimed at patient selection for APBI (5-7). Detailed guidelines concerning the appropriate target definition and quality assurance are also available, especially for MIB APBI technique (25,26). Likewise, long-term outcomes are well documented for MIB (21,22), but less so for other APBI techniques. Finally, the long-term risk of secondary cancer is reduced 2- to 4-fold in MIB with the lowest mean lung dose, compared to other APBI techniques (27).
In the light of such observations, APBI seems to be an advisable postoperative approach in properly selected elderly EBC patients, with its combined advantages of a radical approach that minimizes the risk of undertreatment, and efficient reduction of redundant irradiated volume, treatment toxicity, overall treatment time, staff workload, radiation technique workflow, patient transportation, and potential for non-compliance. APBI has become a standard of care in patients with low-risk EBC, but there is no “one size fits all” technique of APBI. The best technique always depends on willing patients, anatomy, performance status, frailty, comorbid conditions, tumour laterality, and location.
APBI in elderly EBC patients
Although the use of APBI is well described predominantly in women aged 50 years or more, there are several manuscripts concerning the feasibility and results of APBI directly in elderly women with breast cancer, aged 65 years or older.
GERICO-03 prospective phase II trial assessed the feasibility, reproducibility, and impact of APBI on functional status in elderly women aged 70 years or older. Forty-six patients with EBC (T1–2 <30 mm, pN0, median age 74 years) underwent high-dose rate (HDR) brachytherapy with a delivered dose of 34 Gy in 10 fractions over 5 days. The treatment was assessed as feasible and reproducible, with no significant impact on functional dependence, when the Activities of Daily Living and Instrumental Activities of Daily Living scores remained unchanged 6 and 12 months after APBI, compared with baseline values (28).
In 2013, a retrospective SEER (Surveillance, Epidemiology, and End Results) analysis of female patients with EBC aged 65 years or older was published. A cohort of 26,931 eligible patients with BCS and sole adjuvant radiotherapy without chemotherapy was divided into patients who underwent APBI with brachytherapy (1,594 patients, 5.9%), and patients treated with WBI (25,339, 94.1%). According to the analysis, APBI and WBI resulted in similar recurrence-free and overall survival rates in the cohort of elderly EBC patients, even after adjustment for the more favourable characteristics of the patients in the former group (29).
In Florence, a monocentric randomized phase III trial was performed comparing APBI using 5 times 6 Gy non-consecutive daily fractions of IMRT vs. WBI (20). In 2015, a subgroup analysis from this trial was published concerning elderly patients aged 70 or older. A total of 117 patients aged 70 years or more (median 74.4 years, range 70.1–85.3 years) were analysed (58 in the WBI arm, 59 in the APBI arm). At a median follow-up of 5-years, the IBTR rate was 1.9% in both groups. The APBI group presented significantly better results in terms of acute skin toxicity, which could translate in a consistent improvement of overall quality of life and elderly patients’ compliance (30).
In 2012, a nomogram was published to predict the benefit of postoperative breast irradiation for older patients with breast cancer after BCS (31). Based on this model, Sumodhee et al. investigated the position of APBI in the cohort of elderly EBC patients, compared to WBI or endocrine therapy alone. In 79 elderly patients with APBI (median age 77 years, range 66–89 years), the 10-year mastectomy-free survival (MFS) rate after 10 years was 97.4%, compared to nomogram-calculated MFS rate 96.3% with adjuvant WBI, or 92.7% without adjuvant radiotherapy (32). This study supports the position of APBI as a compromise between WBI and omission of radiotherapy in elderly EBC patients.
Intraoperative/perioperative APBI in elderly patients
APBI is generally performed several weeks after surgery to avoid possible wound-healing complications and only after complete results of the pathology examination are available. As mentioned before, only selected patients with strict pathological findings are suitable for APBI and these conditions are not available at the time of surgery. However, perioperative APBI techniques have some undeniable advantages. The main advantage of intraoperative irradiation (IORT) is that it allows the radiation oncologist to visualize the surgical cavity directly before irradiation, and also allows for the rapid completion of both surgery and radiotherapy in 1 day. However, the risk of consecutive adverse histopathological findings after IORT that may disqualify the patient from already accomplished APBI is not negligible.
In the TARGIT study, of the 1,140 patients allocated to targeted IORT in the prepathological stratum, 219 (19%) ultimately received both IORT and WBI because postoperative evaluation revealed high-risk characteristics in that subset of patients (33). In the ELIOT trial, 651 patients treated with electron IORT had significantly higher risk of ipsilateral breast tumour recurrence (IBTR) after 5 years compared to WBI (4.4% vs. 0.4%). Nonetheless, the risk of IBTR after IORT in this trial would be 1.9% if only good candidates for APBI according to GEC-ESTRO recommendations were irradiated intraoperatively (16).
Another perioperative approach is open-cavity MIB or industrial applicator implantation, when one can see without difficulty the cavity that needs to be implanted as well as the distribution and spacing (Figure 4). Moreover, the patient is protected from repeated anaesthesia or invasive procedure at the same time. The APBI itself starts only after definitive completion of the postoperative histopathological report. Therefore, in the case of intra-operative implant, the catheters must stay in place for at least 10 to 15 days (6–8 days to obtain the full postoperative pathological report plus 4 to 5 more days for the treatment itself, plus one more weekend in some cases). This time may be a potential risk for patient discomfort or local infection. In the situation of intra-operative implant, when APBI is not possible due to pathological findings with regard to the GEC-ESTRO recommendations, it is possible to remove catheters and continue with the external beam WBI or systemic therapy. It is also possible to use the implant for the interstitial cavity boost, with consequent WBI.
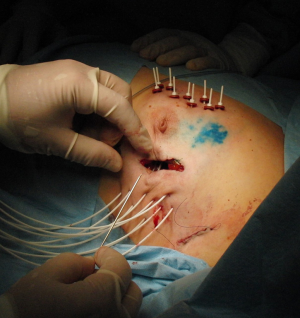
In the study conducted by Cambeiro et al. (34), intra-operative MIB implant for postoperative APBI in low risk EBC was investigated. APBI was performed in 88 from 137 initial candidates (64.2%), in 34 patients (24.8%) the implant was used as the boost to EBRT, and in 15 cases (11%) brachytherapy was not performed. Likewise, the Czech monocentric prospective study evaluated the feasibility of using perioperative MIB (starting 6 days postoperatively) in highly selected EBC patients aged 60 years or more. From 125 patients intended for APBI with perioperative MIB implantation, only 12 patients (9.6%) did not undergo APBI due to unsuitable final histopathological findings (35). Based on the low rate of adverse histological findings in carefully selected elderly patients, APBI with perioperatively implanted MIB seems to be a reasonable approach.
Future perspectives
Further acceleration of adjuvant radiotherapy in EBC is under investigation, both for external beam APBI or brachytherapy. Results from a phase I/II trial for hypofractionated APBI using a 2-day dose schedule was recently published (36). A total of 45 patients (median age 66 years) were treated with balloon-based intracavitary brachytherapy 4 times 7 Gy, twice daily on 2 consecutive days. After 6 years, there was no IBTR recorded, and the chronic toxicities were acceptable according to the authors. In addition, for elderly EBC patients, the feasibility and early clinical outcomes of a single fraction of post-operative MIB were evaluated in a prospective phase I/II trial (37). In 26 patients (aged 70 years or older, median 77 years) after lumpectomy, intraoperative catheter implant was performed for post-operative APBI in a single fraction of 16 Gy.
The results of these trials are encouraging, but need longer follow-up and confirmation on a larger cohort of patients.
Conclusions
APBI seems to be an advisable postoperative approach in properly selected elderly EBC patients, combining advantages of a radical approach that minimizes the risk of undertreatment, with efficient reduction of redundant irradiated volume, treatment toxicity, overall treatment time, staff workload, radiation technique workflow, patient transportation, and potential for non-compliance. Moreover, APBI seems to be an ideal compromise between WBI and the omission of any radiotherapy at all in elderly EBC patients. To date, APBI is considered a standard postoperative treatment in low-risk EBC patients with suitable pathological characteristics. There are currently multiple techniques to deliver APBI, with best evidence favouring multicatheter interstitial brachytherapy. However, there is no “one size fits all” technique of APBI, with the best technique always depending on willing patients, anatomy, performance status, frailty, comorbid conditions, tumor laterality, and location. With ongoing trials, we anticipate further shortening of the treatment time in elderly patients with ultra-hypofractionated or single dose APBI schedules. Unfortunately, accessibility of APBI and specific cost analyses vary across the world and across regions of particular continents and countries.
Acknowledgments
The authors would like to thank Dr. Ian McColl for proof-reading the manuscript.
Funding: The review was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.03). The series “Radiotherapy for Breast Cancer in Advanced Age” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Vincent Vinh-Hung and Nam P Nguyen) for the series “Radiotherapy for Breast Cancer in Advanced Age” published in Translational Cancer Research. The article has undergone external peer review.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sun M, Cole AP, Lipsitz SL, et al. Trends in breast, colorectal, and cervical cancer incidence following the affordable care act: Implications for cancer screening. JAMA Oncol 2018;4:128-9. [Crossref] [PubMed]
- Petera J, Dušek L, Sirák I, et al. Cancer in the elderly in the Czech Republic. Eur J Cancer Care (Engl) 2015;24:163-78. [Crossref] [PubMed]
- Early Breast Cancer Trialists Collaborative Group (EBCTCG). Effect of radiotherapy after breast conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 174 randomized trials. Lancet 2011;378:1707-16. [Crossref] [PubMed]
- Orecchia R, Fossati P. Partial breast irradiation: Ready for routine? Breast 2007;16:S89-97. [Crossref] [PubMed]
- Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement for the American society for radiation oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009;74:987-1001. [Crossref] [PubMed]
- Polgár C, Limbergen EV, Potter R, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: Recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol 2010;94:264-73. [Crossref] [PubMed]
- Shah C, Vicini F, Wazer DF, et al. The American Brachytherapy Society consensus statement for accelerated partial breast irradiation. Brachytherapy 2013;12:267-77. [Crossref] [PubMed]
- Coles CE, Griffin CL, Kirby AM, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017;390:1048-60. [Crossref] [PubMed]
- Kunkler IH, Williams LJ, Jack WL, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol 2015;16:266-73. [Crossref] [PubMed]
- Patnaik JL, Byers T, DiGuiseppi C, et al. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst 2011;103:1101-11. [Crossref] [PubMed]
- Nattinger AB, Kneusel RT, Hoffmann RG, et al. Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst 2001;93:1344-46. [Crossref] [PubMed]
- Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy Plus Tamoxifen With or Without Irradiation in Women Age 70 Years or Older With Early Breast Cancer: Long-Term Follow-Up of CALGB 9343. J Clin Oncol 2013;31:2382-7. [Crossref] [PubMed]
- Blamey RW, Bates T, Chetty U, et al. Radiotherapy or tamoxifen after conserving surgery for breast cancers of excellent prognosis: British Association of Surgical Oncology (BASO) II trial. Eur J Cancer 2013;49:2294-302. [Crossref] [PubMed]
- Kunkler IH, Audisio R, Belkacemi Y, et al. Review of current best practice and priorities for research in radiation oncology in elderly patients with cancer: the International Society of Geriatric Oncology (SIOG) task force. Ann Oncol 2014;25:2134-46. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Older Adult Oncology Guidelines (Version 1.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf. Accessed April 10, 2019.
- Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013;14:1269-77. [Crossref] [PubMed]
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomized trial. Lancet 2014;383:603-13. [Crossref] [PubMed]
- Horst KC, Fasola C, Ikeda D, et al. Five-year results of a prospective clinical trial investigating accelerated partial breast irradiation using 3D conformal radiotherapy after lumpectomy for early stage breast cancer. Breast 2016;28:178-83. [Crossref] [PubMed]
- Olivotto IA, Whelan TJ, Parpia S, et al. Interim Cosmetic and Toxicity Results From RAPID: A Randomized Trial of Accelerated Partial Breast Irradiation Using Three-Dimensional Conformal External Beam Radiation Therapy. J Clin Oncol 2013;31:4038-45. [Crossref] [PubMed]
- Livi L, Meattini I, Marrazzo L, et al. Accelerated partial breast irradiation using intensity modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer 2015;51:451-63. [Crossref] [PubMed]
- Polgár C, Fodor J, Major T. at al. Breast-conserving therapy with partial or whole breast irradiation: Ten-year results of the Budapest randomized trial. Radiother Oncol 2013;108:197-202. [Crossref] [PubMed]
- Strnad V, Ott OJ, Hildebrandt G, et al. 5-year result of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast – conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet 2016;387:229-38. [Crossref] [PubMed]
- Marta GN, Macedo CR, Carvalho HA, et al. Accelerated partial irradiation for breast cancer: Systematic review and meta-analysis of 8653 women in eight randomized trials. Radiotherapy and Oncology 2015;114:42-9. [Crossref] [PubMed]
- Hickey BE, Lehman M, Francis DP, et al. Partial breast irradiation for early breast cancer. Cochrane Database of Systematic Reviews 2016;7:CD007077. [PubMed]
- Strnad V, Hannoun-Levi JM, Guinot JLRecommendations from GEC ESTRO Breast Cancer Working Group, et al. (I): Target definition and target delineation for accelerated or boost Partial Breast Irradiation using multicatheter interstitial brachytherapy after breast conserving closed cavity surgery. Radiother Oncol 2015;115:342-8. [Crossref] [PubMed]
- Strnad V, Major T, Polgar C, et al. ESTRO-ACROP guideline: Interstitial multi-catheter breast brachytherapy as Accelerated Partial Breast Irradiation alone or as boost – GEC-ESTRO Breast Cancer Working Group practical recommendations. Radiother Oncol 2018;128:411-20. [Crossref] [PubMed]
- Hoekstra N, Fleury E, Merino Tara TR, et al. Long-term risks of secondary cancer for various whole and partial breast irradiation techniques. Radiother Oncol 2018;128:428-33. [Crossref] [PubMed]
- Hannoun-Levi JM, Gourgou-Bourgade S, Belkacemi Y, et al. GERICO-03 phase II trial of accelerated and partial breast irradiation in elderly women: Feasibility, reproducibility, and impact on functional status. Brachytherapy 2013;12:285-92. [Crossref] [PubMed]
- Yao N, Mackley HB, Anderson RT, et al. Survival after partial breast brachytherapy in elderly patients with nonmetastatic breast cancer. Brachytherapy 2013;12:293-302. [Crossref] [PubMed]
- Meattini I, Saieva C, Marrazzo L, et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy technique compared to whole breast irradiation for patients aged 70 years or older: subgroup analysis from a randomized phase 3 trial. Breast Cancer Res Treat 2015;153:539-47. [Crossref] [PubMed]
- Albert JM, Liu DD, Shen Y, et al. Nomogram to predict the benefit of radiation for older patients with breast cancer treated with conservative surgery. J Clin Oncol 2012;30:2837-43. [Crossref] [PubMed]
- Sumodhee S, Levy J, Chamorey E, et al. Accelerated partial breast irradiation for elderly women with early breast cancer: A compromise between whole breast irradiation and omission of radiotherapy. Brachytherapy 2017;16:929-34. [Crossref] [PubMed]
- Vaidya JS, Bulsara M, Wenz F, et al. Reduced Mortality With Partial-Breast Irradiation for Early Breast Cancer: A Meta-Analysis of Randomized Trials. Int J Radiat Oncol Biol Phys 2016;96:259-65. [Crossref] [PubMed]
- Cambeiro M, Martinez-Regueira F, et al. Multicatheter breast implant during breast conservative surgery: Novel approach to deliver accelerated partial breast irradiation. Brachytherapy 2016;15:485-94. [Crossref] [PubMed]
- Pohanková D, Sirák I, Jandík P, et al. Accelerated partial breast irradiation with perioperative multicatheter interstitial brachytherapy—A feasibility study. Brachytherapy 2018;17:949-55. [Crossref] [PubMed]
- Wilkinson JB, Chen PY, Wallace MF, et al. Six-Year Results from a Phase I/II Trial for Hypofractionated Accelerated Partial Breast Irradiation Using a 2-Day Dose Schedule. Am J Clin Oncol 2018;41:986-91. [Crossref] [PubMed]
- Hannoun-Lévi JM, Kee DLC, Gal J, et al. Accelerated partial breast irradiation for suitable elderly women using a single fraction of multicatheter interstitial high-dose-rate brachytherapy: Early results of the Single-Fraction Elderly Breast Irradiation (SiFEBI) Phase I/II trial. Brachytherapy 2018;17:407-14. [Crossref] [PubMed]