
Comparison of the effect on the prognosis of HCC in terms of different surgical approaches for hepatic inflow occlusion
Introduction
Seriously threatening human public health, the incidence of hepatocellular carcinoma (HCC) has increased worldwide (1). Hepatectomy is regarded as the first-line curative treatment for HCC and has improved postoperative survival outcomes (2,3). However, the rates of postoperative recurrence and metastasis continue to be high (4,5). Appropriate approaches for treating hepatic inflow occlusion have generally been thought to be important and necessary to guarantee a smooth surgery (6).
Two methods, i.e., the Pringle maneuver and selective hemi-hepatic vascular exclusion (SHVE), have become routine during surgery for HCC. These techniques effectively reduce intraoperative blood loss. The Pringle maneuver prevents bleeding from the liver by clamping the hepatoduodenal ligament and has been used for more than 100 years (7). However, the Pringle maneuver is controversial because of the potential for severe ischemia-reperfusion (IR) injury, which usually causes reversible or irreversible liver and multiple organ dysfunction and other complications (8,9). By occluding the bleeding inflow from the hemi-hepatic tissue in which the tumor is situated, SHVE is useful, but IR injury is also unavoidable (10,11). To decrease blood loss and accelerate the postoperative recovery of patients, surgical approaches for hepatic inflow occlusion have been proposed but remain controversial, especially regarding the effects of these approaches on the prognosis of HCC patients (12-16). Therefore, further exploration of these surgical approaches is necessary and imperative.
Previous studies have reported that hepatic inflow occlusion influences postoperative liver function, but the effects of hepatic inflow occlusion on tumor-free survival (TFS) remain unclear. The Barcelona clinic liver cancer (BCLC) staging system is commonly used to direct treatment at different stages of disease (17). Hence, we explored the effects of hepatic blood flow occlusion on the prognosis of HCC patients undergoing surgery. We performed a retrospective study to investigate whether hepatic inflow occlusion could affect the TFS of HCC patients according to the BCLC stage.
Methods
Patients
In total, 343 patients undergoing R0 resection between March 2013 and June 2018 at our hospital were enrolled, and the follow-up deadline was October 1, 2018. The inclusion criteria were as follows: (I) R0 resection indicated that the macroscopic tumor was completely removed, the resection margin was negative, and no detectable intrahepatic and extrahepatic metastasis lesions remained; (II) the disease stages were distinguished according to the BCLC staging system; (III) a definitive pathological diagnosis of HCC was made based on the World Health Organization (WHO) criteria; (IV) no other treatment was performed before surgery; (V) the patient’s liver function was not beyond the Child-Pugh stage A, and the performance status test (PST) score was less than 1; (VI) the tumor differentiation grade was identified according to the Edmondson-Steiner criteria (18); (VII) finally, the criteria for microvascular invasion (MVI) were that the tumor thrombus was whole or partly covered by the endothelium and visible only under microscopy. The endothelium-covered tumor thrombi had to be located inside the tumor or close to the tumor edge and extend into the portal vein or hepatic vein branches to be considered MVI (19).
Surgical approaches
Hepatectomy (R0) and hepatic inflow occlusion were performed by surgeons with considerable clinical experience. The surgeons occluded the blood flow by blocking the portal blood flow (Pringle maneuver). A flexible tourniquet was used to clamp the whole hepatoduodenal ligament. A relaxed interval of 5 minutes was alternated with intermittent vascular interruption, which lasted less than 25 minutes each time. The right or left Glisson’s pedicle was blocked for SHVE.
Follow-up
Postoperative follow-up was performed every 1–2 months during the first year and every 3 months thereafter. The serum alpha-fetoprotein (AFP) levels were measured regularly, and imaging examinations, including ultrasonography and computed tomography (CT)/magnetic resonance imaging (MRI), were performed regularly. Recurrence was defined as meeting one of the following criteria: (I) AFP levels significantly increased along with one typical imaging finding, (II) two types of imaging showing a lesion simultaneously, or (III) lesion biopsy implemented due to a new episode of recurrence. The final time to recurrence (either intrahepatic or extrahepatic) was estimated by clinical doctors. The final follow-up data included in this study were collected in June 2018.
Statistical analysis
All statistical analyses were performed with IBM SPSS Statistics 23. TFS curves were constructed using the Kaplan-Meier method and compared with a log-rank test. A Cox proportional hazard model was established to evaluate the independent risk factors for postoperative recurrence. A two-tailed P value <0.05 was considered significant.
Results
Patient characteristics
The characteristics of the 343 patients are described in Table 1. The tumor size in 52.77% of the patients was greater than or equal to 5 cm, and 78.70% of the patients had serum AFP levels greater than 400 ng/mL. The positivity rates for hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA were 86.00% and 65.89%, respectively. In total, 122 (35.57%) patients and 221 (64.43%) patients had low Edmonson grades and cirrhosis stages, respectively. The capsule was absent in 114 (33.24%) patients. The tumor number in 30.61% of the patients was greater than one. In total, 211 (61.52%) patients and 54 (15.74%) patients had MVI and a portal vein tumor thrombus (PVTT), respectively. In total, 214 patients and 129 patients had BCLC stage A disease and BCLC stage B–C disease, respectively. The median age of all patients was 47.830±0.587 [24–80] years. The 1-, 2-, and 3-year TFS rates were 54.7%, 39.9%, and 30.6% (median TFS: 14 months), respectively.
Table 1
| Variables | Value |
|---|---|
| Age (years), mean ± SD [range] | 47.830±0.587 [24–80] |
| Sex, n (%) | |
| Male | 298 (86.88) |
| Female | 45 (13.12) |
| HBsAg, n (%) | |
| Positive | 295 (86.00) |
| Negative | 48 (14.00) |
| HBV DNA, n (%) | |
| ≥500 copies/mL | 226 (65.89) |
| <500 copies/mL | 117 (34.11) |
| AFP, n (%) | |
| ≥400 ng/mL | 181 (52.77) |
| <400 ng/mL | 162 (47.23) |
| Tumor size, n (%) | |
| ≥5 cm | 270 (78.70) |
| <5 cm | 73 (21.30) |
| Edmondson grade, n (%) | |
| Low | 122 (35.57) |
| High | 221 (64.43) |
| Capsule, n (%) | |
| Complete | 229 (66.76) |
| Absent | 114 (33.24) |
| Cirrhosis degree, n (%) | |
| Low | 221 (64.43) |
| High | 122 (35.57) |
| MVI, n (%) | |
| Positive | 211 (61.52) |
| Negative | 132 (38.48) |
| PVTT, n (%) | |
| Positive | 54 (15.74) |
| Negative | 289 (84.26) |
| Surgical approaches, n (%) | |
| No occlusion | 107 (31.20) |
| SHVE | 76 (22.16) |
| Pringle maneuver | 160 (46.64) |
| Tumor number, n (%) | |
| Number >1 | 105 (30.61) |
| Number ≤1 | 238 (69.39) |
SD, standard deviation; HBsAg, serum hepatitis B surface antigen; HBV, hepatitis B virus; AFP, alpha-fetoprotein; MVI, microvascular invasion; PVTT, portal vein tumor thrombus; SHVE, selective hemi-hepatic vascular exclusion.
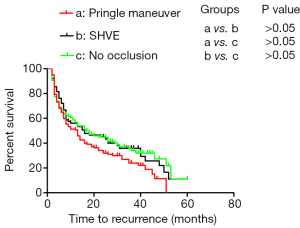
Surgical approaches had no impact on the prognosis of all patients
SHVE and the Pringle maneuver were performed in 76 patients and 160 patients, respectively. Additionally, 107 patients underwent surgery without hepatic inflow occlusion. The 1-, 2-, and 3-year TFS rates in all patients without occlusion were 58.7%, 44.9%, and 35.7% (median TFS: 17 months), respectively, and those in the patients who underwent surgery with SHVE were 56.1%, 45.0%, and 35.9% (median TFS: 16 months), respectively. In addition, the 1-, 2-, and 3-year TFS rates in all patients who underwent surgery with the Pringle maneuver were 51.3%, 34.1%, and 24.0% (median TFS: 13 months), respectively. There was no difference in TFS among the patients who underwent the three different surgical approaches (Figure 1).
Characteristics of the patients with BCLC stage A disease
The characteristics of the patients with BCLC stage A disease are described in Table 2. The median patient age was 49.270±0.770 [24–80] years. Approximately 47.20% and 71.50% of the patients had AFP levels ≥400 ng/mL and a tumor size ≥5 cm, respectively. The positivity rates for HBsAg and HBV DNA were 83.18% and 63.08%, respectively. There were 60 (28.04%) and 137 (64.02%) patients with low Edmonson grade and cirrhosis stage, respectively. The capsule was absent in 45 (21.03%) patients, and MVI was detected in 111 (51.87%) patients. The tumor number in 3.3% of the patients was greater than one. Additionally, SHVE was performed in 59 (27.57%) patients, 97 (45.33%) patients underwent the Pringle maneuver, and 58 (27.10%) patients underwent surgery without hepatic inflow occlusion.
Table 2
| Variables | Value |
|---|---|
| Age (years), mean ± SD [range] | 49.270±0.770 [24–80] |
| Sex, n (%) | |
| Male | 178 (83.18) |
| Female | 36 (16.82) |
| HBsAg, n (%) | |
| Positive | 178 (83.18) |
| Negative | 36 (16.82) |
| HBV DNA, n (%) | |
| ≥500 copies/mL | 135 (63.08) |
| <500 copies/mL | 79 (36.92) |
| AFP, n (%) | |
| ≥400 ng/mL | 101 (47.20) |
| <400 ng/mL | 113 (52.80) |
| Tumor size, n (%) | |
| ≥5 cm | 153 (71.50) |
| <5 cm | 61 (28.50) |
| Edmondson grade, n (%) | |
| Low | 60 (28.04) |
| High | 154 (61.96) |
| Capsule, n (%) | |
| Complete | 169 (78.97) |
| Absent | 45 (21.03) |
| Cirrhosis degree, n (%) | |
| Low | 137 (64.02) |
| High | 77 (35.98) |
| MVI, n (%) | |
| Positive | 111 (51.87) |
| Negative | 103 (48.13) |
| Surgical approaches, n (%) | |
| No occlusion | 58 (27.10) |
| SHVE, | 59 (27.57) |
| Pringle maneuver | 97 (45.33) |
| Tumor number, n (%) | |
| Number >1 | 7 (3.30) |
| Number ≤1 | 207 (96.70) |
BCLC, Barcelona clinic liver cancer; SD, standard deviation; HBsAg, serum hepatitis B surface antigen; HBV, hepatitis B virus; AFP, alpha-fetoprotein; MVI, microvascular invasion; PVTT, portal vein tumor thrombus; SHVE, selective hemi-hepatic vascular exclusion.
Performance of the Pringle maneuver had a negative impact on TFS in patients with BCLC stage A disease
By the end of follow-up, 131 of the 214 patients (61.21%) developed recurrence. Among all patients, the median TFS was 26 months, and the 1-, 2-, and 3-year TFS rates were 69.8%, 51.9%, and 40.4%, respectively. Among the patients with no inflow occlusion, the 1-, 2-, and 3-year TFS were 84.4%, 63.3%, and 51.5%, respectively, and those among the patients who underwent surgery with SHVE were 69.0%, 56.5%, and 45.1%, respectively. Among the patients treated with the Pringle maneuver, the 1-, 2-, and 3-year TFS rates were 61.5%, 42.2%, and 29.4%, respectively. The Kaplan-Meier analysis revealed that the patients treated with the Pringle maneuver had the worst TFS compared with those treated with SHVE and those treated without hepatic inflow occlusion (median: 16 vs. 30 months and 16 vs. 36 months; P<0.05) (Figure 2A). There was no difference in TFS between the patients treated with SHVE and those treated without inflow occlusion (median: 30 vs. 36 months; P>0.05) (Figure 2A). Additionally, as described in Table 3, the multivariate analysis revealed that a tumor size ≥5 cm, an absent capsule, a low Edmondson grade, MVI positivity and the Pringle maneuver were independent risk factors of the prognosis in the patients with BCLC stage A disease.
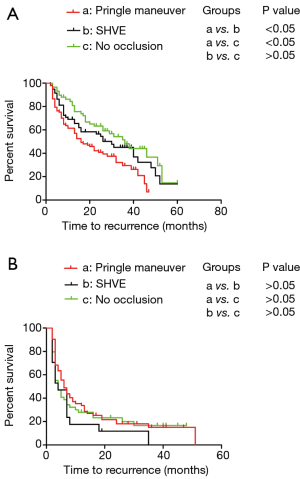
Table 3
| Variables | Kaplan-Meier analysis | Multivariate Cox analysis | ||
|---|---|---|---|---|
| Log rank (mantel-Cox) | HR (95% CI) | P value | ||
| Age (years) | 0.243 | |||
| ≥50 | ||||
| <50 | ||||
| Sex | 0.318 | |||
| Male | ||||
| Female | ||||
| HBsAg | 0.020 | |||
| Positive | ||||
| Negative | ||||
| HBV DNA | 0.050 | |||
| ≥500 copies/mL | ||||
| <500 copies/mL | ||||
| AFP | 0.009 | |||
| ≥400 ng/ml | ||||
| <400 ng/mL | ||||
| Tumor size | 0.004 | 1.857 (1.168–2.979) | 0.009 | |
| ≥5 cm | ||||
| <5 cm | ||||
| Edmondson grade | 0.007 | 1.610 (1.081–2.400) | 0.019 | |
| Low | ||||
| High | ||||
| Capsule | 0.000 | 2.401 (1.549–3.723) | 0.000 | |
| Complete | ||||
| Absent | ||||
| Margin | 0.285 | 0.668 (0.449–0.996) | 0.048 | |
| Positive | ||||
| Negative | ||||
| Cirrhosis degree | 0.560 | |||
| Low | ||||
| High | ||||
| MVI | 0.001 | 1.661 (1.137–2.425) | 0.009 | |
| Positive | ||||
| Negative | ||||
| Hepatic blood flow occlusion | 0.005 | 1.277 (1.005–1.622) | 0.046 | |
| No occlusion | ||||
| SHVE | ||||
| Pringle maneuver | ||||
| Tumor number | 0.640 | |||
| Number >1 | ||||
| Number ≤1 | ||||
TFS, tumor-free survival; BCLC, Barcelona clinic liver cancer; HR, hazard ratio; CI, confidence interval; HBsAg, serum hepatitis B surface antigen; HBV, hepatitis B virus; AFP, alpha-fetoprotein; MVI, microvascular invasion; SHVE, selective hemi-hepatic vascular exclusion.
Characteristics of the patients with BCLC stage B–C disease
The characteristics of the patients with BCLC stage B–C disease are described in Table 4. The median patient age was 45.440±0.870 [20–69] years. Approximately 62.02% and 37.98% of the patients had AFP levels ≥400 ng/mL and a tumor size ≥5 cm, respectively. The positivity rates for HBsAg and HBV DNA were 90.70% and 70.54%, respectively. There were 67 (51.94%) and 84 (65.12%) patients with low Edmonson grades and cirrhosis stages, respectively. The capsule was absent in 69 (54.00%) patients, MVI was detected in 100 (51.90%) patients, and PVTT was detected in 54 (41.86%) patients. The tumor number in 75.97% of the patients was greater than one. Additionally, SHVE was performed in 17 (13.18%) patients, 63 (48.84%) patients underwent the Pringle maneuver, and 49 (37.98%) patients underwent surgery without hepatic inflow occlusion.
Table 4
| Variables | Value |
|---|---|
| Age (years), mean ± SD [range] | 45.440±0.870 [20–69] |
| Sex, n (%) | |
| Male | 120 (93.02) |
| Female | 9 (6.98) |
| HBsAg, n (%) | |
| Positive | 117 (90.70) |
| Negative | 12 (9.30) |
| HBV DNA, n (%) | |
| ≥500 copies/mL | 91 (70.54) |
| <500 copies/mL | 38 (29.46) |
| AFP, n (%) | |
| ≥400 ng/mL | 80 (62.02) |
| <400 ng/mL | 49 (37.98) |
| Tumor size, n (%) | |
| ≥5 cm | 117 (90.70) |
| <5 cm | 12 (9.30) |
| Edmondson grade, n (%) | |
| Low | 67 (51.94) |
| High | 62 (48.06) |
| Capsule, n (%) | |
| Complete | 60 (46.51) |
| Absent | 69 (54.00) |
| Cirrhosis degree, n (%) | |
| Low | 84 (65.12) |
| High | 45 (34.88) |
| MVI, n (%) | |
| Positive | 100 (51.90) |
| Negative | 29 (48.10) |
| PVTT, n (%) | |
| Positive | 54 (41.86) |
| Negative | 75 (58.14) |
| Hepatic blood flow occlusion, n (%) | |
| No occlusion | 49 (37.98) |
| SHVE | 17 (13.18) |
| Pringle maneuver | 63 (48.84) |
| Tumor number, n (%) | |
| Number >1 | 98 (75.97) |
| Number ≤1 | 31 (24.03) |
BCLC, Barcelona clinic liver cancer; SD, standard deviation; HBsAg, serum hepatitis B surface antigen; HBV, hepatitis B virus; AFP, alpha-fetoprotein; MVI, microvascular invasion; PVTT, portal vein tumor thrombus; SHVE, selective hemi-hepatic vascular exclusion.
Effect of hepatic inflow occlusion on TFS in the patients with BCLC stage B–C disease
By the end of follow-up, 107 of the 129 patients (82.95%) developed recurrence. The median TFS rate was 5 months, and the 1-, 2-, and 3-year TFS rates were 30.1%, 20.8%, and 13.7%, respectively. Among the patients without inflow occlusion, the 1-, 2-, and 3-year TFS rates were 27.9%, 23.3%, and 16.6%, respectively, and those among the patients who underwent surgery with SHVE were 17.6%, 11.8%, 0%, respectively. Among the patients treated with the Pringle maneuver, the 1-, 2-, and 3-year TFS rates were 35.4%, 21.6%, and 15.0%, respectively. As displayed, the Kaplan-Meier analysis revealed that there was no significant difference in TFS among the patients with BCLC stage B–C disease treated with the different approaches for hepatic inflow occlusion (Figure 2B).
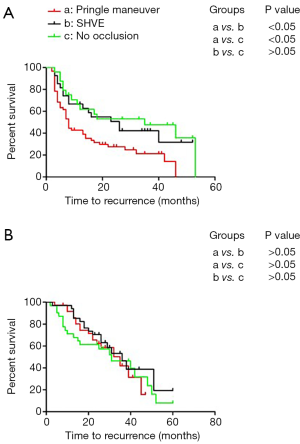
Performance of the Pringle maneuver had a negative impact on the TFS of patients with MVI with BCLC stage A disease
In this analysis, the 214 patients were subdivided into an MVI-positive subgroup (n=111) and an MVI-negative subgroup (n=103). The Kaplan-Meier method was used to analyze the relationship between the different surgical approaches for hepatic inflow occlusion and the prognosis of the HCC patients with MVI. In the MVI-positive group, the 1-, 2-, and 3-year TFS rates were 53.7%, 38.9%, and 32.6%, respectively (median TFS: 13 months). In the MVI-negative group, the 1-, 2-, and 3-year TFS rates were 87.2%, 65.9%, and 47.8%, respectively (median TFS: 32 months). In the MVI-positive group, SHVE was performed in 27 (24.32%) patients, 60 (54.05%) patients underwent the Pringle maneuver, and 24 (21.63%) patients underwent surgery without hepatic inflow occlusion. In the MVI-negative group, SHVE was performed in 32 (31.07%) patients, 37 (35.92%) patients underwent the Pringle maneuver, and 34 (33.01%) patients underwent surgery without hepatic inflow occlusion. As described, in the MVI-positive subgroup, the patients treated with Pringle maneuver had the worst TFS compared to those treated with SHVE and those treated without blood flow occlusion (median: 8 vs. 26 months/8 vs. 35 months) (Figure 3A). No difference was observed in TFS between the patients treated with SHVE and the patients treated without inflow occlusion (P>0.05). However, there was no significant difference in TFS among the patients treated with different approaches for hepatic inflow occlusion in the MVI-negative subgroup (P>0.05) (Figure 3B).
Discussion
It has been one hundred years since the Pringle maneuver was developed, and this technique still has both pros and cons. With the introduction of precision therapy, SHVE has also been frequently used during hepatectomy, and sometimes, performing surgery without hepatic vascular occlusion is feasible (10). However, the effect of the surgical approach on the prognosis of HCC patients has been continuously disputed.
In contrast to a previous study, this study separated patients into three groups, including a Pringle maneuver group, hemi-hepatic vascular occlusion group and no hepatic vascular occlusion group (20). Special analyses of patients with early stage disease and those with intermediate/advanced disease were performed. The present study demonstrated that there was no significant difference in the recurrence rate or TFS among the three groups. These results are consistent with the outcomes reported in a previous study (20). We also found that the patients treated with the Pringle maneuver had the worst median TFS among the three groups, although no significant association was found between the surgical approach and prognosis. Hence, further analysis involving patients with different BCLC stages was performed. In the present study, there was no significant difference in TFS among the BCLC B–C stage patients treated with different approaches for hepatic inflow occlusion. This result indicated that surgical approaches for hepatic inflow occlusion have no influence on the prognosis of HCC patients with intermediate/advanced disease. High intracorporal tumor dissemination and PVTT are commonly found in patients with BCLC B–C stage disease, and both are indicative of more serious disease before surgery, which may offset the effect of the operative method. Therefore, it seems reasonable that there was no significant difference in TFS among the three groups of patients with advanced stage disease (BCLC stage B–C).
However, among the patients with BCLC A stage disease, a significant difference in prognosis was observed between the patients treated with the Pringle maneuver and those treated with SHVE or without hepatic inflow occlusion. However, there was no significant difference in TFS between the patients treated with SHVE and those treated without hepatic inflow occlusion. These outcomes indicate that the surgical approach possibly had an impact on the survival of HCC patients with early-stage disease. The presence of MVI ranges from 15% to 57% in HCC patients after liver resection or liver transplantation and significantly shorten prognostic survival for HCC patients even in early stage (21,22). In the present study, the early-stage HCC patients were stratified according to the MVI status, and a subgroup analysis was performed. We found that compared with SHVE or without hepatic inflow occlusion, the Pringle maneuver in liver resection significantly decreased the TFS in patients with MVI. However, in the case of patients without MVI in our study, the surgical approaches for hepatic inflow occlusion did not significantly affect TFS. Among the patients with BCLC stage A disease, the Pringle maneuver had an impact on the prognosis of the patients with MVI but not on those without MVI. Currently, there is no clear reason to explain the phenomenon. MVI may promote metastasis by acting as a “seed” that gives rise to micro-metastases in the liver parenchyma (23). Changes in the hepatic microenvironment caused by IR promote “seeding” and the development of metastases. Furthermore, the proinflammatory response induced by IR may play an important role in the formation and growth of metastases at both local and remote sites through changes in the hepatic microenvironment (24,25). In addition, The level of reactive oxygen species (ROS) was upregulated by hypoxia caused by the Pringle maneuver, spurring the development of remaining tumor lesions and promoting HCC recurrence after hepatectomy (26). Therefore, we inferred that IR injury and hypoxia caused by the Pringle maneuver harmed liver function and had an adverse impact on the postoperative prognosis of the HCC patients in the early stage (8,27). Because MVI was undetectable preoperatively, given our findings, no hepatic inflow occlusion or the SHVE approach rather than the Pringle maneuver should be considered first during hepatectomy for all patients with BCLC stage A disease.
The limitations of our study should be discussed. First, our study claimed that hepatic inflow occlusion and the surgical approach may have an impact on HCC prognosis in patients with BCLC stage A disease, especially those with MVI positivity. However, our sample size is not large enough, our conclusion may lack persuasive power in a way. In the future, we will continue to expand the sample size and use propensity score to avoid bias. Second, among the patients with BCLC stage B–C disease, those treated with SHVE had shorter median TFS than those treated with the Pringle maneuver; however, no statistically significant difference was observed between the groups. We believe that a limited number of cases of patients with BCLC stage B–C disease were selected. However, the Pringle maneuver contributed to a poor prognosis in the HCC patients with BCLC stage A disease, especially those with MVI, as demonstrated in our study.
Conclusions
Hepatic inflow occlusion and the surgical approach may have an impact on HCC prognosis in patients with BCLC stage A disease, especially those with MVI positivity. No hepatic inflow occlusion or SHVE rather than the Pringle maneuver should be considered first during hepatectomy for patients with BCLC stage A disease.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.44). The authors have no conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. In accordance with the Declaration of Helsinki (as revised in 2013), this study was approved by the Institutional Review Board of the Affiliated Tumor Hospital of Guangxi Medical University (No. LW2019019). Informed consent was obtained from all voluntary participants for their clinical cases to be used in the present study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Bell R, Pandanaboyana S, Lodge JPA, et al. Primary liver resection for patients with cirrhosis and hepatocellular carcinoma: the role of surgery in BCLC early (A) and intermediate stages (B). Langenbecks Arch Surg 2017;402:575-83. [Crossref] [PubMed]
- Ye JZ, Wang YY, Bai T, et al. Surgical resection for hepatocellular carcinoma with portal vein tumor thrombus in the Asia-Pacific region beyond the Barcelona clinic liver cancer treatment algorithms: a review and update. Oncotarget 2017;8:93258-78. [PubMed]
- Chan AC, Fan ST, Poon RT, et al. Evaluation of the seventh edition of the American Joint Committee on Cancer tumour-node-metastasis (TNM) staging system for patients undergoing curative resection of hepatocellular carcinoma: implications for the development of a refined staging system. HPB (Oxford) 2013;15:439-48.
- Choi GH, Kim DH, Kang CM, et al. Prognostic factors and optimal treatment strategy for intrahepatic nodular recurrence after curative resection of hepatocellular carcinoma. Ann Surg Oncol 2008;15:618-29. [Crossref] [PubMed]
- Huntington JT, Royall NA, Schmidt CR. Minimizing blood loss during hepatectomy: a literature review. J Surg Oncol 2014;109:81-8. [Crossref] [PubMed]
- Pringle JH V.. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg 1908;48:541-9. [Crossref] [PubMed]
- van Riel WG, van Golen RF, Reiniers MJ, et al. How much ischemia can the liver tolerate during resection? Hepatobiliary Surg Nutr 2016;5:58-71. [PubMed]
- Delva E, Camus Y, Nordlinger B, et al. Vascular occlusions for liver resections. Operative management and tolerance to hepatic ischemia: 142 cases. Ann Surg 1989;209:211-8. [Crossref] [PubMed]
- Yang Y, Zhao LH, Fu SY, et al. Selective hepatic vascular exclusion versus pringle maneuver in partial hepatectomy for liver hemangioma compressing or involving the major hepatic veins. Am Surg 2014;80:236-40. [PubMed]
- Fu SY, Lau WY, Li GG, et al. A prospective randomized controlled trial to compare Pringle maneuver, hemihepatic vascular inflow occlusion, and main portal vein inflow occlusion in partial hepatectomy. Am J Surg 2011;201:62-9. [Crossref] [PubMed]
- Katz SC, Shia J, Liau KH, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg 2009;249:617-23. [Crossref] [PubMed]
- Lee SH, Culberson C, Korneszczuk K, et al. Differential mechanisms of hepatic vascular dysregulation with mild vs. moderate ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol 2008;294:G1219-26. [Crossref] [PubMed]
- Tong Y, Yang JM, Lai EC, et al. Complete hemihepatic vascular exclusion versus pringle maneuver for liver resection: a comparative study. Hepatogastroenterology 2011;58:1307-11. [Crossref] [PubMed]
- Xu W, Xu H, Yang H, et al. Continuous pringle maneuver does not affect outcomes of patients with hepatocellular carcinoma after curative resection. Asia Pac J Clin Oncol 2017;13:e321-30. [Crossref] [PubMed]
- Guo X, Liu G, Zhang X. Meta-analysis of ischemic preconditioning (IP) on postoperative outcomes after liver resections. Medicine (Baltimore) 2017;96:e8217. [Crossref] [PubMed]
- Bruix J, Sherman MAmerican Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 1954;7:462-503. [Crossref] [PubMed]
- Rodríguez-Perálvarez M, Luong TV, Andreana L, et al. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol 2013;20:325-39. [Crossref] [PubMed]
- Jiang JH, Wang KX, Zhu JY, et al. Comparison of hepatectomy with or without hepatic inflow occlusion in patients with hepatocellular carcinoma: a single-center experience. Minerva Med 2017;108:324-33. [PubMed]
- Shen J, Wen J, Li C, et al. The prognostic value of microvascular invasion in early-intermediate stage hepatocelluar carcinoma: a propensity score matching analysis. BMC Cancer 2018;18:278. [Crossref] [PubMed]
- Du M, Chen L, Zhao J, et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer 2014;14:38. [Crossref] [PubMed]
- Hirokawa F, Hayashi M, Asakuma M, et al. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol 2016;25:24-9. [Crossref] [PubMed]
- Lim C, Broqueres-You D, Brouland JP, et al. Hepatic ischemia-reperfusion increases circulating bone marrow-derived progenitor cells and tumor growth in a mouse model of colorectal liver metastases. J Surg Res 2013;184:888-97. [Crossref] [PubMed]
- Man K, Ng KT, Lo CM, et al. Ischemia-reperfusion of small liver remnant promotes liver tumor growth and metastases--activation of cell invasion and migration pathways. Liver Transpl 2007;13:1669-77. [Crossref] [PubMed]
- Chiarugi P, Pani G, Giannoni E, et al. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J Cell Biol 2003;161:933-44. [Crossref] [PubMed]
- Orci LA, Lacotte S, Oldani G, et al. The role of hepatic ischemia-reperfusion injury and liver parenchymal quality on cancer recurrence. Dig Dis Sci 2014;59:2058-68. [Crossref] [PubMed]


