Rare sites of breast cancer metastasis: a review
Introduction
Breast cancer (BC) metastasis accounts for the majority of deaths from BC. The rate of metastasis even in sites known as uncommon is on the rise. On one side this is due to the more effective therapy which have prolonged the overall survival of BC patients, on the other side due to the development of new imaging techniques and early detection (1). The American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline recommends that primary care clinicians should educate and counsel all women about the signs and symptoms of recurrence, including new lumps, pain in the bone, chest or abdomen, dyspnea and constant headaches (2). There is no evidence that routine laboratory tests or imaging (exception for mammography, when indicated) in asymptomatic patients gives any survival advantage, thus advanced imaging should be offered when recurrence is suspected (3,4). The evaluation of patient-reported symptoms is essential in detecting a recurrence as early as possible, which may impact survival (5,6). Hence, the knowledge of even the rare sites of BC metastasis is of paramount importance for the clinical interpretation of new symptoms in BC survivors.
BC can metastasize to several organs, the most frequent metastatic sites include bone, lungs, liver and skin (7). Additionally, more and more sites of BC metastasis have been reported in literature. The definition of “unusual metastasis” is not universally accepted, however it defines a systemic failure with a frequency of ≤1% at each site and according to this unusual metastasis involve the central nervous system, secretory/endocrine organs and glands, internal organs and structures, and gynaecological organs.
This review reports on the rare anatomical regions where BC can spread forming metastases.
Methods
The literature search was performed using the electronic database PubMed up to December 2018, with the following key words: {[rare(Title/Abstract)] OR [unusual(Title/Abstract)] OR [unconventional(Title/Abstract)]} AND {[metastases(Title/Abstract)] OR [metastasis(Title/Abstract)]} AND {[breast(Title/Abstract)]} AND {[cancer(Title/Abstract)] OR [tumor(Title/Abstract)] OR [tumour(Title/Abstract)] OR [neoplasm(Title/Abstract)]}. The search did not include editorials, letters, comments, conference letters, systematic reviews and meta-analyses and it was limited to papers in English language.
All studies on rare sites of metastases from BC were eligible to be included.
All data were extracted in a standard pre-determined format including information on: first author’s name, publication year, number of cases and site of metastases. We considered rare the sites different from nodes, bone, lung, liver and skin.
Results
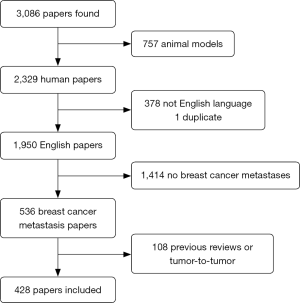
The flow chart of the study selection was shown in Figure 1. Of the 3,086 papers found, 757 were excluded as reporting animal models, 378 were not in English language, 1 was a duplicate of the same research, 1,414 did not report on BC metastases, 108 were previous review on BC or tumour to tumour metastases, 428 papers were included in this review. Table 1 shows the distribution per sites of all cases of rare metastases reported in literature.
Table 1
| Anatomical region | Site | Reference | Number | Tot. |
|---|---|---|---|---|
| Eye | Orbital soft tissue | Reeves et al., 2002 (8) | 1 | |
| Asproudis et al., 2004 (9) | 1 | |||
| Eckardt et al., 2011 (10) | 1 | |||
| Mora-Guzmán et al., 2018 (11) | 1 | |||
| Maliepaard et al., 2017 (12) | 2 | |||
| Spraker et al., 2017 (13) | 1 | |||
| Pinto Proença et al., 2018 (14) | 1 | |||
| Gondim et al., 2017 (15) | 5 | |||
| Kim et al., 2011 (16) | 1 (bilateral) | |||
| Pierson et al., 2016 (17) | 6 | |||
| Raap et al., 2015 (18) | 8 | |||
| Zwicker et al., 2008 (19) | 7 | |||
| Torres et al., 2007 (20) | 2 | |||
| Schick et al., 2006 (21) | 3 | |||
| Dieing et al., 2004 (22) | 2 | |||
| Amemiya et al., 2002 (23) | 20 | |||
| Rossi et al., 2014 (24) | 1 | 61 | ||
| Intraocular | Bajcsay et al., 2003 (25) | 11 | 11 | |
| Extraocular muscles | Murthy et al., 2011 (26) | 1 | ||
| Chang et al., 2017 (27) | 1 | |||
| Nifosì et al., 2018 (28) | 1 | |||
| Framarino-Dei-Malatesta et al., 2019 (29) | 1 | |||
| Weiss et al., 1984 (30) | 2 | |||
| Lell et al., 2004 (31) | 1 | |||
| Kouvaris et al., 2008 (32) | 1 (bilateral) | |||
| Spitzer et al., 2005 (33) | 1 | |||
| Van der Heijden et al., 1991 (34) | 1 | |||
| Glazer et al., 1991 (35) | 1 | |||
| Pierson et al., 2016 (17) | 6 | 17 | ||
| Retina | Correa de Mello et al., 2017 (36) | 1 | ||
| Shields et al., 2014 (37) | 2 | |||
| Shah et al., 2017 (38) | 1 | |||
| Pierson et al., 2016 (17) | 9 | |||
| Biswas et al., 2007 (39) | 1 (bilateral) | |||
| Sirimaharaj et al., 2006 (40) | 1 | |||
| Schlaen et al., 1986 (41) | 1 | 16 | ||
| Conjunctiva | Skalicky et al., 2007 (42) | 1 | ||
| Sánchez Orgaz et al., 2017 (43) | 1 | |||
| Radovanović et al., 2013 (44) | 1 | |||
| Kiratli et al., 1996 (45) | 4 | 7 | ||
| Eyelid | Kaur et al., 2005 (46) | 1 | ||
| Martorell-Calatayud et al., 2010 (47) | 1 (bilateral) | |||
| Goodier et al., 2010 (48) | 1 | |||
| Kuo et al., 2008 (49) | 1 | |||
| Douglas et al., 2002 (50) | 1 (bilateral) | |||
| Claessens et al., 2000 (51) | 1 | |||
| Rosenblum et al., 1983 (52) | 2 | |||
| Lawton et al., 1980 (53) | 1 | 9 | ||
| Iris and ciliar body | Ozturk et al., 2007 (54) | 1 | ||
| Shields et al., 1995 (37) | 16 | |||
| Mennel et al., 2001 (55) | 2 | |||
| Reddy et al., 2000 (56) | 1 | 20 | ||
| Choroid | Williams et al., 2000 (57) | 1 | ||
| Solav et al., 2010 (58) | 1 | |||
| Arya et al., 2018 (59) | 1 | |||
| Luo et al., 2018 (60) | 1 | |||
| Antosz et al., 2014 (61) | 1 | |||
| Liu et al., 2012 (62) | 1 | |||
| Hood et al., 2011 (63) | 3 | |||
| Oleksy et al., 2010 (64) | 1 | |||
| Kosmas et al., 2000 (65) | 1 | |||
| Kreusel et al., 1999 (66) | 2 | |||
| Chen et al., 1998 (67) | 1 | |||
| Paoli et al., 1998 (68) | 1 | |||
| Gupta et al., 1991 (69) | 1 | |||
| Thatcher et al., 1975 (70) | 42 | 58 | ||
| Genital organs | Cervix | Pambuccian et al., 2000 (71) | 1 | |
| Nair et al., 2009 (72) | 1 | |||
| Proença et al., 2016 (73) | 2 | |||
| Toyoshima et al., 2015 (74) | 1 | |||
| Bogliolo et al., 2010 (75) | 1 | |||
| Mousavi et al., 2007 (76) | 1 | |||
| Green et al., 2004 (77) | 1 | |||
| Pauer et al., 2003 (78) | 1 | |||
| Sinkre et al., 2000 (79) | 1 | |||
| Kesavan et al., 2000 (80) | 1 | |||
| Hepp et al., 1999 (81) | 1 | |||
| Kennebeck et al., 1998 (82) | 2 | |||
| Taxy et al., 1994 (83) | 1 | |||
| Sanuki-Fujimoto et al., 2008 (84) | 4 | 19 | ||
| Endometrium | Kemp et al., 1997 (85) | 1 | ||
| Moey et al., 2016 (86) | 1 | |||
| Çift et al., 2016 (87) | 1 | |||
| Rahmani et al., 2018 (88) | 1 | |||
| Aytekin et al., 2018 (89) | 1 | |||
| Briki et al., 2018 (90) | 2 | |||
| Franco-Màrquez et al., 2019 (91) | 1 | |||
| Ertas et al., 2012 (92) | 1 | |||
| D’souza et al., 2010 (93) | 1 | |||
| Karvouni et al., 2009 (94) | 1 | |||
| Scopa et al., 2005 (95) | 2 | |||
| Chehal et al., 2002 (96) | 1 | |||
| Horn et al., 2000 (97) | 1 | 16 | ||
| Vulva | Gandhi et al., 2015 (98) | 1 | ||
| Engelstaedter et al., 2011 (99) | 1 | |||
| Sheen-Chen et al., 2004 (100) | 1 | |||
| Curtin et al., 1997 (101) | 1 | |||
| Valenzano Menada et al., 2003 (102) | 1 | 5 | ||
| Vagina | Pineda et al., 1978 (103) | 1 | ||
| Bellati et al., 2012 (104) | 1 | 2 | ||
| Ovaries | Pambuccian et al., 2000 (71) | 1 | ||
| Durga et al., 2018 (105) | 1 | |||
| Fujii et al., 2006 (106) | 1 | |||
| Sanuki-Fujimoto et al., 2008 (84) | 6 | 9 | ||
| Clitoris | Julien et al., 2012 (107) | 1 | 1 | |
| Placenta | Alexander et al., 2003 (108) | 15 | ||
| Froehlich et al., 2018 (109) | 1 | |||
| Eltorky et al., 1995 (110) | 2 | |||
| Sanuki-Fujimoto et al., 2008 (84) | 1 | 19 | ||
| Soft tissue | Skeletal muscle | Liu et al., 2015 (111) | 1 | |
| Khettab et al., 2017 (112) | 1 | |||
| Almusarhed et al., 2017 (113) | 1 | |||
| Salemis et al., 2015 (114) | 1 | |||
| Khandelwal et al., 2012 (115) | 10 | |||
| Surov et al., 2010 (116) | 2 | 16 | ||
| Subcutaneous tissue | Purkayastha et al., 2016 (117) | 1 | ||
| Rao et al., 2015 (118) | 1 | |||
| Metere et al., 2012 (119) | 1 | |||
| Plaza et al., 2008 (120) | 13 | 16 | ||
| Intra-articular | Jaffe et al., 2016 (121) | 1 | 1 | |
| Head and neck | Paranasal sinus | Monserez et al., 2001 (122) | 1 | |
| Pignataro et al., 2001 (123) | 1 | |||
| Asproudis et al., 2004 (9) | 1 | |||
| Fyrmpas et al., 2008 (124) | 1 | |||
| Reimann et al., 2011 (125) | 1 | |||
| Xiong et al., 2017 (126) | 1 | |||
| Walker et al., 2013 (127) | 1 | |||
| Marchioni et al., 2004 (128) | 1 | |||
| Pitkäranta et al., 2001 (129) | 1 | |||
| Austin et al., 1995 (130) | 9 | |||
| Gondim et al., 2017 (15) | 2 | |||
| Tiwari et al., 2014 (131) | 1 | |||
| Namad et al., 2014 (132) | 1 | |||
| Imre et al., 2013 (133) | 1 | |||
| Atasoy et al., 2013 (134) | 1 | 24 | ||
| Parotid gland | Ando et al., 2011 (135) | 1 | ||
| Agrawal et al., 2018 (136) | 1 | |||
| Cao et al., 2018 (137) | 1 | |||
| Rawet et al., 2017 (138) | 1 | |||
| Sellinger et al., 2011 (139) | 1 | |||
| Dangore-K et al., 2009 (140) | 1 | |||
| Perez-Fidalgo et al., 2007 (141) | 1 | |||
| Zhang et al., 2003 (142) | 1 | |||
| Sanuki-Fujimoto et al., 2008 (84) | 3 | 11 | ||
| Minor Salivary glands | Erra et al., 2011 (143) | 1 | ||
| Cain et al., 2001 (144) | 1 | 2 | ||
| Tongue and lip | Suárez Roa et al., 2007 (145) | 2 | ||
| Owosho et al., 2016 (146) | 1 | 3 | ||
| Tonsils | Bar et al., 2011 (147) | 1 | ||
| Sera et al., 2017 (148) | 1 | |||
| Maruzzo et al., 2012 (149) | 1 | |||
| Barton et al., 1980 (150) | 1 | |||
| Gondim et al., 2017 (15) | 1 | 5 | ||
| Pharynx and parapharyngeal space | Saab et al., 1987 (151) | 1 | ||
| Copson et al., 2018 (152) | 1 | |||
| Murhekar et al., 2015 (153) | 1 | |||
| Raut et al., 2001 (154) | 1 | |||
| Nguyen et al., 1983 (155) | 1 | |||
| Agrawal et al., 2015 (156) | 1 | 6 | ||
| Larynx | Schuler et al., 2010 (157) | 1 | ||
| Wanamaker et al., 1993 (158) | 1 | 2 | ||
| Nasal cavity | Weng et al., 2014 (159) | 1 | ||
| Gondim et al., 2017 (15) | 1 | |||
| Wanamaker et al., 1993 (158) | 1 | 3 | ||
| Ear | Pusiol et al., 2013 (160) | 1 | ||
| Marques et al., 2002 (161) | 1 | 2 | ||
| Glomus | Çelik et al., 2017 (162) | 1 | 1 | |
| Intravascular carcinomatosis | Takei et al., 2015 (163) | 1 | 1 | |
| Lacrimal gland | Sanuki-Fujimoto et al., 2008 (84) | 1 | 1 | |
| Oral cavity | Cooney et al., 1988 (164) | 1 | ||
| Scipio et al., 2001 (165) | 1 | |||
| Malhotra et al., 2006 (166) | 1 | |||
| Eichhorn et al., 2010 (167) | 1 | |||
| Kechagias et al., 2012 (168) | 1 | |||
| Gondim et al., 2017 (15) | 3 | |||
| Friedrich et al., 2010 (169) | 6 | |||
| Rajesh et al., 1998 (170) | 1 | 15 | ||
| Thyroid | Yang et al., 2014 (171) | 1 | ||
| Bourcier et al., 2018 (172) | 1 | |||
| Ghias et al., 2019 (173) | 1 | |||
| Kho et al., 2018 (174) | 1 | |||
| Plonczak et al., 2017 (175) | 1 | |||
| Debnam et al., 2017 (176) | 8 | |||
| Magers et al., 2016 (177) | 1 | |||
| Rossi et al., 2015 (178) | 5 | |||
| HooKim et al., 2015 (179) | 3 | |||
| Kolarević et al., 2012 (180) | 1 | |||
| Calzolari et al., 2008 (181) | 1 | |||
| Saber et al., 2007 (182) | 1 | |||
| Leboeuf et al., 2006 (183) | 1 | |||
| Gong et al., 2005 (184) | 1 | |||
| Lam et al., 1998 (185) | 7 | |||
| Ferrara et al., 1997 (186) | 1 | |||
| Rosen et al., 1978 (187) | 1 | 39 | ||
| Sanuki-Fujimoto et al., 2008 (84) | 3 | |||
| Parathyroid glands | Lee et al., 2013 (188) | 1 | ||
| Watanabe et al., 1983 (189) | 1 | 2 | ||
| Pituitary gland | Ghosn et al., 1991 et al., (190) | 1 | ||
| Fukunaga et al., 2014 (191) | 1 | |||
| Rozen et al., 2007 (192) | 1 | |||
| Poursadegh Fard et al., 2011 (193) | 1 | |||
| Nose et al., 2018 (194) | 1 | |||
| Castle-Kirszbaum et al., 2018 (195) | 4 | |||
| Kam et al., 2017 (196) | 1 | |||
| Ravnik et al., 2016 (197) | 1 | |||
| Burkhardt et al., 2016 (198) | 6 | |||
| Gormally et al., 2014 (199) | 1 | |||
| Magalhães et al., 2014 (200) | 1 | |||
| Spinelli et al., 2012 (201) | 1 | |||
| Naqi et al., 2012 (202) | 1 | |||
| Dogan et al., 2008 (203) | 1 | |||
| Gołkowski et al., 2007 (204) | 1 | |||
| Kurkjian et al., 2005 (205) | 2 | |||
| Sturm et al., 2004 (206) | 1 | |||
| Ruiz Hernández et al., 1996 (207) | 1 | |||
| Paulus et al., 1990 (208) | 1 | |||
| Yap et al., 1979 (209) | 39 | |||
| Sanuki-Fujimoto et al., 2008 (84) | 2 | |||
| Teears et al., 1975 (210) | 35 | 104 | ||
| Jugular foramen (Villaret syndrome) | Flis et al., 2015 (211) | 1 | 1 | |
| Cavernous sinus | Khaw et al., 2012 (212) | 1 | 1 | |
| Thoracic organs | Mediastinum | Kim et al., 2018 (213) | 1 | |
| Rampado et al., 2007 (214) | 25 | 26 | ||
| Thymus | Fukunaga et al., 2017 (215) | 1 | ||
| Fujioka et al., 2013 (216) | 1 | |||
| Park et al., 2007 (217) | 1 | 3 | ||
| Heart | Bhojwani et al., 2016 (218) | 1 | ||
| Sandhu et al., 2017 (219) | 1 | |||
| Bhambhani et al., 2014 (220) | 1 | |||
| Katalinic et al., 2013 (221) | 1 | |||
| Eminowicz et al., 2011 (222) | 1 | |||
| Garg et al., 2011 (223) | 1 | |||
| Kawase et al., 2009 (224) | 1 | |||
| Broom et al., 2006 (225) | 1 | |||
| Lieberman et al., 1993 (226) | 1 | |||
| Labib et al., 1992 (227) | 2 | 11 | ||
| Gastrointestinal tract | Esophagus | Wada et al., 2009 (228) | 1 | |
| Anaya et al., 2006 (229) | 1 | |||
| Koike et al., 2005 (230) | 2 | |||
| McLemore et al., 2005 (231) | 4 | |||
| Sunada et al., 2005 (232) | 1 | |||
| Erman et al., 2002 (233) | 1 | |||
| Simchuk et al., 2001 (234) | 4 | |||
| Varanasi et al., 1995 (235) | 4 | |||
| Herrera et al., 1992 (236) | 1 | |||
| Biller et al., 1982 (237) | 2 | |||
| Sanuki-Fujimoto et al., 2008 (84) | 2 | 23 | ||
| Stomach | Ghosn et al., 1991 (190) | 1 | ||
| Malhotra et al., 2009 (238) | 1 | |||
| Hara et al., 2010 (239) | 1 | |||
| Dòria et al., 2015 (240) | 1 | |||
| Geredeli et al., 2015 (241) | 1 | |||
| Ricciuti et al., 2016 (242) | 1 | |||
| Dos Santos Fernandes et al., 2016 (243) | 4 | |||
| Villa Guzmán et al., 2017 (244) | 1 | |||
| Jmour et al., 2017 (245) | 4 | |||
| Khan et al., 2017 (246) | 1 | |||
| Kliiger et al., 2017 (247) | 1 | |||
| Ulmer et al., 2018 (248) | 1 | |||
| Bushan et al., 2018 (249) | 1 | |||
| Güler et al., 2019 (250) | 1 | |||
| Woo et al., 2018 (251) | 1 | |||
| Kim et al., 2018 (213) | 1 | |||
| Gurzu et al., 2018 (252) | 2 | |||
| Yim et al., 2017 (253) | 1 | |||
| Choi et al., 2017 (254) | 1 | |||
| Mullally et al., 2017 (255) | 1 | |||
| Rodrigues et al., 2016 (256) | 1 | |||
| Zuhair et al., 2015 (257) | 1 | |||
| Rachan Shetty et al., 2015 (258) | 2 | |||
| Wysocka et al., 2011 (259) | 1 | |||
| Critchley et al., 2011 (260) | 1 | |||
| Almubarak et al., 2011 (261) | 35 | |||
| Ghirarduzzi et al., 2010 (262) | 3 | |||
| Yamamoto et al., 2010 (263) | 1 | |||
| Trouillet et al., 2010 (264) | 4 | |||
| Ellis et al., 2009 (265) | 4 | |||
| Ciulla et al., 2008 (266) | 1 | |||
| Jhaveri et al., 2006 (267) | 1 | |||
| Savanis et al., 2006 (268) | 1 | |||
| Whitty et al., 2005 (269) | 1 | |||
| Akcali et al., 2005 (270) | 1 | |||
| Tremblay et al., 2002 (271) | 1 | |||
| Oda et al., 2001 (272) | 61 (autopsy) | |||
| Pera et al., 2001 (273) | 1 | |||
| Washington et al., 1995 (274) | 20 | |||
| McLemore et al., 2005 (231) | 11 | |||
| Sanuki-Fujimoto et al., 2008 (84) | 6 | 185 | ||
| Duodenum | Houghton et al., 1987 (275) | 1 | ||
| Sarkar et al., 2002 (276) | 1 | |||
| Sato et al., 2007 (277) | 1 | |||
| Jones et al., 2015 (278) | 1 | |||
| Giestas et al., 2016 (279) | 1 | |||
| Lin et al., 2019 (280) | 1 | |||
| Wang et al., 2018 (281) | 1 | |||
| Zhao et al., 2012 (282) | 1 | |||
| Asoglu et al., 2006 (283) | 1 | |||
| Lottini et al., 2002 (284) | 1 | |||
| Titus et al., 1997 (285) | 1 | 11 | ||
| Small bowel | Hernández et al., 2000 (286) | 1 | ||
| Choi et al., 2011 (287) | 1 | |||
| Khan et al., 2017 (246) | 1 | |||
| Liu et al., 2018 (288) | 1 | |||
| Bilen et al., 2012 (289) | 1 | |||
| Cho et al., 2011 (290) | 1 | |||
| Mouawad et al., 2011 (291) | 1 | |||
| Kelly et al., 2009 (292) | 1 | |||
| Oyasiji et al., 2009 (293) | 1 | |||
| Al-Qahatani et al., 2007 (294) | 1 | |||
| McLemore et al., 2005 (231) | 14 | 24 | ||
| Colon-rectum | Wang et al., 2014 (159) | 1 | ||
| Haberstich et al., 2005 (295) | 1 | |||
| Malhotra et al., 2009 (238) | 1 | |||
| Titi et al., 2010 (296) | 1 | |||
| Okido et al., 2011 (297) | 1 | |||
| Saranovic et al., 2011 (298) | 1 | |||
| Mistrangelo et al., 2011 (299) | 1 | |||
| Villa Guzmán et al., 2016 (244) | 1 | |||
| Cherian et al., 2017 (300) | 1 | |||
| Khan et al., 2017 (246) | 1 | |||
| Falco et al., 2018 (301) | 1 | |||
| Blachman-Braun et al., 2019 (302) | 1 | |||
| Samra et al., 2019 (303) | 1 | |||
| Schellenberg et al., 2018 (304) | 1 | |||
| Ruymbeke et al., 2018 (305) | 1 | |||
| Tsujimura et al., 2017 (306) | 1 | |||
| Buka et al., 2016 (307) | 1 | |||
| Rengifo et al., 2016 (308) | 1 | |||
| Mroz et al., 2015 (309) | 1 | |||
| Zhou et al., 2012 (310) | 1 | |||
| Takeuchi et al., 2012 (311) | 1 | |||
| Bochicchio et al., 2012 (312) | 1 | |||
| Gerova et al., 2012 (313) | 1 | |||
| Nikolic et al., 2012 (314) | 1 | |||
| Amin et al., 2011 (315) | 1 | |||
| Razzetta et al., 2011 (316) | 1 | |||
| Critchley et al., 2011 (260) | 1 | |||
| Efthimiadis et al., 2011 (317) | 1 | |||
| Balja et al., 2010 (318) | 1 | |||
| Thèraux et al., 2009 (319) | 1 | |||
| Birla et al., 2008 (320) | 1 | |||
| Uygun et al., 2006 (321) | 1 | |||
| Savanis et al., 2006 (268) | 1 | |||
| Signorelli et al., 2005 (322) | 1 | |||
| McLemore et al., 2005 (231) | 18 | |||
| Law et al., 2003 (323) | 1 | |||
| Dhar et al., 2003 (324) | 1 | |||
| Bamias et al., 2001 (325) | 1 | |||
| Koutsomanis et al., 2000 (326) | 1 | |||
| Flamme et al., 1994 (327) | 1 | |||
| Rabau et al., 1988 (328) | 1 | |||
| Balibrea et al., 2007 (329) | 1 | 59 | ||
| Abdominal organs | Appendix | Dirksen et al., 2010 (330) | 1 | 1 |
| Pancreas | Hardt et al., 1993 (331) | 1 | ||
| Stoeckler et al., 2007 (332) | 1 | |||
| Bonapasta et al., 2010 (333) | 1 | |||
| Takamizawa et al., 2011 (334) | 1 | |||
| Inoue et al., 2018 (335) | 1 | |||
| Sun et al., 2017 (336) | 1 | |||
| Song et al., 2014 (337) | 1 | |||
| Molino et al., 2014 (338) | 1 | |||
| Razzetta et al., 2011 (316) | 1 | |||
| Lam et al., 2011 (339) | 1 | |||
| Mourra et al., 2010 (340) | 1 | |||
| Ang et al., 2007 (341) | 1 | |||
| Crippa et al., 2006 (342) | 3 | |||
| Haque et al., 2005 (343) | 1 | |||
| Z'graggen et al., 1998 (344) | 1 | |||
| Mountney et al., 1997 (345) | 1 | |||
| Sanuki-Fujimoto et al., 2008 (84) | 6 | |||
| Kliiger et al., 2017 (247) | 1 | 25 | ||
| Peritoneum | Saranovic et al., 2011 (298) | 1 | ||
| Shan et al., 2016 (346) | 1 | |||
| Osaku et al., 2015 (347) | 1 | |||
| Cardi et al., 2013 (348) | 5 | |||
| D’Annibale et al., 2007 (349) | 1 | |||
| Kobayashi et al., 2007 (350) | 1 | |||
| McLemore et al., 2005 (231) | 50 | |||
| Sanuki-Fujimoto et al., 2008 (84) | 27 | 87 | ||
| Gallbladder | Doval et al., 2006 (351) | 1 | ||
| Markelov et al., 2011 (352) | 1 | |||
| Di Vita et al., 2011 (353) | 1 | |||
| Urade et al., 2019 (354) | 1 | |||
| Zamkowski et al., 2017 (355) | 1 | |||
| Abdelilah et al., 2014 (356) | 1 | |||
| Manouras et al., 2008 (357) | 1 | |||
| Zagouri et al., 2007 (358) | 1 | 8 | ||
| Spleen | Bartolotti et al., 2012 (359) | 1 | ||
| El Fadli et al., 2017 (360) | 1 | |||
| Sufficool et al., 2013 (361) | 1 | |||
| Foroudi et al., 1999 (362) | 1 | |||
| Chapel et al., 1999 (363) | 1 | |||
| Cummings et al., 1992 (364) | 2 | |||
| Sanuki-Fujimoto et al., 2008 (84) | 2 | 9 | ||
| Kidney | Kykalos et al., 2010 (365) | 1 | ||
| Mhamdi et al., 2017 (366) | 1 | |||
| Nasu et al., 2015 (367) | 1 | |||
| Karczmarek-Borowska et al., 2015 (368) | 1 | |||
| Wu et al., 2015 (369) | 6 | |||
| Herzberg et al., 1991 (370) | 1 | |||
| Sanuki-Fujimoto et al., 2008 (84) | 1 | 12 | ||
| Bladder | Al Ibraheemi et al., 2016 (371) | 1 | ||
| Jordan et al., 2018 (372) | 1 | |||
| Kase et al., 2018 (373) | 1 | |||
| Xiao et al., 2012 (374) | 3 | |||
| Vulcano et al., 2010 (375) | 1 | |||
| Gatti et al., 2005 (376) | 1 | |||
| Soon et al., 2004 (377) | 1 | |||
| Elia et al., 1999 (378) | 1 | 10 | ||
| Ureter | Gabsi et al., 2018 (379) | 1 | ||
| Zunarelli et al., 1995 (380) | 1 | 2 | ||
| Adrenal gland | Mizuyama et al., 2013 (381) | 1 | ||
| Andjelic-Dekic et al., 2014 (382) | 1 | |||
| Paunovic et al., 2014 (383) | 1 | |||
| Demirci et al., 2011 (384) | 1 | |||
| Liu et al., 2010 (385) | 1 | |||
| Bausewein et al., 2006 (386) | 1 | |||
| Davì et al., 1996 (387) | 1 | |||
| Sanuki-Fujimoto et al., 2008 (84) | 2 | 9 | ||
| Retroperitoneum | Sanuki-Fujimoto et al., 2008 (84) | 16 | ||
| Kim et al., 2018 (213) | 1 | 17 | ||
| Pelvis | Shan et al., 2016 (346) | 1 | ||
| Colak et al., 2005 (388) | 1 | 2 | ||
| Nervous System | Meninges | Kashiwagi et al., 2012 (389) | 4 | |
| Laurencet et al., 2000 (390) | 1 | |||
| Higashi et al., 2000 (391) | 1 | |||
| Mego et al., 2011 (392) | 2 | |||
| Rao et al., 2017 (393) | 15 | |||
| Alnajar et al., 2017 (394) | 19 | |||
| Seki et al., 2016 (395) | 1 | |||
| Pan et al., 2015 (396) | 1 | |||
| Meattini et al., 2012 (397) | 33 | |||
| Tseng et al.,H. 2003 (398) | 1 | |||
| Kosmas et al., 2000 (65) | 1 | |||
| Jayson et al., 1994 (399) | 35 | |||
| Sanuki-Fujimoto et al., 2008 (84) | 16 | |||
| Madgula et al., 2014 (400) | 1 | 125 | ||
| Intramedullary spinal cord | Higashi et al., 2000 (391) | 1 | ||
| Gasser et al., 2001 (401) | 1 | |||
| Choi et al., 2010 (402) | 1 | |||
| Garcia et al., 2016 (403) | 1 | |||
| Aiello et al., 2017 (404) | 1 | |||
| Payer et al., 2015 (405) | 3 | |||
| Hsu et al., 2013 (406) | 1 | |||
| Zebrowski et al., 2010 (407) | 3 | |||
| Watanabe et al., 2006 (408) | 1 | |||
| Villegas et al., 2004 (409) | 1 | |||
| Chen et al., 1995 (410) | 1 | |||
| Schwechheimer et al., 1985 (411) | 1 | |||
| Moffie et al., 1980 (412) | 1 | |||
| West et al., 1979 (413) | 1 | 18 | ||
| Intraventricular | Della Puppa et al., 2010 (414) | 1 | ||
| Sajko et al., 2009 (415) | 1 | 2 | ||
| Cerebellum | Saha et al., 2018 (416) | 1 | ||
| Singh et al., 2015 (417) | 1 | |||
| Rowe et al., 2015 (418) | 1 | |||
| Higashi et al., 2000 (391) | 1 | 4 | ||
| Peripheral nervous system | Artico et al., 1991 (419) | 1 | ||
| Backhouse et al., 1998 (420) | 1 | |||
| Cherekaev et al., 2013 (421) | 1 | |||
| Suryanarayanan et al., 2005 (422) | 1 | |||
| Ito et al., 2010 (423) | 1 | |||
| Schulz et al., 2009 (424) | 1 | |||
| Zingale et al., 2002 (425) | 1 | |||
| Hirota et al., 1998 (426) | 1 | |||
| Breadon et al., 1977 (427) | 1 | 9 | ||
| Brainstem | Reyes et al., 2011 (428) | 1 | 1 | |
| Brain | de Ceuster et al., 2016 (429) | 1 | ||
| Chakrabarti et al., 2013 (430) | 1 | |||
| Kashiwagi et al., 2012 (389) | 3 | |||
| Low et al., 2012 (431) | 1 | |||
| Modi et al., 2006 (432) | 1 | |||
| Saisho et al., 2005 (433) | 2 | |||
| Higashi et al., 2000 (391) | 4 | |||
| Chou et al., 1998 (434) | 1 | |||
| Koller et al., 1986 (435) | 1 | 15 |
Discussion
In the war against BC, metastasis remains a primary clinical challenge as it is unpredictable in onset and it exponentially increases the clinical impact to the patient. The process of tumour metastasis is still controversial. Starting from Paget’s hypothesis of “seed and soil” (436), numerous studies have partially confirmed his observations and integrated new findings with the idea of a multistage process dependent on both the intrinsic properties of the tumour cells and the host response (437). In 1889 the surgeon Stephen Paget had already raised the question of “What is that decides what organs shall suffer in a case of disseminated cancer?” and contradicted the prevailing theory of Virchow (438) on metastasis originating from the arrest of tumour-cell emboli in the vasculature. Paget’s hypothesis is now widely accepted and enriched by new studies. The current definition of “seed” is a progenitor cell, cancer stem cell, or metastatic cell within the heterogeneous subpopulations forming the primary tumour, and the “soil” is the host factor, stroma, niche or organ microenvironment where selected metastatic cells can live and grow (439,440).
Translating Paget’s theory into clinical practice, findings show that BC preferentially spreads to some organs, whilst it is less common to find BC metastasis in other remote sites. In this review we mainly focused on anatomical sites where few cases of metastases have been reported in literature.
In clinical practice the knowledge of common sites of metastases helps the physician to detect early symptoms corresponding to secondary lesions, however when BC metastasizes in less expected sites the diagnosis could be delayed.
BC is amongst the most common tumours to metastasize to the head and neck, it constitutes ~15–20% of all metastases to this region and has been described in almost every head and neck anatomic subsite. However, since metastases to the head and neck are uncommon to begin with, breast carcinoma metastases are still relatively rare in clinical practice. Parenchymal metastases to the submandibular and parotid glands can be difficult to distinguish from new primary tumors arising there, specifically salivary duct carcinoma. In these cases immuno-histochemistry only can help differential diagnosis (15). Among metastatic orbital neoplasms, BC is the most common primary tumour, in particular, 55 cases were reported as affecting orbital soft tissue and 55 cases choroid, being bilateral in less than 1% of cases (1/55). A median time to onset of orbital metastases from BC diagnosis is 4.5–6.5 years and the majority of these patients have ER positive Her2 positive BC. A biological explanation for this tropism might be that steroid hormones, needed for tear production are produced in the orbital fat or in alternative, orbital metastases could be a late complication in patients with slow growing ER positive disease (17). Whether there is a tropism of lobular cancer cells to the orbital tissue or this is purely due to the more infiltrative nature of this tumor type along with its general tendency for spreading to myriad body sites is a matter of speculation (18). Prognosis in cases of orbital metastases from BC is determined by metastatic burden. A mean survival time of 5–22 months after orbital diagnosis has been reported in the literature. Furthermore, orbital metastases can cause various local problems, such as exophthalmos, exposure keratitis, optic neuropathy, and limited extraocular muscle motion. As enucleation confers no benefit in terms of survival, external beam radiotherapy is the most commonly used palliative treatment (16). Although BC rarely metastasizes to the head and neck region, awareness should be raised when BC patients experience headache or have sinus-related symptoms. The most of these patients have very poor prognosis, however chemotherapy and radiotherapy may be effective to prolong survival (126). Despite the principle that metastatic deposits have a predilection for highly vascularized organs, the thyroid is rarely a metastatic site. We found 39 cases of BC metastasis to the thyroid gland reported in literature, the true prevalence oscillates from 3% to 34% of all thyroid metastases, whilst it is much more common to find synchronous or metachronous malignancies (84,170-187). The suspicion of metastatic disease should be raised by thyroid lumps in BC survivors, although indicating poor prognosis, the role of surgery in these lesions should be considered for local disease to control, palliate and prevent the potential morbidity on the airway (441,442).
Despite the well-known BC propensity to spread to the central nervous system, the prevalence of symptomatic central nervous system metastases among patients with BC ranges from 5% to 16%, although autoptic studies have reported prevalence rates of up to 30% (414). In our review we found 18 cases of intramedullary spinal cord metastases (see Table 1). The mechanism of intramedullary spread is not well established but it may involve lymphatic or haematological transit, direct spread from the vertebrae or ‘drop-down’ metastases from the brain. The most common symptoms are paresis and dysesthesia or bladder dysfunction, both surgery and radiotherapy are recommended both for diagnosis and treatment of brain metastases (405). Interestingly, Zebrowski et al. (407) suggested a potential association between the inability of trastuzumab to cross the blood-brain barrier and the occurrence of intramedullary metastases in Her2 positive disease. Additionally, some studies report a predisposition of lobular histotype to leptomeningeal carcinomatosis, whilst in the case of ductal carcinoma spreading to the meninges a high histological grade and triple-negative biology seems to be prevalent (394).
Although very rare (9 cases reported), metastasis to the spinal nerve root ganglion can be the first manifestation of distant hematogenous metastases of BC. The clinical course is characterized by increasing radicular symptoms-especially intractable pain. Surgical intervention with tumour debulking followed by radiotherapy provides local tumour control and palliation from pain (422-425).
Soft tissue metastasis from any primary malignancy is considered very rare, it can be in subcutaneous and muscular tissue (117). In this review we found 33 cases of soft tissues metastases involving skeletal muscle or subcutaneous tissue or both of the upper and lower limbs, trunk, shoulders, and buttocks (117-120). Some studies have reported a frequency of 0.8% based on autopsies while few reported an incidence of 0.2% based on clinical studies (443,444). This rarity can be due to the fact that soft tissues produce anti-carcinogenic factors like lactic acid, beta adrenergic receptors or protease inhibitors which serve as a deterrent for metastatic invasion (116,445,446). As for other metastatic sites a multimodality approach generally is adopted depending upon the performance status of the patient, any comorbid condition, type of primary malignancy, site and size of the metastatic lesion. RT and chemotherapy have been generally considered the primary modality of therapy either in combination or separately while surgery is reserved for patients not responding to radiation or chemotherapy. A high degree of clinical suspicion and immuno-histopathological confirmation are required to identify and diagnose any soft tissue swelling of the body in a previously treated primary breast cancer patient to prevent any inappropriate treatment causing undue morbidity or even mortality (117).
Gastrointestinal (GI) tract metastases from BC are also considered rare, their incidence in autopsy series varied from 8% to 35% (274,447). This localization can easily simulate a primary GI cancer and any region of the GI tract can be involved, from the tongue (145) to the anus (448). Most series report a greater propensity of lobular carcinoma to metastasize to the GI tract and peritoneum, but data on this tropism are poor and inconclusive (231,449,450). The most common site of GI tract metastasis is the stomach, 185 cases in our review. Colon involvement is quite common (59 cases), whilst small intestine involvement has been reported in 24 cases and is more frequently diagnosed at autopsy. In particular, ductal carcinoma seems to produce nodular stomach lesions, while lobular BC tend to cause more diffuse disease (451). The GI tract metastases are uncommon and peculiar, the main issue is to identify symptoms like nausea, vomiting, diarrhoea or abdominal pain and start the differential diagnosis with CT scan, endoscopy and biopsy. According to literature reports, surgery is crucial when possible, nevertheless chemotherapy and endocrine therapy were commonly used, very rarely radiotherapy can also play a role (452). Other abdominal organs are also rare sites of BC metastasis often mimicking primary tumours, so the final diagnosis occurs after biopsy or surgical excision.
BC metastases can also involve genital organs and the placenta. Most cases of uterine metastases presents as vaginal bleeding or abdominal discomfort, although the vast majority of these have been diagnosed during autopsy (78,453). Studies have showed that the incidence of ovarian metastases in BC patients is 13–47%. It seems that the metastatic lesions reach the ovaries and genital organs through the lymphatic and blood vessels, or through trans-coelomic spread, then the reciprocal interaction between intrinsic BC molecular characteristics and the local microenvironment explains the metastatic organotropism (103,454). The most common sign of uterine or vaginal metastases is vaginal bleeding, while ovarian metastases frequently present as an asymptomatic ovarian mass (455,456). In any case, the central task is to differentiate primary versus secondary tumours, as this will affect the clinical decision process, treatment and prognosis. The surgical metastasis excision, hysterectomy or oophorectomy not only provide diagnostic information, but they could also have a curative effect along with systemic therapy (104). The local control of bleeding can also benefit from radiation therapy, uterine artery embolization and conisation (73). However, genital tract and ovarian metastases represent the late stage of a systemic disease, for instance BC patients with ovarian metastases have a 6–26% 5-year survival rate (106,454,457). The lack of symptoms and poor outcomes emphasize the relevance of a regular gynaecological surveillance in all BC survivors, despite guidelines citing different approaches (73). In 2003, Alexander et al. (108) have reported the most comprehensive review of placental involvement (15 cases), where BC cells were located in the intervillous space without any involvement of the fetal villous stroma and vascular circulation. Fetal immune response seems to play a key role in avoiding the fetal involvement when possible, although maternal prognosis remains very poor (109,110). Malignant disease in pregnancy provoke a challenging situation for gynaecologists, breast surgeons, oncologists and neonatologists, all placentas should be evaluated by pathologists and each single case of metastasis should be discussed within a multidisciplinary team (109).
The analysis of BC metastatic pattern has shown that lobular tumours were more likely to metastasize to the peritoneum, adrenal glands, uterus and pleural surface (458). Additionally, the aggressiveness of lobular carcinoma seems to be associated to metastasis at unusual sites, in particular meningeal dissemination was more frequently associated to lobular BC (459-462). A retrospective study on 3783 patients about the distribution and frequency of metastases at unusual sites showed that the majority of unusual metastases are associated with prior metastases at more unusual sites, which appear, on average, 1 year before. The prognosis of metastatic BC patients was the same irrespective of the metastasis site and no risk factors for unusual metastasis were identified (84). In general, it is uncommon to find isolated rare metastases, the vast majority of the rare metastases described develop together with metastases in other sites, thus highlighting a worsening systemic disease.
Conclusions
Despite the improvements in diagnosis, surgical techniques, general patient care, and local and systemic adjuvant therapies, most deaths from cancer result from metastases that are resistant to conventional therapies. The process of cancer metastasis is sequential and selective and incorporates stochastic elements, hence the growth of metastases represents the endpoint of the interplay of tumour cells with host factors.
On the research level, the recent advances in our understanding of the metastatic process at the cellular and molecular level provide unprecedented potential for the improvement and the development of effective adjuvant therapies.
On the clinical level, the early diagnosis of secondary lesions represents the only chance to control the disease and prolong survival, hence the knowledge of the common as well as rare sites of metastases can help the physician to detect symptoms and plan the most appropriate treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Emanuela Esposito and Michelino De Laurentiis) for the focused issue “Rare Tumors of the Breast” published in Translational Cancer Research. This article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.24). The focused issue “Rare Tumors of the Breast” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol 2016;34:611-35. [Crossref] [PubMed]
- Rosselli Del Turco M, Palli D, Cariddi A, et al. Intensive diagnostic follow-up after treatment of primary breast cancer. A randomized trial. National Research Council Project on Breast Cancer follow-up. JAMA 1994;271:1593-7. [Crossref] [PubMed]
- Palli D, Russo A, Saieva C, et al. Intensive vs clinical follow-up after treatment of primary breast cancer: 10-year update of a randomized trial. National Research Council Project on Breast Cancer Follow-up. JAMA 1999;281:1586. [Crossref] [PubMed]
- Gradishar WJ, Anderson BO, Balassanian R, et al. Breast Cancer, Version 4.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:310-20. [Crossref] [PubMed]
- Khatcheressian JL, Hurley P, Bantug E, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:961-5. [Crossref] [PubMed]
- Fauci AS, Hauser SL, Jameson JL, et al. Harrison's Manual of Medicine, 19e. 19th ed. McGraw-Hill's AccessMedicine. New York, NY: McGraw-Hill Education LLC, 2016.
- Reeves D, Levine MR, Lash R. Nonpalpable breast carcinoma presenting as orbital infiltration: case presentation and literature review. Ophthalmic Plast Reconstr Surg 2002;18:84-8. [Crossref] [PubMed]
- Asproudis I, Gorezis S, Charalabopoulos K, et al. Breast carcinoma metastasis to the orbit and paranasal sinuses: a case report. Exp Oncol 2004;26:246-8. [PubMed]
- Eckardt AM, Rana M, Essig H, et al. Orbital metastases as first sign of metastatic spread in breast cancer: case report and review of the literature. Head Neck Oncol 2011;3:37. [Crossref] [PubMed]
- Mora-Guzmán I, Maqueda González R, Doblado Cardellach B, et al. Isolated orbital metastasis as an initial presentation of a breast cancer. Cir Esp 2018;96:119. [PubMed]
- Maliepaard M, Mesham M, Aleksic Z, et al. Ocular metastasis as initial presentation in breast cancer. S Afr Med J 2017;107:694-6. [Crossref] [PubMed]
- Spraker MB, Francis CE, Korde L, et al. Solitary Orbital Metastasis 35 Years after a Diagnosis of Lobular Carcinoma in Situ. Cureus 2017;9:e1404. [PubMed]
- Pinto Proença R, Fernandes J, Burnier MN, et al. Orbital metastasis from an occult breast carcinoma (T0, N1, M1). BMJ Case Rep 2018; [Crossref] [PubMed]
- Gondim DD, Chernock R, El-Mofty S, et al. The Great Mimicker: Metastatic Breast Carcinoma to the Head and Neck with Emphasis on Unusual Clinical and Pathologic Features. Head Neck Pathol 2017;11:306-13. [Crossref] [PubMed]
- Kim JH, Choi SY, Cho CK, et al. Bilateral orbital metastases from breast cancer: a case report of successful palliation using stereotactic radiotherapy. Breast J 2011;17:669-71. [Crossref] [PubMed]
- Pierson TM, Tebit EV, El Sayed A, et al. Orbital Metastases from Breast Cancer: Retrospective Analysis at an Academic Cancer Center. Breast J 2016;22:447-50. [Crossref] [PubMed]
- Raap M, Antonopoulos W, Dämmrich M, et al. High frequency of lobular breast cancer in distant metastases to the orbit. Cancer Med 2015;4:104-11. [Crossref] [PubMed]
- Zwicker F, Herfarth K, Welzel T, et al. Palliative radiotherapy of retrobulbar orbit metastases due to breast cancer. Onkologie 2008;31:529-33. [Crossref] [PubMed]
- Torres JJ, Medel R, Alonso T, et al. Orbital metastases from male breast cancer in two cases. Ophthalmic Plast Reconstr Surg 2007;23:154-6. [Crossref] [PubMed]
- Schick U, Lermen O, Hassler W. Management of orbital metastases. Zentralbl Neurochir 2006;67:1-7. [Crossref] [PubMed]
- Dieing A, Schulz CO, Schmid P, et al. Orbital metastases in breast cancer: report of two cases and review of the literature. J Cancer Res Clin Oncol 2004;130:745-8. [Crossref] [PubMed]
- Amemiya T, Hayashida H, Dake Y. Metastatic orbital tumors in Japan: a review of the literature. Ophthalmic Epidemiol 2002;9:35-47. [Crossref] [PubMed]
- Rossi L, Zancla S, Civitelli L, et al. An unusual orbital metastasis of breast cancer. Breast Dis 2014;34:173-6. [Crossref] [PubMed]
- Bajcsay A, Kontra G, Récsán Z, et al. Lens-sparing external beam radiotherapy of intraocular metastases: our experiences with twenty four eyes. Neoplasma 2003;50:459-64. [PubMed]
- Murthy R, Gupta A, Hegde S, et al. Bilateral multiple extraocular muscle metastasis from breast carcinoma. Indian J Ophthalmol 2011;59:381-2. [Crossref] [PubMed]
- Chang YC, Hsieh TC, Kao CH. Single Ophthalmic Metastasis After Definitive Therapy of Locoregional Breast Cancer. Am J Med Sci 2017;354:440. [Crossref] [PubMed]
- Nifosí G, Zuccarello M. Unilateral localized extraocular muscle metastasis by lobular breast carcinoma. BMJ Case Rep 2018; [Crossref] [PubMed]
- Framarino-Dei-Malatesta M, Chiarito A, Bianciardi F, et al. Metastases to extraocular muscles from breast cancer: case report and up-to-date review of the literature. BMC Cancer 2019;19:36. [Crossref] [PubMed]
- Weiss R, Grisold W, Jellinger K, et al. Metastasis of solid tumors in extraocular muscles. Acta Neuropathol 1984;65:168-71. [Crossref] [PubMed]
- Lell M, Schulz-Wendtland R, Hafner A, et al. Bilateral orbital tumour as the presentation of mammographically occult breast cancer. Neuroradiology 2004;46:682-5. [Crossref] [PubMed]
- Kouvaris JR, Gkongkou PV, Papadimitriou CA, et al. Bilateral metastases to extraocular muscles from lobular breast carcinoma. Onkologie 2008;31:387-9. [Crossref] [PubMed]
- Spitzer SG, Bersani TA, Mejico LJ. Multiple bilateral extraocular muscle metastases as the initial manifestation of breast cancer. J Neuroophthalmol 2005;25:37-9. [Crossref] [PubMed]
- van der Heijden A, Twijnstra A, Lamers WP, et al. An unusual cause of diplopia in a cancer patient. Eur J Cancer 1991;27:1315-6. [Crossref] [PubMed]
- Glazer LC, Harris GJ, Simons KB. Orbital metastasis as the presenting sign of adenocarcinoma of the breast. Ophthalmic Plast Reconstr Surg 1991;7:252-5. [Crossref] [PubMed]
- Correa de Mello P, Brasil OFM. Isolated Retinal Metastasis From Breast Cancer. Retina 2017;37:e125-e7. [Crossref] [PubMed]
- Shields JA, Shields CL, Kiratli H, et al. Metastatic tumors to the iris in 40 patients. Am J Ophthalmol 1995;119:422-30. [Crossref] [PubMed]
- Shah RK, Lamichhane S. Ocular metastasis from breast carcinoma simulating anterior scleritis: a case report. J Med Case Rep 2017;11:249. [Crossref] [PubMed]
- Biswas J, Ho TC, Bhavsar K. Bilateral metastasis to the retina, choroids and optic nerve from breast cancer: a clinicopathological case. Indian J Ophthalmol 2007;55:71-2. [Crossref] [PubMed]
- Sirimaharaj M, Hunyor AP, Chan WC, et al. Unsual ocular metastasis from breast cancer. Clin Exp Ophthalmol 2006;34:74-6. [Crossref] [PubMed]
- Schlaen ND, Naves AE. Orbital and choroidal metastases from carcinoma of the male breast. Arch Ophthalmol 1986;104:1344-6. [Crossref] [PubMed]
- Skalicky SE, Hirst LW, Conway RM. Metastatic breast carcinoma presenting as a conjunctival lesion. Clin Exp Ophthalmol 2007;35:767-9. [Crossref] [PubMed]
- Sánchez Orgaz M, Gonzalez Pessolani T, Pozo Kreilinger JJ, et al. Orbital and conjunctival metastasis from lobular breast carcinoma. Orbit 2017;36:197-200. [Crossref] [PubMed]
- Radovanović AB, Rasić D, Buta M, et al. Breast cancer metastasis to the conjunctiva. Vojnosanit Pregl 2013;70:331-4. [Crossref] [PubMed]
- Kiratli H, Shields CL, Shields JA, et al. Metastatic tumours to the conjunctiva: report of 10 cases. Br J Ophthalmol 1996;80:5-8. [Crossref] [PubMed]
- Kaur G, Ismail R, Harun H. Metastatic mucinous carcinoma of the eyelid. Malays J Pathol 2005;27:117-8. [PubMed]
- Martorell-Calatayud A, Requena C, Díaz-Recuero JL, et al. Mask-like metastasis: report of 2 cases of 4 eyelid metastases and review of the literature. Am J Dermatopathol 2010;32:9-14. [Crossref] [PubMed]
- Goodier MA, Jordan JR. Metastatic breast cancer to the lower eyelid. Laryngoscope 2010;120:S129. [Crossref] [PubMed]
- Kuo SC, Hsiao SC, Chiou CC, et al. Metastatic carcinoma of the breast: a case with the unusual presentation of unilateral periorbital edema. Jpn J Ophthalmol 2008;52:305-7. [Crossref] [PubMed]
- Douglas RS, Goldstein SM, Einhorn E, et al. Metastatic breast cancer to 4 eyelids: a clinicopathologic report. Cutis 2002;70:291-3. [PubMed]
- Claessens N, Rakic L, Arrese JE, et al. Breast cancer metastatic to the eyelids. Eur J Dermatol 2000;10:473-4. [PubMed]
- Rosenblum GA. Metastatic breast cancer in the eyelid. Cutis 1983;31:411-5, 7.
- Lawton RL, Hobbs B, Jochimsen P. Metastases to eyelids: breast. J Surg Oncol 1980;13:117-20. [Crossref] [PubMed]
- Ozturk B, Buyukberber S, Coskun U, et al. Solitary iris metastasis from breast cancer with dramatic course: case report. Med Oncol 2007;24:463-5. [Crossref] [PubMed]
- Mennel RG. Ocular metastases from breast cancer. Clin Breast Cancer 2001;1:318-9. [Crossref] [PubMed]
- Reddy SC, Madhavan M, Mutum SS. Anterior uveal and episcleral metastases from carcinoma of the breast. Ophthalmologica 2000;214:368-72. [Crossref] [PubMed]
- Williams NJ, Leris AC, Kouriefs C, et al. Choroidal metastasis-the initial presentation of breast carcinoma. Eur J Surg Oncol 2000;26:817-8. [Crossref] [PubMed]
- Solav S, Bhandari R, Sowani A, et al. Choroidal metastasis from carcinoma of breast detected on F18-FDG PET CT scan: A case report and review of literature. Indian J Nucl Med 2010;25:160-3. [PubMed]
- Arya M, Duker JS. Vinorelbine-induced regression of a choroidal metastasis from primary breast carcinoma. Int J Retina Vitreous 2018;4:17. [Crossref] [PubMed]
- Luo Z, Cai Q, Zhao Y, et al. Late distant recurrence of breast carcinoma and metastasis to the main bronchus and choroid: A case report. Medicine (Baltimore) 2018;97:e10754. [Crossref] [PubMed]
- Antosz ZS, Walocha J, Poręba R, et al. Sudden loss of vision due to breast cancer metastasis to the eyeball. Neuro Endocrinol Lett 2014;35:249-51. [PubMed]
- Liu T, Xu Y, Wan L, et al. Choroid as the first recurrence site: 13 years after breast carcinoma. J Cancer Res Ther 2012;8:639-40. [Crossref] [PubMed]
- Hood CT, Budd GT, Zakov ZN, et al. Male breast carcinoma metastatic to the choroid: report of 3 cases and review of the literature. Eur J Ophthalmol 2011;21:459-67. [Crossref] [PubMed]
- Oleksy P, Pogrzebielski A, Karska-Basta I, et al. A case of choroidal metastasis in a male breast cancer. Klin Oczna 2010;112:311-3. [PubMed]
- Kosmas C, Malamos NA, Antonopoulos M. Complete regression of choroidal metastases from breast cancer after docetaxel-based systemic chemotherapy. Med Pediatr Oncol 2000;34:229-30. [Crossref] [PubMed]
- Kreusel KM, Heimann H, Wiegel T, et al. Choroidal metastasis in men with metastatic breast cancer. Am J Ophthalmol 1999;128:253-5. [Crossref] [PubMed]
- Chen YR, Lin TH, Chan SM, et al. Bilateral choroidal metastases as the initial presentation of a small breast carcinoma: a case report. Zhonghua Yi Xue Za Zhi (Taipei) 1998;61:99-103. [PubMed]
- Paoli D. Regression of choroidal metastasis from a carcinoma of the male breast: case report. Ophthalmologica 1998;212:74-6. [Crossref] [PubMed]
- Gupta RK, Lallu S, McHutchison AG, et al. Fine needle aspiration of Sister Mary Joseph's nodule. Cytopathology 1991;2:311-4. [Crossref] [PubMed]
- Thatcher N, Thomas PR. Choroidal metastases from breast carcinoma: a survey of 42 patients and the use of radiation therapy. Clin Radiol 1975;26:549-53. [Crossref] [PubMed]
- Pambuccian SE, Bachowski GJ, Twiggs LB. Signet ring cell lobular carcinoma of the breast presenting in a cervicovaginal smear. A case report. Acta Cytol 2000;44:824-30. [Crossref] [PubMed]
- Nair RR, Conroy MD, Jeyarajah AR. Obstructive uropathy secondary to parametrial metastasis: an unusual presentation of breast carcinoma. Eur J Gynaecol Oncol 2009;30:214-5. [PubMed]
- Proença S, Reis MI, Cominho J, et al. Metastatic Breast Cancer in Uterine Cervix: A Rare Presentation. J Low Genit Tract Dis 2016;20:e1-3. [Crossref] [PubMed]
- Toyoshima M, Iwahashi H, Shima T, et al. Solitary uterine metastasis of invasive lobular carcinoma after adjuvant endocrine therapy: a case report. J Med Case Rep 2015;9:47. [Crossref] [PubMed]
- Bogliolo S, Morotti M, Valenzano Menada M, et al. Breast cancer with synchronous massive metastasis in the uterine cervix: a case report and review of the literature. Arch Gynecol Obstet 2010;281:769-73. [Crossref] [PubMed]
- Mousavi A, Karimi Zarchi M. Isolated cervical metastasis of breast cancer: a case report and literature review. J Low Genit Tract Dis 2007;11:276-8. [Crossref] [PubMed]
- Green AE, Biscotti C, Michener C, et al. Isolated cervical metastasis of breast cancer: a case report and review of the literature. Gynecol Oncol 2004;95:267-9. [Crossref] [PubMed]
- Pauer HU, Viereck V, Burfeind P, et al. Uterine cervical metastasis of breast cancer: a rare complication that may be overlooked. Onkologie 2003;26:58-60. [PubMed]
- Sinkre P, Milchgrub S, Miller DS, et al. Uterine metastasis from a heterologous metaplastic breast carcinoma simulating a primary uterine malignancy. Gynecol Oncol 2000;77:216-8. [Crossref] [PubMed]
- Kesavan S, Lee IW. An unusual tumour metastasis to the cervix. Ann Acad Med Singapore 2000;29:780-2. [PubMed]
- Hepp HH, Hoos A, Leppien G, et al. Breast cancer metastatic to the uterine cervix: analysis of a rare event. Cancer Invest 1999;17:468-73. [Crossref] [PubMed]
- Kennebeck CH, Alagoz T. Signet ring breast carcinoma metastases limited to the endometrium and cervix. Gynecol Oncol 1998;71:461-4. [Crossref] [PubMed]
- Taxy JB, Trujillo YP. Breast cancer metastatic to the uterus. Clinical manifestations of a rare event. Arch Pathol Lab Med 1994;118:819-21. [PubMed]
- Sanuki-Fujimoto N, Takeda A, Amemiya A, et al. Pattern of tumor recurrence in initially nonmetastatic breast cancer patients: distribution and frequency of metastases at unusual sites. Cancer 2008;113:677-82. [Crossref] [PubMed]
- Kemp B, Schröder W, Hermann A, et al. Uterine metastasis of invasive lobular breast carcinoma. Case report and review of the literature with reference to differential diagnostic problems and clinical consequences. Zentralbl Gynakol 1997;119:500-2. [PubMed]
- Moey MY, Hassan OA, Papageorgiou CN, et al. The potential role of HER2 upregulation in metastatic breast cancer to the uterus: a case report. Clin Case Rep 2016;4:928-34. [Crossref] [PubMed]
- Çift T, Aslan B, Bulut B, et al. Unusual uterine metastasis of invasive ductal carcinoma: A case report. Turk J Obstet Gynecol 2016;13:164-6. [Crossref] [PubMed]
- Rahmani M, Nili F, Tabibian E. Endometrial Metastasis from Ductal Breast Carcinoma: A Case Report with Literature Review. Am J Case Rep 2018;19:494-9. [Crossref] [PubMed]
- Aytekin A, Bilgetekin I, Ciltas A, et al. Lobular breast cancer metastasis to uterus during adjuvant tamoxifen treatment: A case report and review of the literature. J Cancer Res Ther 2018;14:1135-7. [Crossref] [PubMed]
- Briki R, Cherif O, Bannour B, et al. Uncommon metastases of invasive lobular breast cancer to the endometrium: a report of two cases and review of the literature. Pan Afr Med J 2018;30:268. [Crossref] [PubMed]
- Franco-Márquez R, Torres-Gaytán AG, Narro-Martinez MA, et al. Metastasis of Breast Lobular Carcinoma to Endometrium Presenting as Recurrent Abnormal Uterine Bleeding: A Case Report and Review of Literature. Case Rep Pathol 2019;2019:5357194. [PubMed]
- Ertas IE, Sayhan S, Karagoz G, et al. Signet-ring cell carcinoma of the breast with uterine metastasis treated with extensive cytoreductive surgery: a case report and brief review of the literature. J Obstet Gynaecol Res 2012;38:948-52. [Crossref] [PubMed]
- D'souza MM, Sharma R, Tripathi M, et al. Cervical and uterine metastasis from carcinoma of breast diagnosed by PET/CT: an unusual presentation. Clin Nucl Med 2010;35:820-3. [Crossref] [PubMed]
- Karvouni E, Papakonstantinou K, Dimopoulou C, et al. Abnormal uterine bleeding as a presentation of metastatic breast disease in a patient with advanced breast cancer. Arch Gynecol Obstet 2009;279:199-201. [Crossref] [PubMed]
- Scopa CD, Aletra C, Lifschitz-Mercer B, et al. Metastases of breast carcinoma to the uterus. Report of two cases, one harboring a primary endometrioid carcinoma, with review of the literature. Gynecol Oncol 2005;96:543-7. [Crossref] [PubMed]
- Chehal A, Seoud M, Taher A, et al. Endometrial metastasis from signet-ring breast carcinoma: case report. Eur J Gynaecol Oncol 2002;23:563-4. [PubMed]
- Horn LC, Einenkel J, Baier D. Endometrial metastasis from breast cancer in a patient receiving tamoxifen therapy. Gynecol Obstet Invest 2000;50:136-8. [Crossref] [PubMed]
- Gandhi AK, Roy S, Mridha AR, et al. Vulvar metastasis from carcinoma breast unveiling distant metastasis: Exploring an unusual metastatic pattern. J Egypt Natl Canc Inst 2015;27:243-6. [Crossref] [PubMed]
- Engelstaedter V, Mylonas I. Lower genital tract metastases at time of first diagnosis of mammary invasive lobular carcinoma. Arch Gynecol Obstet 2011;283:93-5. [Crossref] [PubMed]
- Sheen-Chen SM, Eng HL, Huang CC. Breast cancer metastatic to the vulva. Gynecol Oncol 2004;94:858-60. [Crossref] [PubMed]
- Curtin WM, Murthy B. Vulvar metastasis of breast carcinoma. A case report. J Reprod Med 1997;42:61-3. [PubMed]
- Valenzano Menada M, Papadia A, Lorenzi P, et al. Breast cancer metastatic to the vulva after local recurrence occurring on a rectus abdominis myocutaneous flap: a case report and review of the literature. Eur J Gynaecol Oncol 2003;24:577-9. [PubMed]
- Pineda A, Sall S. Metastasis to the vagina from carcinoma of the breast. J Reprod Med 1978;20:243-5. [PubMed]
- Bellati F, Palaia I, Gasparri ML, et al. First case of isolated vaginal metastasis from breast cancer treated by surgery. BMC Cancer 2012;12:479. [Crossref] [PubMed]
- Durga G, Gandhi JS, Mehta A. Malignant phyllodes tumor metastatic to bilateral ovaries: A Krukenberg-like presentation. J Cancer Res Ther 2018;14:1138-41. [Crossref] [PubMed]
- Fujii M, Okino M, Fujioka K, et al. Pseudo-Meigs' syndrome caused by breast cancer metastasis to both ovaries. Breast Cancer 2006;13:344-8. [Crossref] [PubMed]
- Julien V, Labadie M, Gauthier G, et al. Clitoral metastasis from ductal breast cancer revealing metastases in multiple sites and review of the literature. J Low Genit Tract Dis 2012;16:66-9. [Crossref] [PubMed]
- Alexander A, Samlowski WE, Grossman D, et al. Metastatic melanoma in pregnancy: risk of transplacental metastases in the infant. J Clin Oncol 2003;21:2179-86. [Crossref] [PubMed]
- Froehlich K, Stensheim H, Markert UR, et al. Breast carcinoma in pregnancy with spheroid-like placental metastases-a case report. APMIS 2018;126:448-52. [Crossref] [PubMed]
- Eltorky M, Khare VK, Osborne P, et al. Placental metastasis from maternal carcinoma. A report of three cases. J Reprod Med 1995;40:399-403. [PubMed]
- Liu CH, Chang C, Sy E, et al. Metaplastic breast carcinoma with multiple muscle metastasis: a case report. Medicine (Baltimore) 2015;94:e662. [Crossref] [PubMed]
- Khettab M, Barrascout E, Lamuraglia M. Sternocleidomastoid muscle metastasis of breast cancer: case report. Eur J Gynaecol Oncol 2017;38:113-4. [PubMed]
- Almusarhed M, Eldeeb H. Solitary biceps muscle metastasis from breast cancer. BMJ Case Rep 2017; [Crossref] [PubMed]
- Salemis NS. Skeletal muscle metastasis from breast cancer: management and literature review. Breast Dis 2015;35:37-40. [Crossref] [PubMed]
- Khandelwal AR, Takalkar AM, Lilien DL, et al. Skeletal muscle metastases on FDG PET/CT imaging. Clin Nucl Med 2012;37:575-9. [Crossref] [PubMed]
- Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol 2010;20:649-58. [Crossref] [PubMed]
- Purkayastha A, Sharma N. Extremely rare presentation of soft tissue metastasis from carcinoma breast as a massive swelling of upper extremity. Ann Palliat Med 2016;5:145-8. [Crossref] [PubMed]
- Rao MY, Wu JB. Invasive ductal breast cancer with extensive subcutaneous metastases in trunk: a case report. Eur Rev Med Pharmacol Sci 2015;19:4101-4. [PubMed]
- Metere A, Di Cosimo C, Chiesa C, et al. An unusual subcutaneous breast cancer metastasis in a 86-year-old woman. Eur Rev Med Pharmacol Sci 2012;16:562-4. [PubMed]
- Plaza JA, Perez-Montiel D, Mayerson J, et al. Metastases to soft tissue: a review of 118 cases over a 30-year period. Cancer 2008;112:193-203. [Crossref] [PubMed]
- Jaffe D, Kim E, Aboulafia A. Erosive Breast Cancer Metastasis to the Ankle: A Case Report. J Foot Ankle Surg 2016;55:1297-301. [Crossref] [PubMed]
- Monserez D, Vlaminck S, Kuhweide R, et al. Symmetrical ethmoidal metastases from ductal carcinoma of the breast, suggesting transcribrosal spread. Acta Otorhinolaryngol Belg 2001;55:251-7. [PubMed]
- Pignataro L, Peri A, Ottaviani F. Breast carcinoma metastatic to the ethmoid sinus: a case report. Tumori 2001;87:455-7. [Crossref] [PubMed]
- Fyrmpas G, Televantou D, Papageorgiou V, et al. Unsuspected breast carcinoma presenting as orbital complication of rhinosinusitis. Eur Arch Otorhinolaryngol 2008;265:979-82. [Crossref] [PubMed]
- Reimann K, Schulze M, Adam P, et al. Metastasis to the paranasal sinuses from primary breast cancer. HNO 2011;59:915-7. [Crossref] [PubMed]
- Xiong J, Chen J, Zheng L, et al. Rare metastasis to paranasal sinuses from triple-negative breast cancer: A case report and literature review. Medicine (Baltimore) 2017;96:e8718. [Crossref] [PubMed]
- Walker DT, Barbur S, Mathew R, et al. Sinus involvement in breast cancer: case report. J Laryngol Otol 2013;127:619-20. [Crossref] [PubMed]
- Marchioni D, Monzani D, Rossi G, et al. Breast carcinoma metastases in paranasal sinuses, a rare occurrence mimicking a primary nasal malignancy. case report. Acta Otorhinolaryngol Ital 2004;24:87-91. [PubMed]
- Pitkäranta A, Markkola A, Malmberg H. Breast cancer metastasis presenting as ethmoiditis. Rhinology 2001;39:107-8. [PubMed]
- Austin JR, Kershiznek MM, McGill D, et al. Breast carcinoma metastatic to paranasal sinuses. Head Neck 1995;17:161-5. [Crossref] [PubMed]
- Tiwari V, Pande SC, Verma K, et al. Paranasal sinus and retro-orbital metastasis in a case of breast carcinoma: a clinicoradiological review. BMJ Case Rep 2014; [Crossref] [PubMed]
- Namad T, Benbrahim Z, Najib R, et al. Maxillofacial metastasis from breast cancer. Pan Afr Med J 2014;19:156. [Crossref] [PubMed]
- Imre A, Sakarya EU, Imre SS, et al. Orbital apex syndrome as a sign of unsuspected breast carcinoma. J Craniofac Surg 2013;24:1476-8. [Crossref] [PubMed]
- Atasoy BM, Cetin IA, Bozkurt SU, et al. Metastasis to paranasal sinuses and orbita of breast cancer with a rare metachronous tumor of the uterine cervix. J Craniofac Surg 2013;24:e64-5. [Crossref] [PubMed]
- Ando K, Masumoto N, Sakamoto M, et al. Parotid Gland Metastasis of Breast Cancer: Case Report and Review of the Literature. Breast Care (Basel) 2011;6:471-3. [Crossref] [PubMed]
- Agarwal G, Sonthineni C, Mayilvaganan S, et al. Surgical Outcomes of Primary Versus Post-Neoadjuvant Chemotherapy Breast Conservation Surgery: A Comparative Study from a Developing Country. World J Surg 2018;42:1364-74. [Crossref] [PubMed]
- Cao XS, Cong BB, Yu ZY. Parotid gland metastasis from carcinoma of the breast detected by PET/CT: Case report and review. Medicine (Baltimore) 2018;97:e10616. [Crossref] [PubMed]
- Rawet T, Jegannathen A, Soumian S. Parotid gland: an unusual site of breast cancer metastasis. BMJ Case Rep 2017; [Crossref] [PubMed]
- Sellinger M, Neubauer K, William M, et al. Contralateral metastasis of parotid gland in advanced breast cancer with peripheral facial paralysis. Arch Gynecol Obstet 2011;284:1557-60. [Crossref] [PubMed]
- Dangore-Khasbage SB, Degwekar SS, Bhowate RR, et al. Metastatic involvement of parotid from carcinoma of the breast--a case report. Oral Maxillofac Surg 2009;13:49-53. [Crossref] [PubMed]
- Perez-Fidalgo JA, Chirivella I, Laforga J, et al. Parotid gland metastasis of a breast cancer. Clin Transl Oncol 2007;9:264-5. [Crossref] [PubMed]
- Zhang JZ, Gu M. Malignant phyllodes tumor of the breast metastatic to the parotid gland diagnosed by fine needle aspiration biopsy. A case report. Acta Cytol 2003;47:253-8. [Crossref] [PubMed]
- Erra S, Costamagna D. Breast cancer metastatic to the submandibular gland. Case report. G Chir 2011;32:194-8. [PubMed]
- Cain AJ, Goodlad J, Denholm SW. Metachronous bilateral submandibular gland metastases from carcinoma of the breast. J Laryngol Otol 2001;115:683-4. [Crossref] [PubMed]
- Suárez Roa Mde L, Ruiz Godoy Rivera LM, Vela Chávez T, et al. Breast malignant phyllodes tumour metastasising to soft tissues of oral cavity. Clin Transl Oncol 2007;9:258-61. [Crossref] [PubMed]
- Owosho AA, Xu B, Kadempour A, et al. Metastatic solid tumors to the jaw and oral soft tissue: A retrospective clinical analysis of 44 patients from a single institution. J Craniomaxillofac Surg 2016;44:1047-53. [Crossref] [PubMed]
- Bar R, Netzer A, Ostrovsky D, et al. Abrupt tonsillar hemorrhage from a metastatic hemangiosarcoma of the breast: case report and literature review. Ear Nose Throat J 2011;90:116-20. [Crossref] [PubMed]
- Sera T, Kashiwagi S, Takashima T, et al. Multiple metastatic malignant phyllodes tumor of the breast with tonsillar metastasis: a case report. BMC Res Notes 2017;10:55. [Crossref] [PubMed]
- Maruzzo M, Giorgi CA, Marioni G, et al. Late onset (22 years) of simultaneous tonsillar and cervical lymph node metastases from breast ductal carcinoma. Am J Otolaryngol 2012;33:627-30. [Crossref] [PubMed]
- Barton TK, Kesterson GH, Wellman D, et al. Tonsillar metastasis from carcinoma of the breast with ultrastructural and steroid receptor analyses. Laryngoscope 1980;90:477-85. [Crossref] [PubMed]
- Saab GA, Abdul-Karim FW, Samara M. Breast carcinoma metastatic to the nasopharynx. J Laryngol Otol 1987;101:723-5. [Crossref] [PubMed]
- Copson B, Pratap U, McLean C, et al. Nasopharyngeal metastasis of breast carcinoma with HER 2 discordance: a case report. ANZ J Surg 2018;88:508-9. [Crossref] [PubMed]
- Murhekar K, Majhi U, Krishnamurthy A, et al. Diagnostic dilemma involving a mass in the parapharyngeal space: A metastatic breast carcinoma masquerading as a malignant salivary gland tumor. Indian J Nucl Med 2015;30:248-50. [Crossref] [PubMed]
- Raut V, Sinnathuray AR, McClean G, et al. Metastatic breast carcinoma in the parapharyngeal space. J Laryngol Otol 2001;115:750-2. [Crossref] [PubMed]
- Nguyen CH, Weitzner S. Metastatic carcinoma of breast in the hypopharynx. South Med J 1983;76:1590-1. [Crossref] [PubMed]
- Agrawal S, Jayant K, Agarwal RK, et al. An unusual case of metastatic male breast cancer to the nasopharynx-review of literature. Ann Palliat Med 2015;4:233-8. [PubMed]
- Schuler PJ, Heikaus S, Friebe-Hoffmann U, et al. Breast cancer metastases in the head and neck region. HNO 2010;58:859-65. [Crossref] [PubMed]
- Wanamaker JR, Kraus DH, Eliachar I, et al. Manifestations of metastatic breast carcinoma to the head and neck. Head Neck 1993;15:257-62. [Crossref] [PubMed]
- Weng B, Wang Q, Lin S, et al. Nasal cavity metastasis of breast cancer: a case report and review of the literature. Int J Clin Exp Pathol 2014;7:7028-33. [PubMed]
- Pusiol T, Franceschetti I, Bonfioli F, et al. Middle ear metastasis from dormant breast cancer as the initial sign of disseminated disease 20 years after quadrantectomy. Ear Nose Throat J 2013;92:121-4. [Crossref] [PubMed]
- Marques E, Brandis A, Samii M, et al. Late metastasis of breast adenocarcinoma into internal auditory canal and cerebellopontine angle: case report. Arq Neuropsiquiatr 2002;60:639-42. [Crossref] [PubMed]
- Çelik M, Şahin B, Tepe M, et al. Breast Cancer Metastasis on the Neck Mimicking a Glomus Tumor. J Craniofac Surg 2017;28:e271-3. [Crossref] [PubMed]
- Takei H, Rouah E, Barrios R. Intravascular carcinomatosis of central nervous system due to metastatic inflammatory breast cancer: A case report. Neuropathology 2015;35:456-61. [Crossref] [PubMed]
- Cooney BM, Ruth GJ, Behrman DA, et al. Malignant cystosarcoma phyllodes of the breast metastatic to the oral cavity: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol 1988;66:599-604. [Crossref] [PubMed]
- Scipio JE, Murti PR, Al-Bayaty HF, et al. Metastasis of breast carcinoma to mandibular gingiva. Oral Oncol 2001;37:393-6. [Crossref] [PubMed]
- Malhotra G, Nair N, Awasare S. F-18 FDG PET scan findings in a case of carcinoma of the breast with a rare site of metastases to the gingival region. Clin Nucl Med 2006;31:820-1. [Crossref] [PubMed]
- Eichhorn W, Wehrmann M, Blessmann M, et al. Metastases in odontogenic cysts: literature review and case presentation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;109:582-6. [Crossref] [PubMed]
- Kechagias N, Ntomouchtsis A, Patrikidou A, et al. Metastasis of a ductal breast carcinoma to the buccal mucosa of the mandible with tooth involvement. Oral Maxillofac Surg 2012;16:377-81. [Crossref] [PubMed]
- Friedrich RE, Abadi M. Distant metastases and malignant cellular neoplasms encountered in the oral and maxillofacial region: analysis of 92 patients treated at a single institution. Anticancer Res 2010;30:1843-8. [PubMed]
- Rajesh KS, Varma BR, Bhat KM. Metastasis to maxillary gingiva from carcinoma of breast. A case report. Indian J Dent Res 1998;9:23-7. [PubMed]
- Yang SI, Park KK, Kim JH. Thyroid metastasis from breast carcinoma accompanied by papillary thyroid carcinoma. Case Rep Oncol 2014;7:528-33. [Crossref] [PubMed]
- Bourcier K, Fermeaux V, Leobon S, et al. Lobular Breast Carcinoma Metastasis to the Thyroid Gland: Case Report and Literature Review. J Breast Cancer 2018;21:463-7. [Crossref] [PubMed]
- Ghias AF, Epps G, Cottrill E, Mardekian SK. Multifocal Metastatic Breast Carcinoma to the Thyroid Gland Histologically Mimicking C Cell Lesions. Case Rep Pathol 2019;2019:9890716. [Crossref] [PubMed]
- Kho MR, Abelardo AD. Isolated thyroid metastasis from benign phyllodes tumour. BMJ Case Rep 2018; [Crossref] [PubMed]
- Plonczak AM, DiMarco AN, Dina R, et al. Breast cancer metastases to the thyroid gland - an uncommon sentinel for diffuse metastatic disease: a case report and review of the literature. J Med Case Rep 2017;11:269. [Crossref] [PubMed]
- Debnam JM, Kwon M, Fornage BD, et al. Sonographic Evaluation of Intrathyroid Metastases. J Ultrasound Med 2017;36:69-76. [Crossref] [PubMed]
- Magers MJ, Dueber JC, Lew M, et al. Metastatic ductal carcinoma of the breast to the thyroid gland diagnosed with fine needle aspiration: A case report with emphasis on morphologic and immunophenotypic features. Diagn Cytopathol 2016;44:530-4. [Crossref] [PubMed]
- Rossi ED, Martini M, Straccia P, et al. Is thyroid gland only a "land" for primary malignancies? role of morphology and immunocytochemistry. Diagn Cytopathol 2015;43:374-80. [Crossref] [PubMed]
- HooKim K. Secondary tumors involving the thyroid gland: A multi-institutional analysis of 28 cases diagnosed on fine-needle aspiration. Diagn Cytopathol 2015;43:904-11. [Crossref] [PubMed]
- Kolarević D, Tomasević Z, Marković I, et al. Rare localisation of breast cancer metastasis to thyroid gland. Vojnosanit Pregl 2012;69:1106-8. [Crossref] [PubMed]
- Calzolari F, Sartori PV, Talarico C, et al. Surgical treatment of intrathyroid metastases: preliminary results of a multicentric study. Anticancer Res 2008;28:2885-8. [PubMed]
- Saber A, Ramzy S, Gouda I. Metastasis to the thyroid gland; unusual site of metastasis. Gulf J Oncolog 2007;1:51-7. [PubMed]
- Leboeuf R, Bénard F, Langlois MF. Thyroid cancer presenting as a PET incidentaloma in a patient with concomitant breast cancer metastases to the thyroid. Clin Nucl Med 2006;31:382-5. [Crossref] [PubMed]
- Gong Y, Jalali M, Staerkel G. Fine needle aspiration cytology of a thyroid metastasis of metaplastic breast carcinoma: a case report. Acta Cytol 2005;49:327-30. [Crossref] [PubMed]
- Lam KY, Lo CY. Metastatic tumors of the thyroid gland: a study of 79 cases in Chinese patients. Arch Pathol Lab Med 1998;122:37-41. [PubMed]
- Ferrara G, Ianniello GP, Nappi O. Thyroid metastases from a ductal carcinoma of the breast. A case report. Tumori 1997;83:783-7. [Crossref] [PubMed]
- Rosen IB, Bedard YC, Walfish PG, et al. Metastasis of cancer of the thyroid gland as a cause of goitre. Can Med Assoc J 1978;118:1265-8. [PubMed]
- Lee SH, Kim BH, Bae MJ, et al. Concurrence of primary hyperparathyroidism and metastatic breast carcinoma affected a parathyroid gland. J Clin Endocrinol Metab 2013;98:3127-30. [Crossref] [PubMed]
- Watanabe T, Adachi I, Kimura S, et al. A case of advanced breast cancer associated with hypocalcemia. Jpn J Clin Oncol 1983;13:441-8. [PubMed]
- Ghosn M, Ghayad E, Biagini J, et al. Hypothalamo-hypophyseal and gastric metastasis of a breast neoplasm. Clinical case and a review of the literature. Bull Cancer 1991;78:1071-3. [PubMed]
- Fukunaga A, Yazaki T, Shimizu K, et al. A case of pituitary metastasis in a patient with male breast cancer developing anterior lobe dysfunction successfully treated by using hormone replacement therapy. No Shinkei Geka 2014;42:629-33. [PubMed]
- Rozen WM, Mann GB. Angiosarcoma arising in an unirradiated breast with subsequent pituitary metastasis. Clin Breast Cancer 2007;7:811-3. [Crossref] [PubMed]
- Poursadegh Fard M, Borhani Haghighi A, Bagheri MH. Breast cancer metastasis to pituitary infandibulum. Iran J Med Sci 2011;36:141-4. [PubMed]
- Nose K, Ogata T, Tsugawa J, et al. Pituitary metastasis of breast cancer mimicking IgG4-related hypophysitis. eNeurologicalSci 2018;14:13-5. [Crossref] [PubMed]
- Castle-Kirszbaum M, Goldschlager T, Ho B, et al. Twelve cases of pituitary metastasis: a case series and review of the literature. Pituitary 2018;21:463-73. [Crossref] [PubMed]
- Kam J, Mann GB, Phillips C, et al. Solitary pituitary metastasis from HER2-positive breast cancer. Asia Pac J Clin Oncol 2017;13:e181-e4. [Crossref] [PubMed]
- Ravnik J, Smigoc T, Bunc G, et al. Hypophyseal metastases: A report of three cases and literature review. Neurol Neurochir Pol 2016;50:511-6. [Crossref] [PubMed]
- Burkhardt T, Henze M, Kluth LA, et al. Surgical management of pituitary metastases. Pituitary 2016;19:11-8. [Crossref] [PubMed]
- Gormally JF, Izard MA, Robinson BG, et al. Pituitary metastasis from breast cancer presenting as diabetes insipidus. BMJ Case Rep 2014; [Crossref] [PubMed]
- Magalhães JF, Bacchin RP, Costa PS, et al. Breast cancer metastasis to the pituitary gland. Arq Bras Endocrinol Metabol 2014;58:869-72. [Crossref] [PubMed]
- Spinelli GP, Lo Russo G, Miele E, et al. Breast cancer metastatic to the pituitary gland: a case report. World J Surg Oncol 2012;10:137. [Crossref] [PubMed]
- Naqi R, Azeemuddin M. Solitary metastasis of breast carcinoma in the infundibulum. J Pak Med Assoc 2012;62:169-70. [PubMed]
- Dogan M, Karakilic E, Oz II, et al. Breast cancer with diabetes insipidus. Exp Oncol 2008;30:324-6. [PubMed]
- Gołkowski F, Trofimiuk M, Czepko R, et al. Two rare cases of pituitary metastases from breast and kidney cancers. Exp Clin Endocrinol Diabetes 2007;115:537-40. [Crossref] [PubMed]
- Kurkjian C, Armor JF, Kamble R, et al. Symptomatic metastases to the pituitary infundibulum resulting from primary breast cancer. Int J Clin Oncol 2005;10:191-4. [Crossref] [PubMed]
- Sturm I, Kirschke S, Krahl D, et al. Panhypopituitarism in a patient with breast cancer. Onkologie 2004;27:480-2. [PubMed]
- Ruiz Hernández G, Chesa-Jiménez J, San Martín Ciges E, et al. Panhypopituitarism as a consequence of metastase of breast cancer in sella turcica. Nuklearmedizin 1996;35:143-5. [Crossref] [PubMed]
- Paulus P, Paridaens R, Mockel J, et al. Argyrophilic breast carcinoma, single metastasis to the pituitary gland. Bull Cancer 1990;77:377-84. [PubMed]
- Yap HY, Tashima CK, Blumenschein GR, et al. Diabetes insipidus and breast cancer. Arch Intern Med 1979;139:1009-11. [Crossref] [PubMed]
- Teears RJ, Silverman EM. Clinicopathologic review of 88 cases of carcinoma metastatic to the putuitary gland. Cancer 1975;36:216-20. [Crossref] [PubMed]
- Flis DW, Shah AT, Tracy JC, et al. Metastatic breast carcinoma of the jugular foramen: a rare case of Villaret syndrome. Head Neck 2015;37:E146-9. [Crossref] [PubMed]
- Khaw K, Ramli N, Rahmat K. Ptosis due to cavernous sinus syndrome as a rare presentation of advanced breast metastasis in a patient with delayed diagnosis. Malays Fam Physician 2012;7:31-3. [PubMed]
- Kim DH, Son SM, Choi YJ. Gastric metastasis from invasive lobular breast cancer, mimicking primary gastric cancer: A case report. Medicine (Baltimore) 2018;97:e0258. [Crossref] [PubMed]
- Rampado S, Ruol A, Guido M, et al. Mediastinal carcinosis involving the esophagus in breast cancer: the "breast-esophagus" syndrome: report on 25 cases and guidelines for diagnosis and treatment. Ann Surg 2007;246:316-22. [Crossref] [PubMed]
- Fukunaga A, Sasamura Y, Takada A, et al. Solitary thymic metastasis of breast cancer 13 years after surgery. Asian Cardiovasc Thorac Ann 2017;25:469-71. [Crossref] [PubMed]
- Fujioka S, Nakamura H, Miwa K, et al. Thymic metastasis of breast cancer 22 years after surgery: a case report. Asian J Endosc Surg 2013;6:330-2. [Crossref] [PubMed]
- Park SB, Kim HH, Shin HJ, et al. Thymic metastasis in breast cancer: a case report. Korean J Radiol 2007;8:360-3. [Crossref] [PubMed]
- Bhojwani N, Harwani A, Hartman JB, et al. Metastatic Breast Carcinoma to the Coronaries: A Rare Cause of Acute Myocardial Infarction. Methodist Debakey Cardiovasc J 2016;12:179-82. [Crossref] [PubMed]
- Sandhu HS, Mahendrakar SKM, Ladhani SS, et al. Superior Vena Cava as Gateway to Heart: Metastatic Breast Carcinoma Causing Ball in a Loop Metastasis to Right Atrium. J Clin Diagn Res 2017;11:XD03-5. [PubMed]
- Bhambhani A, Ayyagari S, Mohapatra T, et al. Malignant phyllodes tumor of the left atrium. Indian Heart J 2014;66:241-3. [Crossref] [PubMed]
- Katalinic D, Stern-Padovan R, Ivanac I, et al. Symptomatic cardiac metastases of breast cancer 27 years after mastectomy: a case report with literature review--pathophysiology of molecular mechanisms and metastatic pathways, clinical aspects, diagnostic procedures and treatment modalities. World J Surg Oncol 2013;11:14. [Crossref] [PubMed]
- Eminowicz G, Achilleos K, Payne S, et al. Solitary atrial metastases: a rare clinical challenge. BMJ Case Rep 2011; [Crossref] [PubMed]
- Garg N, Moorthy N, Agrawal SK, et al. Delayed cardiac metastasis from phyllodes breast tumor presenting as cardiogenic shock. Tex Heart Inst J 2011;38:441-4. [PubMed]
- Kawase T, Fujii H, Nakahara T, et al. Intense accumulation of Tc-99m MDP in pericardial metastasis from breast cancer. Clin Nucl Med 2009;34:173-4. [Crossref] [PubMed]
- Broom RJ, Harvey VJ, Lee KM. A durable complete remission between two isolated presentations of metastatic breast cancer, the second with intracardiac disease. Clin Breast Cancer 2006;7:162-3. [Crossref] [PubMed]
- Lieberman EB, Arthur J, Steenbergen C, et al. Antemortem diagnosis of an endomyocardial breast cancer metastasis by transvenous endomyocardial biopsy. Chest 1993;103:1280-1. [Crossref] [PubMed]
- Labib SB, Schick EC, Isner JM. Obstruction of right ventricular outflow tract caused by intracavitary metastatic disease: analysis of 14 cases. J Am Coll Cardiol 1992;19:1664-8. [Crossref] [PubMed]
- Wada Y, Harada N, Ohara K, et al. Esophageal metastasis of breast carcinoma. Breast Cancer 2009;16:151-6. [Crossref] [PubMed]
- Anaya DA, Yu M, Karmy-Jones R. Esophageal perforation in a patient with metastatic breast cancer to esophagus. Ann Thorac Surg 2006;81:1136-8. [Crossref] [PubMed]
- Koike M, Akiyama S, Kodera Y, et al. Breast carcinoma metastasis to the esophagus: report of two cases. Hepatogastroenterology 2005;52:1116-8. [PubMed]
- McLemore EC, Pockaj BA, Reynolds C, et al. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol 2005;12:886-94. [Crossref] [PubMed]
- Sunada F, Yamamoto H, Kita H, et al. A case of esophageal stricture due to metastatic breast cancer diagnosed by endoscopic mucosal resection. Jpn J Clin Oncol 2005;35:483-6. [Crossref] [PubMed]
- Erman M, Karaoğlu A, Oksüzoğlu B, et al. Solitary esophageal metastasis of breast cancer after 11 years: a case report. Med Oncol 2002;19:171-5. [Crossref] [PubMed]
- Simchuk EJ, Low DE. Direct esophageal metastasis from a distant primary tumor is a submucosal process: a review of six cases. Dis Esophagus 2001;14:247-50. [Crossref] [PubMed]
- Varanasi RV, Saltzman JR, Krims P, et al. Breast carcinoma metastatic to the esophagus: clinicopathological and management features of four cases, and literature review. Am J Gastroenterol 1995;90:1495-9. [PubMed]
- Herrera JL. Case report: esophageal metastasis from breast carcinoma presenting as achalasia. Am J Med Sci 1992;303:321-3. [Crossref] [PubMed]
- Roostaeian J, Yoon AP, Sanchez IS, et al. The effect of prior abdominal surgery on abdominally based free flaps in breast reconstruction. Plast Reconstr Surg 2014;133:247e-55e. [Crossref] [PubMed]
- Malhotra A, Guturu P, Basim MS, et al. A rare case of breast cancer metastasis presenting as linitis plastica of the stomach and colon (with videos). Gastrointest Endosc 2009;70:552-3; discussion 553. [Crossref] [PubMed]
- Hara F, Kiyoto S, Takabatake D, et al. Metastatic Breast Cancer to the Stomach Resembling Early Gastric Cancer. Case Rep Oncol 2010;3:142-7. [Crossref] [PubMed]
- Dória MT, Maesaka JY, Martins SN, et al. Gastric metastasis as the first manifestation of an invasive lobular carcinoma of the breast. Autops Case Rep 2015;5:49-53. [Crossref] [PubMed]
- Geredeli C, Dogru O, Omeroglu E, et al. Gastric Metastasis of Triple Negative Invasive Lobular Carcinoma. Rare Tumors 2015;7:5764. [Crossref] [PubMed]
- Ricciuti B, Leonardi GC, Ravaioli N, et al. Ductal Breast Carcinoma Metastatic to the Stomach Resembling Primary Linitis Plastica in a Male Patient. J Breast Cancer 2016;19:324-9. [Crossref] [PubMed]
- Dos Santos Fernandes G, Batista Bugiato Faria LD, de Assis Pereira I, et al. Gastric Metastasis of Breast Cancer: A Case Series. Rare Tumors 2016;8:6305. [Crossref] [PubMed]
- Villa Guzmán JC, Espinosa J, Cervera R, et al. Gastric and colon metastasis from breast cancer: case report, review of the literature, and possible underlying mechanisms. Breast Cancer (Dove Med Press) 2016;9:1-7. [Crossref] [PubMed]
- Jmour O, Belaïd A, Mghirbi F, et al. Gastric metastasis of bilateral breast cancer. J Gastrointest Oncol 2017;8:E16-20. [Crossref] [PubMed]
- Khan I, Malik R, Khan A, et al. Breast Cancer Metastases to the Gastrointestinal Tract Presenting with Anemia and Intra-abdominal Bleed. Cureus 2017;9:e1429. [PubMed]
- Kliiger J, Gorbaty M. Metastasis to the pancreas and stomach from a breast cancer primary: a case report. J Community Hosp Intern Med Perspect 2017;7:234-7. [Crossref] [PubMed]
- Ulmer LL, Cormier I, Jha LK, et al. Use of Endoscopic Ultrasound in a Diagnostic Dilemma: Metastatic Breast Cancer to the Stomach. Case Rep Gastrointest Med 2018;2018:2820352. [PubMed]
- Bushan K, Kammar P, Singh C, et al. Infiltrating Lobular Breast Cancer Presenting as Isolated Gastric Metastasis: a Case Report. Indian J Surg Oncol 2018;9:318-22. [Crossref] [PubMed]
- Güler SA, Şimşek T, Pösteki G, et al. A Very Rare Reason for Gastric Perforation, Caused by Gastric Metastasis of Breast Cancer: Case Presentation. Eur J Breast Health 2018;15:59-62. [Crossref] [PubMed]
- Woo J, Lee JH, Lee KE, et al. Gastric Metastasis as the First Presentation One Year Before Diagnosis of Primary Breast Cancer. Am J Case Rep 2018;19:354-9. [Crossref] [PubMed]
- Gurzu S, Banias L, Bara T, et al. The Epithelial-Mesenchymal Transition Pathway in Two Cases with Gastric Metastasis Originating from Breast Carcinoma, One with a Metachronous Primary Gastric Cancer. Recent Pat Anticancer Drug Discov 2018;13:118-24. [Crossref] [PubMed]
- Yim K, Ro SM, Lee J. Breast cancer metastasizing to the stomach mimicking primary gastric cancer: A case report. World J Gastroenterol 2017;23:2251-7. [Crossref] [PubMed]
- Choi DI, Chi HS, Lee SH, et al. A Rare Case of Phyllodes Tumor Metastasis to the Stomach Presenting as Anemia. Cancer Res Treat 2017;49:846-9. [Crossref] [PubMed]
- Mullally WJ, O'Súilleabháin CB, Brady C, et al. Vinorelbine induced perforation of a metastatic gastric lesion. Ir J Med Sci 2017;186:571-5. [Crossref] [PubMed]
- Rodrigues MV, Tercioti-Junior V, Lopes LR, et al. Breast cancer metastasis in the stomach: when the gastrectomy is indicated? Arq Bras Cir Dig 2016;29:86-9. [Crossref] [PubMed]
- Zuhair AR, Maron AR. Occult bilateral invasive lobular carcinoma of the breast presenting as gastroduodenal metastases: a case report. Breast Dis 2015;35:63-5. [Crossref] [PubMed]
- Rachan Shetty KS, Challa VR, Lakshmaiah KC, et al. Gastric metastases from breast cancer: A report of two cases and review of literature. J Cancer Res Ther 2015;11:660. [Crossref] [PubMed]
- Wysocka K, Okoń K, Matyja A. Breast cancer metastatic to the gastric wall. Pol J Pathol 2011;62:282-5. [PubMed]
- Critchley AC, Harvey J, Carr M, et al. Synchronous gastric and colonic metastases of invasive lobular breast carcinoma: case report and review of the literature. Ann R Coll Surg Engl 2011;93:e49-50. [Crossref] [PubMed]
- Almubarak MM, Laé M, Cacheux W, et al. Gastric metastasis of breast cancer: a single centre retrospective study. Dig Liver Dis 2011;43:823-7. [Crossref] [PubMed]
- Ghirarduzzi A, Sivelli R, Martella E, et al. Gastric metastasis from breast carcinoma. Report of three cases, diagnostic-therapeutic critical close examination and literature review. Ann Ital Chir 2010;81:141-6. [PubMed]
- Yamamoto D, Yoshida H, Sumida K, et al. Gastric tumor from metastasis of breast cancer. Anticancer Res 2010;30:3705-8. [PubMed]
- Trouillet N, Robert B, Charfi S, et al. Gastric metastases. An endoscopic series of ten cases. Gastroenterol Clin Biol 2010;34:305-9. [Crossref] [PubMed]
- Ellis MC, Mason T, Barnett J, et al. Gastric malignancies in breast cancer survivors: pathology and outcomes. Am J Surg 2009;197:633-6. [Crossref] [PubMed]
- Ciulla A, Castronovo G, Tomasello G, et al. Gastric metastases originating from occult breast lobular carcinoma: diagnostic and therapeutic problems. World J Surg Oncol 2008;6:78. [Crossref] [PubMed]
- Jhaveri A, Nagral A, Dhaber B, et al. Chronic diarrhea--an unusual presentation of metastatic invasive lobular cancer of breast. Indian J Gastroenterol 2006;25:312-3. [PubMed]
- Savanis G, Simatos G, Tzaida O, et al. Gastrointestinal tract metastasis as first presentation of breast cancer. J BUON 2006;11:79-81. [PubMed]
- Whitty LA, Crawford DL, Woodland JH, et al. Metastatic breast cancer presenting as linitis plastica of the stomach. Gastric Cancer 2005;8:193-7. [Crossref] [PubMed]
- Akcali Z, Sakalli H, Ozyilkan O, et al. Prognostically favorable abdominal breast cancer metastases with stomach involvement. Onkologie 2005;28:270-2. [PubMed]
- Tremblay F, Jamison B, Meterissian S. Breast cancer masquerading as a primary gastric carcinoma. J Gastrointest Surg 2002;6:614-6. [Crossref] [PubMed]
- Oda Kondo H. Metastatic tumors to the stomach: analysis of 54 patients diagnosed at endoscopy and 347 autopsy cases. Endoscopy 2001;33:507-10. [Crossref] [PubMed]
- Pera M, Riera E, Lopez R, et al. Metastatic carcinoma of the breast resembling early gastric carcinoma. Mayo Clin Proc 2001;76:205-7. [Crossref] [PubMed]
- Washington K, McDonagh D. Secondary tumors of the gastrointestinal tract: surgical pathologic findings and comparison with autopsy survey. Mod Pathol 1995;8:427-33. [PubMed]
- Houghton AD, Pheils P. Isolated duodenal metastasis from breast carcinoma. Eur J Surg Oncol 1987;13:367-9. [PubMed]
- Sarkar N, Kejariwal D, Roy S. Isolated duodenal metastasis from breast carcinoma. J Assoc Physicians India 2002;50:962-3. [PubMed]
- Sato T, Muto I, Hasegawa M, et al. Breast signet-ring cell lobular carcinoma presenting with duodenal obstruction and acute pancreatitis. Asian J Surg 2007;30:220-3. [Crossref] [PubMed]
- Jones C, Tong AW, Mir M, et al. Lobular carcinoma of the breast with gastrointestinal metastasis. Proc (Bayl Univ Med Cent) 2015;28:50-3. [Crossref] [PubMed]
- Giestas S, Lopes S, Souto P, et al. Ampullary Metastasis From Breast Cancer: A Rare Cause of Obstructive Jaundice. GE Port J Gastroenterol 2016;23:300-3. [Crossref] [PubMed]
- Lin Y, Wong SI, Wang Y, et al. Periampullary Metastases from Breast Cancer: A Case Report and Literature Review. Case Rep Oncol Med 2019;2019:3479568. [PubMed]
- Wang X, Jin M, Ye Q, et al. Solitary duodenum metastasis from breast cancer with 8 years' latency: A case report. Medicine (Baltimore) 2018;97:e9550. [Crossref] [PubMed]
- Zhao R, Li Y, Yu X, et al. Duodenal metastasis from recurrent invasive lobular carcinoma of breast: a case report and literature review. Int J Clin Oncol 2012;17:160-4. [Crossref] [PubMed]
- Asoglu O, Karanlik H, Barbaros U, et al. Malignant phyllode tumor metastatic to the duodenum. World J Gastroenterol 2006;12:1649-51. [Crossref] [PubMed]
- Lottini M, Neri A, Vuolo G, et al. Duodenal obstruction from isolated breast cancer metastasis: a case report. Tumori 2002;88:427-9. [Crossref] [PubMed]
- Titus AS, Baron TH, Listinsky CM, et al. Solitary breast metastasis to the ampulla and distal common bile duct. Am Surg 1997;63:512-5. [PubMed]
- Hernández V, Flor-Lorente B, Burgués O, et al. Anasarca as presentation of lobular breast carcinoma. Gastroenterol Hepatol 2000;23:338-40. [PubMed]
- Choi JE, Park SY, Jeon MH, et al. Solitary small bowel metastasis from breast cancer. J Breast Cancer 2011;14:69-71. [Crossref] [PubMed]
- Liu M, Zhang L, Guo L, et al. Intestinal metastasis from breast invasive ductal carcinoma after a long latency: case report and literature review. Onco Targets Ther 2018;11:8599-603. [Crossref] [PubMed]
- Bilen MA, Laucirica R, Rimawi MF, et al. Jejunal intussusception due to malignant phyllodes tumor of the breast. Clin Breast Cancer 2012;12:219-21. [Crossref] [PubMed]
- Cho DH, Jeon YS, Choi MY, et al. Ileal metastasis of breast cancer in a patient with a BRCA2 gene mutation: report of a case. Surg Today 2011;41:1665-9. [Crossref] [PubMed]
- Mouawad NJ, Cleary RK. Small bowel obstruction as the primary presentation of undiagnosed metastatic lobular breast carcinoma. Breast Dis 2011;33:35-40. [Crossref] [PubMed]
- Kelly RJ, Barrett C, Swan N, et al. Metastatic phyllodes tumor causing small-bowel obstruction. Clin Breast Cancer 2009;9:193-5. [Crossref] [PubMed]
- Oyasiji T, Shoemake P, Bakhos C, et al. Small bowel obstruction from metastatic breast cancer masquerading as an obstructed incisional hernia. Conn Med 2009;73:403-6. [PubMed]
- Al-Qahtani MS. Gut metastasis from breast carcinoma. Saudi Med J 2007;28:1590-2. [PubMed]
- Haberstich R, Tuech JJ, Wilt M, et al. Anal localization as first manifestation of metastatic ductal breast carcinoma. Tech Coloproctol 2005;9:237-8. [Crossref] [PubMed]
- Titi MA, Anabtawi A, Newland AD. Isolated gastrointestinal metastasis of breast carcinoma: a case report. Case Rep Med 2010;2010:615923. [PubMed]
- Okido M, Seo M, Hamada Y, et al. Metastatic breast carcinoma simulating linitis plastica of the colon: report of a case. Surg Today 2011;41:542-5. [Crossref] [PubMed]
- Saranovic D, Kovac JD, Knezevic S, et al. Invasive lobular breast cancer presenting an unusual metastatic pattern in the form of peritoneal and rectal metastases: a case report. J Breast Cancer 2011;14:247-50. [Crossref] [PubMed]
- Mistrangelo M, Cassoni P, Castellano I, et al. Obstructive colon metastases from lobular breast cancer: report of a case and review of the literature. Tumori 2011;97:800-4. [Crossref] [PubMed]
- Cherian N, Qureshi NA, Cairncross C, et al. Invasive lobular breast carcinoma metastasising to the rectum. BMJ Case Rep 2017;2017. [PubMed]
- Falco G, Mele S, Zizzo M, et al. Colonic metastasis from breast carcinoma detection by CESM and PET/CT: A case report. Medicine (Baltimore) 2018;97:e10888. [Crossref] [PubMed]
- Blachman-Braun R, Felemovicius I, Barker K, et al. Widespread metastatic breast cancer to the bowel: an unexpected finding during colonoscopy. Oxf Med Case Reports 2019;2019:omy133. [PubMed]
- Samra B, Ghanem S, Ilyas G, et al. Screening Colonoscopy Unmasking Colonic Metastasis from an Occult Breast Ductal Carcinoma: A Case Report and Review of the Literature. Case Rep Oncol Med 2019;2019:8432079. [Crossref] [PubMed]
- Schellenberg AE, Wood ML, Baniak N, et al. Metastatic ductal carcinoma of the breast to colonic mucosa. BMJ Case Rep 2018; [Crossref] [PubMed]
- Ruymbeke H, Harlet L, Stragier B, et al. Anorectal metastasis from breast carcinoma: a case report and review of the literature. BMC Res Notes 2018;11:268. [Crossref] [PubMed]
- Tsujimura K, Teruya T, Kiyuna M, et al. Colonic metastasis from breast carcinoma: a case report. World J Surg Oncol 2017;15:124. [Crossref] [PubMed]
- Buka D, Dvořák J, Richter I, et al. Gastric and Colorectal Metastases of Lobular Breast Carcinoma: A Case Report. Acta Medica (Hradec Kralove) 2016;59:18-21. [Crossref] [PubMed]
- Rengifo C, Titi S, Walls J. Anal metastasis as the sentinel and isolated presentation of invasive ductal breast carcinoma. Ann R Coll Surg Engl 2016;98:e68-70. [Crossref] [PubMed]
- Mroz A, Kiedrowski M. An unusual case of colonic adenocarcinoma development in the region of disseminating lobular breast carcinoma infiltration: diagnostic approach and review of the literature. Int J Clin Exp Pathol 2015;8:7470-4. [PubMed]
- Zhou XC, Zhou H, Ye YH, et al. Invasive ductal breast cancer metastatic to the sigmoid colon. World J Surg Oncol 2012;10:256. [Crossref] [PubMed]
- Takeuchi H, Hiroshige S, Yoshikawa Y, et al. A case of synchronous metastasis of breast cancer to stomach and colon. Anticancer Res 2012;32:4051-5. [PubMed]
- Bochicchio A, Tartarone A, Ignomirelli O, et al. Anal metastasis from breast cancer: a case report and review of the literature. Future Oncol 2012;8:333-6. [Crossref] [PubMed]
- Gerova VA, Tankova LT, Mihova AA, et al. Gastrointestinal metastases from breast cancer: report of two cases. Hepatogastroenterology 2012;59:178-81. [PubMed]
- Nikolić I, Ivković-Kapicl T, Kukić B, et al. Uncommon metastatic site from breast cancer. Vojnosanit Pregl 2012;69:806-8. [Crossref] [PubMed]
- Amin AA, Reddy A, Jha M, et al. Rectal metastasis from breast cancer: an interval of 17 years. BMJ Case Rep 2011; [Crossref] [PubMed]
- Razzetta F, Tassara E, Saro F, et al. Rare abdominal metastases from occult lobular breast cancer: report of two cases. Updates Surg 2011;63:129-33. [Crossref] [PubMed]
- Efthimiadis C, Kosmidis C, Fotiadis P, et al. Breast cancer metastatic to the rectum: a case report. Tech Coloproctol 2011;15:S91-3. [Crossref] [PubMed]
- Balja MP, Vrdoljak DV, Stanec M, et al. Rectal metastasis from lobular carcinoma of the breast: a case report. Coll Antropol 2010;34:719-21. [PubMed]
- Théraux J, Bretagnol F, Guedj N, et al. Colorectal breast carcinoma metastasis diagnosed as an obstructive colonic primary tumor. A case report and review of the literature. Gastroenterol Clin Biol 2009;33:1114-7. [Crossref] [PubMed]
- Birla R, Mahawar KK, Orizu M, et al. Caecal metastasis from breast cancer presenting as intestinal obstruction. World J Surg Oncol 2008;6:47. [Crossref] [PubMed]
- Uygun K, Kocak Z, Altaner S, et al. Colonic metastasis from carcinoma of the breast that mimics a primary intestinal cancer. Yonsei Med J 2006;47:578-82. [Crossref] [PubMed]
- Signorelli C, Pomponi-Formiconi D, Nelli F, et al. Single colon metastasis from breast cancer: a clinical case report. Tumori 2005;91:424-7. [Crossref] [PubMed]
- Law WL, Chu KW. Scirrhous colonic metastasis from ductal carcinoma of the breast: report of a case. Dis Colon Rectum 2003;46:1424-7. [Crossref] [PubMed]
- Dhar S, Kulaylat MN, Gordon K, et al. Solitary papillary breast carcinoma metastasis to the large bowel presenting as primary colon carcinoma: case report and review of the literature. Am Surg 2003;69:799-803. [PubMed]
- Bamias A, Baltayiannis G, Kamina S, et al. Rectal metastases from lobular carcinoma of the breast: report of a case and literature review. Ann Oncol 2001;12:715-8. [Crossref] [PubMed]
- Koutsomanis D, Renier JF, Ollivier R, et al. Colonic metastasis of breast carcinoma. Hepatogastroenterology 2000;47:681-2. [PubMed]
- Flamme F, Jacobowitz D, Feoli F, et al. Diminutive polyp: a rare presentation of breast cancer metastases to the colon. Acta Gastroenterol Belg 1994;57:260-3. [PubMed]
- Rabau MY, Alon RJ, Werbin N, et al. Colonic metastases from lobular carcinoma of the breast. Report of a case. Dis Colon Rectum 1988;31:401-2. [Crossref] [PubMed]
- Balibrea JM, Cantero R, García-Calvo M, et al. Perianal metastases from lobular breast carcinoma. Clin Transl Oncol 2007;9:606-9. [Crossref] [PubMed]
- Dirksen JL, Souder MG, Burick AJ. Metastatic Breast Carcinoma Presenting as Perforated Appendicitis. Breast Care (Basel) 2010;5:409-10. [Crossref] [PubMed]
- Hardt W, Parent B, Januschka J, et al. A rare case of an isolated metastasis of the head of the pancreas of a breast cancer with obstruction of the efferent bile ducts. Pathologe 1993;14:167-71. [PubMed]
- Stoeckler F, Hagmüller E, Rumpelt HJ, et al. A rare cause of distal bile duct stenosis. J Gastrointest Cancer 2007;38:157-9. [Crossref] [PubMed]
- Bonapasta SA, Gregori M, Lanza R, et al. Metastasis to the Pancreas from Breast Cancer: Difficulties in Diagnosis and Controversies in Treatment. Breast Care (Basel) 2010;5:170-3. [Crossref] [PubMed]
- Takamizawa J, Kuze S, Kyokane T, et al. A case of pancreatic metastasis from breast cancer during multimodality therapy. Gan To Kagaku Ryoho 2011;38:301-3. [PubMed]
- Inoue M, Ohta T, Shioya H, et al. Inflammatory myofibroblastic tumors of the breast with simultaneous intracranial, lung, and pancreas involvement: ultrasonographic findings and a review of the literature. J Med Ultrason (2001) 2018;45:331-5. [Crossref] [PubMed]
- Sun X, Zuo K, Huang D, et al. Pancreatic metastasis from invasive pleomorphic lobular carcinoma of the breast: a rare case report. Diagn Pathol 2017;12:52. [Crossref] [PubMed]
- Song SW, Cheng JF, Liu N, et al. Diagnosis and treatment of pancreatic metastases in 22 patients: a retrospective study. World J Surg Oncol 2014;12:299. [Crossref] [PubMed]
- Molino C, Mocerino C, Braucci A, et al. Pancreatic solitary and synchronous metastasis from breast cancer: a case report and systematic review of controversies in diagnosis and treatment. World J Surg Oncol 2014;12:2. [Crossref] [PubMed]
- Lam WW, Loke KS, Loi HY, et al. Pancreatic metastasis detected by F-18 FDG PET/CT in a patient with breast cancer. Clin Nucl Med 2011;36:479-80. [Crossref] [PubMed]
- Mourra N, Arrive L, Balladur P, et al. Isolated metastatic tumors to the pancreas: Hôpital St-Antoine experience. Pancreas 2010;39:577-80. [Crossref] [PubMed]
- Ang TL, Ng VW, Fock KM, et al. Diagnosis of a metastatic phyllodes tumor of the pancreas using EUS-FNA. JOP 2007;8:35-8. [PubMed]
- Crippa S, Angelini C, Mussi C, et al. Surgical treatment of metastatic tumors to the pancreas: a single center experience and review of the literature. World J Surg 2006;30:1536-42. [Crossref] [PubMed]
- Haque S, Gopaldas RR, Plymyer MR, et al. Pancreatic mass of unusual etiology: case report of metastatic disease after a prolonged lag phase. Am Surg 2005;71:1082-5. [PubMed]
- Z'graggen K, Fernández-del Castillo C, Rattner DW, et al. Metastases to the pancreas and their surgical extirpation. Arch Surg 1998;133:413-7; discussion 418-9. [Crossref] [PubMed]
- Mountney J, Maury AC, Jackson AM, et al. Pancreatic metastases from breast cancer: an unusual cause of biliary obstruction. Eur J Surg Oncol 1997;23:574-6. [Crossref] [PubMed]
- Shan J, Zhang S, Wang Z, et al. Breast malignant phyllodes tumor with rare pelvic metastases and long-term overall survival: A case report and literature review. Medicine (Baltimore) 2016;95:e4942. [Crossref] [PubMed]
- Osaku T, Ogata H, Magoshi S, et al. Metastatic nonpalpable invasive lobular breast carcinoma presenting as rectal stenosis: a case report. J Med Case Rep 2015;9:88. [Crossref] [PubMed]
- Cardi M, Sammartino P, Framarino ML, et al. Treatment of peritoneal carcinomatosis from breast cancer by maximal cytoreduction and HIPEC: a preliminary report on 5 cases. Breast 2013;22:845-9. [Crossref] [PubMed]
- D'Annibale M, Esposito A, Boschetto A, et al. Solitary peritoneal metastases from ductal breast cancer. Chir Ital 2007;59:191-6. [PubMed]
- Kobayashi T, Adachi S, Matsuda Y, et al. A case of metastatic lobular breast carcinoma with detection of the primary tumor after ten years. Breast Cancer 2007;14:333-6. [Crossref] [PubMed]
- Doval DC, Bhatia K, Pavithran K, et al. Breast carcinoma with metastasis to the gallbladder: an unusual case report with a short review of literature. Hepatobiliary Pancreat Dis Int 2006;5:305-7. [PubMed]
- Markelov A, Taheri H, Vunnamadala K, et al. Biliary dyskinesia as a rare presentation of metastatic breast carcinoma of the gallbladder: a case report. Case Rep Pathol 2011;2011:806570. [PubMed]
- Di Vita M, Zanghì A, Lanzafame S, et al. Gallbladder metastases of breast cancer: from clinical-pathological patterns to diagnostic and therapeutic strategy. Clin Ter 2011;162:451-6. [PubMed]
- Urade T, Oka S, Iimori S, et al. A resected case of gallbladder metastasis with symptoms of acute cholecystitis in multiple metastatic ductal carcinoma of the breast. Clin J Gastroenterol 2019;12:52-6. [Crossref] [PubMed]
- Zamkowski M, Kąkol M, Makarewicz W, et al. Patient with metastatic breast cancer presenting as acute cholecystitis with one-year survival on hormonotherapy. Pol Przegl Chir 2017;89:46-9. [Crossref] [PubMed]
- Abdelilah B, Mohamed O, Yamoul R, et al. Acute cholecystitis as a rare presentation of metastatic breast carcinoma of the gallbladder: A case report and review of the literature. Pan Afr Med J 2014;17:216. [Crossref] [PubMed]
- Manouras A, Lagoudianakis EE, Genetzakis M, et al. Metastatic breast carcinoma initially presenting as acute cholecystitis: a case report and review of the literature. Eur J Gynaecol Oncol 2008;29:179-81. [PubMed]
- Zagouri F, Sergentanis TN, Koulocheri D, et al. Bilateral synchronous breast carcinomas followed by a metastasis to the gallbladder: a case report. World J Surg Oncol 2007;5:101. [Crossref] [PubMed]
- Bartolotti M, Franceschi E, Di Battista M, et al. Cytologically confirmed splenic metastases in breast cancer. Future Oncol 2012;8:1495-500. [Crossref] [PubMed]
- El Fadli M, Kerrou K, Alaoui Mhamdi H, et al. Breast cancer metastasis to the spleen: a case report and literature review. Oxf Med Case Reports 2017;2017:omx069. [PubMed]
- Sufficool K, Wang J, Doherty S. Isolated splenic metastasis from carcinoma of the breast: a case report. Diagn Cytopathol 2013;41:914-6. [PubMed]
- Foroudi F, Ahern V, Peduto A. Splenosis mimicking metastases from breast carcinoma. Clin Oncol (R Coll Radiol) 1999;11:190-2. [Crossref] [PubMed]
- Chapel F, Baume D, Bereder JM. Unusual vascular changes in the red pulp of the spleen accompanying breast carcinoma metastasis. Pathol Res Pract 1999;195:53-6; discussion 57-8. [Crossref] [PubMed]
- Cummings OW, Mazur MT. Breast carcinoma diffusely metastatic to the spleen. A report of two cases presenting as idiopathic thrombocytopenic purpura. Am J Clin Pathol 1992;97:484-9. [Crossref] [PubMed]
- Kykalos S, Mantas D, Dimitroulis D, et al. A rare case of breast cancer metastasis. Breast Dis 2010;31:53-5. [Crossref] [PubMed]
- Mhamdi HA, Kourie HR, Jungels C, et al. Adenoid cystic carcinoma of the breast - an aggressive presentation with pulmonary, kidney, and brain metastases: a case report. J Med Case Rep 2017;11:303. [Crossref] [PubMed]
- Nasu H, Miura K, Baba M, et al. Breast cancer metastatic to the kidney with renal vein involvement. Jpn J Radiol 2015;33:107-11. [Crossref] [PubMed]
- Karczmarek-Borowska B, Bukala A, Syrek-Kaplita K, et al. A Rare Case of Breast Malignant Phyllodes Tumor With Metastases to the Kidney: Case Report. Medicine (Baltimore) 2015;94:e1312. [Crossref] [PubMed]
- Wu AJ, Mehra R, Hafez K, et al. Metastases to the kidney: a clinicopathological study of 43 cases with an emphasis on deceptive features. Histopathology 2015;66:587-97. [Crossref] [PubMed]
- Herzberg AJ, Bossen EH, Walther PJ. Adenoid cystic carcinoma of the breast metastatic to the kidney. A clinically symptomatic lesion requiring surgical management. Cancer 1991;68:1015-20. [Crossref] [PubMed]
- Al Ibraheemi AA. Case report of metastatic invasive breast lobular carcinoma to the urinary bladder. Int J Hematol Oncol Stem Cell Res 2016;10:51-5. [PubMed]
- Jordan LA, Green L. Late breast cancer metastasis to the urinary bladder presenting with bilateral hydronephrosis. Radiol Case Rep 2018;13:1238-41. [Crossref] [PubMed]
- Kase AM, Menke D, Tan W. Breast cancer metastasis to the bladder: a literature review. BMJ Case Rep 2018; [Crossref] [PubMed]
- Xiao GQ, Chow J, Unger PD. Metastatic tumors to the urinary bladder: clinicopathologic study of 11 cases. Int J Surg Pathol 2012;20:342-8. [Crossref] [PubMed]
- Vulcano E, Montesano M, Battista C, et al. Urinary complications from breast cancer metastasis: case report and review of the literature. G Chir 2010;31:243-5. [PubMed]
- Gatti G, Zurrida S, Gilardi D, et al. Urinary bladder metastases from breast carcinoma: review of the literature starting from a clinical case. Tumori 2005;91:283-6. [Crossref] [PubMed]
- Soon PS, Lynch W, Schwartz P. Breast cancer presenting initially with urinary incontinence: a case of bladder metastasis from breast cancer. Breast 2004;13:69-71. [Crossref] [PubMed]
- Elia G, Stewart S, Makhuli ZN, et al. Metastatic breast cancer diagnosed during a work-up for urinary incontinence: a case report. Int Urogynecol J Pelvic Floor Dysfunct 1999;10:39-42. [Crossref] [PubMed]
- Gabsi A, Yahiaoui Y, Zenhani A, et al. Ureteral metastasis in carcinoma of the breast. Urol Case Rep 2018;21:38-40. [Crossref] [PubMed]
- Zunarelli E, Pollastri CA, Calo M, et al. Metastatic breast carcinoma presenting as urinoma. Pathologica 1995;87:712-4. [PubMed]
- Mizuyama Y, Shinto O, Tamura T, et al. A case of solitary adrenal metastasis from breast cancer successfully resected by laparoscopy. Gan To Kagaku Ryoho 2013;40:2351-3. [PubMed]
- Andjelić-Dekić N, Božović-Spasojević I, Milošević S, et al. A rare case of isolated adrenal metastasis of invasive ductal breast carcinoma. Srp Arh Celok Lek 2014;142:597-601. [Crossref] [PubMed]
- Paunovic I, Zivaljevic V, Diklic A, et al. Prognostic parameters after surgery for adrenal metastases: a single institution experience. Acta Chir Belg 2014;114:198-202. [Crossref] [PubMed]
- Demirci U, Buyukberber S, Cakir T, et al. Isolated mucinous adrenal metastasis in a breast cancer patient. J Oncol Pharm Pract 2011;17:444-7. [Crossref] [PubMed]
- Liu XJ, Shen P, Wang XF, et al. Solitary adrenal metastasis from invasive ductal breast cancer: an uncommon finding. World J Surg Oncol 2010;8:7. [Crossref] [PubMed]
- Bausewein C, Kühnbach R, Haberland B. Adrenal insufficiency caused by bilateral adrenal metastases -- a rare treatable cause for recurrent nausea and vomiting in metastatic breast cancer. Onkologie 2006;29:203-5. [PubMed]
- Davì MV, Francia G, Brazzarola P, et al. An unusual case of adrenal failure due to isolated metastases of breast cancer. J Endocrinol Invest 1996;19:488-9. [Crossref] [PubMed]
- Colak T, Akca T, Dirlik M, et al. Signet ring cell carcinoma of the breast as a source of pelvic floor metastatic mass. A case report. Acta Chir Belg 2005;105:224-6. [Crossref] [PubMed]
- Kashiwagi S, Asano Y, Watanabe M, et al. Four cases of meningeal metastasis originating from breast cancer. Gan To Kagaku Ryoho 2012;39:1917-9. [PubMed]
- Laurencet FM, Anchisi S, Tullen E, et al. Mental neuropathy: report of five cases and review of the literature. Crit Rev Oncol Hematol 2000;34:71-9. [Crossref] [PubMed]
- Higashi H, Fukutomi T, Watanabe T, et al. Seven cases of breast cancer recurrence limited to the central nervous system without other visceral metastases. Breast Cancer 2000;7:153-6. [Crossref] [PubMed]
- Mego M, Sycova-Mila Z, Obertova J, et al. Intrathecal administration of trastuzumab with cytarabine and methotrexate in breast cancer patients with leptomeningeal carcinomatosis. Breast 2011;20:478-80. [Crossref] [PubMed]
- Rao R, Hoda SA, Marcus A, et al. Metastatic Breast Carcinoma in Cerebrospinal Fluid: A Cytopathological Review of 15 Cases. Breast J 2017;23:456-60. [Crossref] [PubMed]
- Alnajar H, Rosen L, Javidiparsijani S, et al. Prognostic Markers and Histologic Subtypes in Patients with Meningeal Carcinomatosis in Breast Cancer. Acta Cytol 2017;61:140-4. [Crossref] [PubMed]
- Seki S, Kamide T, Tamase A, et al. Intraparenchymal hemorrhage from dural metastasis of breast cancer mimicking meningioma. Neuroradiol J 2016;29:179-82. [Crossref] [PubMed]
- Pan Z, Yang G, Yuan T, et al. 'Hot cross bun' sign with leptomeningeal metastases of breast cancer: a case report and review of the literature. World J Surg Oncol 2015;13:43. [Crossref] [PubMed]
- Meattini I, Livi L, Saieva C, et al. Prognostic factors and clinical features in patients with leptominengeal metastases from breast cancer: a single center experience. J Chemother 2012;24:279-84. [Crossref] [PubMed]
- Tseng SH, Liao CC, Lin SM, et al. Dural metastasis in patients with malignant neoplasm and chronic subdural hematoma. Acta Neurol Scand 2003;108:43-6. [Crossref] [PubMed]
- Jayson GC, Howell A, Harris M, et al. Carcinomatous meningitis in patients with breast cancer. An aggressive disease variant. Cancer 1994;74:3135-41. [Crossref] [PubMed]
- Madgula IM, Hemmerdinger CM, Clark P. Metastatic breast cancer presenting as sequential cranial nerve palsy: a case report. J Med Case Rep 2014;8:430. [Crossref] [PubMed]
- Gasser TG, Pospiech J, Stolke D, et al. Spinal intramedullary metastases. Report of two cases and review of the literature. Neurosurg Rev 2001;24:88-92. [Crossref] [PubMed]
- Choi HC, Yoon DH, Kim SC, et al. Two separate episodes of intramedullary spinal cord metastasis in a single patient with breast cancer. J Korean Neurosurg Soc 2010;48:162-5. [Crossref] [PubMed]
- Garcia R, Sallabanda K, Santa-Olalla I, et al. Robotic Radiosurgery for the Treatment of Intramedullary Spinal Cord Metastases: A Case Report and Literature Review. Cureus 2016;8:e609. [PubMed]
- Aiello D, Mazzola R, Gregucci F, et al. Surprising complete response of intramedullary spinal cord metastasis from breast cancer: a case report and literature review. Tumori 2017;103:e28-30. [Crossref] [PubMed]
- Payer S, Mende KC, Westphal M, et al. Intramedullary spinal cord metastases: an increasingly common diagnosis. Neurosurg Focus 2015;39:E15. [Crossref] [PubMed]
- Hsu KC, Li TY, Chu HY, et al. Conus medullaris metastasis in breast cancer: report of a case and a review of the literature. Surg Today 2013;43:910-4. [Crossref] [PubMed]
- Zebrowski A, Wilson L, Lim A, et al. Intramedullary spinal cord metastases in breast cancer are associated with improved longer-term systemic control. Future Oncol 2010;6:1517-9. [Crossref] [PubMed]
- Watanabe M, Nomura T, Toh E, et al. Intramedullary spinal cord metastasis: a clinical and imaging study of seven patients. J Spinal Disord Tech 2006;19:43-7. [Crossref] [PubMed]
- Villegas AE, Guthrie TH. Intramedullary spinal cord metastasis in breast cancer: clinical features, diagnosis, and therapeutic consideration. Breast J 2004;10:532-5. [Crossref] [PubMed]
- Chen YJ, Fan FS, Chen PM. Intramedullary spinal cord metastasis: a case report. Zhonghua Yi Xue Za Zhi (Taipei) 1995;56:58-61. [PubMed]
- Schwechheimer K, Lemminger JM. Intramedullary metastases: report of 4 cases and review of the literature. Clin Neuropathol 1985;4:28-37. [PubMed]
- Moffie D, Stefanko SZ. Intramedullary metastasis. Clin Neurol Neurosurg 1980;82:199-202. [Crossref] [PubMed]
- West CG. Spinal subarchnoid metastatic spread from non-neuraxial primary neoplasms. Case report. J Neurosurg 1979;51:251-3. [Crossref] [PubMed]
- Della Puppa A, Dal Pos S, Zovato S, et al. Solitary intra-ventricular brain metastasis from a breast carcinoma. J Neurooncol 2010;97:123-6. [Crossref] [PubMed]
- Sajko T, Rotim K, Kudelić N, et al. Intraventricular location of metastatic breast carcinoma: a case report. Acta Clin Croat 2009;48:55-8. [PubMed]
- Saha S, Kumar A, Kumar C, et al. Paraneoplastic cerebellar degeneration as a manifestation of metastatic recurrent carcinoma breast: rare scenario. BMJ Case Rep 2018; [Crossref] [PubMed]
- Singh J, Majumdar K, Gupta R, et al. Cerebellar metastases of recurrent phyllodes tumor breast; a rare phenomenon reflecting the unpredictable outcome. J Cancer Res Ther 2015;11:1035. [Crossref] [PubMed]
- Rowe JJ, Prayson RA. Metastatic malignant phyllodes tumor involving the cerebellum. J Clin Neurosci 2015;22:226-7. [Crossref] [PubMed]
- Artico M, Scarpinati M, Salvati M, et al. Late intraneural metastasis of the brachial plexus from mammary carcinoma. Report of a case. J Neurosurg Sci 1991;35:51-3. [PubMed]
- Backhouse O, Simmons I, Frank A, et al. Optic nerve breast metastasis mimicking meningioma. Aust N Z J Ophthalmol 1998;26:247-9. [Crossref] [PubMed]
- Cherekaev VA, Lasunin NV, Stepanian MA, et al. Breast carcinoma metastasis to the optic nerve: case report and review of literature. Zh Vopr Neirokhir Im N N Burdenko 2013;77:42-8; discussion 48. [PubMed]
- Suryanarayanan R, Dezso A, Ramsden RT, et al. Metastatic carcinoma mimicking a facial nerve schwannoma: the role of computerized tomography in diagnosis. J Laryngol Otol 2005;119:1010-2. [Crossref] [PubMed]
- Ito K, Miyahara T, Goto T, et al. Solitary metastatic cauda equina tumor from breast cancer -case report-. Neurol Med Chir (Tokyo) 2010;50:417-20. [Crossref] [PubMed]
- Schulz M, Lamont D, Muthu T, et al. Metastasis of breast cancer to a lumbar spinal nerve root ganglion. Spine (Phila Pa 1976) 2009;34:E735-9. [Crossref] [PubMed]
- Zingale A, Ponzo G, Ciavola G, et al. Metastatic breast cancer delayed brachial plexopathy. A brief case report. J Neurosurg Sci 2002;46:147-9. [PubMed]
- Hirota N, Fujimoto T, Takahashi M, et al. Isolated trigeminal nerve metastases from breast cancer: an unusual cause of trigeminal mononeuropathy. Surg Neurol 1998;49:558-61. [Crossref] [PubMed]
- Breadon GE, Cody DT, Weiland LH. Facial palsy: unusual etiology. Laryngoscope 1977;87:26-34. [Crossref] [PubMed]
- Reyes KB, Lee HY, Ng I, et al. Abducens (sixth) nerve palsy presenting as a rare case of isolated brainstem metastasis from a primary breast carcinoma. Singapore Med J 2011;52:e220-2. [PubMed]
- de Ceuster LM, de Bruijn SF, Hoffmann CF. Miliary cerebral calcifications: A rare presentation of breast cancer metastasis. Neurology 2016;86:879. [Crossref] [PubMed]
- Chakrabarti I, Ghosh N, Giri A. Cytologic diagnosis of undifferentiated high grade pleomorphic sarcoma of breast presenting with brain metastasis. J Neurosci Rural Pract 2013;4:188-90. [Crossref] [PubMed]
- Low YY, Thomas J, Wan WK, et al. Brain metastases as a cause of malignant cerebrospinal fluid ascites: case report and review of the literature. CNS Oncol 2012;1:29-37. [Crossref] [PubMed]
- Modi M, Singla V, Bhatia R, et al. Isolated nuclear oculomotor nerve palsy due to a solitary midbrain metastasis: a rare presentation. Indian J Ophthalmol 2006;54:286-7. [Crossref] [PubMed]
- Saisho S, Takashima S, Ohsumi S, et al. Two cases with long-term disease-free survival after resection and radiotherapy for solitary brain metastasis from breast cancer with extensive nodal metastases. Breast Cancer 2005;12:221-5. [Crossref] [PubMed]
- Chou TM, Demer JL. Isolated inferior rectus palsy caused by a metastasis to the oculomotor nucleus. Am J Ophthalmol 1998;126:737-40. [Crossref] [PubMed]
- Koller M, Ram Z, Findler G, et al. Brain metastasis: a rare manifestation of adenoid cystic carcinoma of the breast. Surg Neurol 1986;26:470-2. [Crossref] [PubMed]
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 1989;8:98-101. [PubMed]
- Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 2010;70:5649-69. [Crossref] [PubMed]
- Virchow R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI--Atheromatous affection of arteries. 1858. Nutr Rev 1989;47:23-5. [Crossref] [PubMed]
- Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev 2007;28:297-321. [Crossref] [PubMed]
- Ribatti D, Mangialardi G, Vacca A. Stephen Paget and the 'seed and soil' theory of metastatic dissemination. Clin Exp Med 2006;6:145-9. [Crossref] [PubMed]
- Cichoń S, Anielski R, Konturek A, et al. Metastases to the thyroid gland: seventeen cases operated on in a single clinical center. Langenbecks Arch Surg 2006;391:581-7. [Crossref] [PubMed]
- Chen H, Nicol TL, Udelsman R. Clinically significant, isolated metastatic disease to the thyroid gland. World J Surg 1999;23:177-80; discussion 181. [Crossref] [PubMed]
- Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology 1959;9:757-66. [Crossref] [PubMed]
- James JJ, Evans AJ, Pinder SE, et al. Bone metastases from breast carcinoma: histopathological - radiological correlations and prognostic features. Br J Cancer 2003;89:660-5. [Crossref] [PubMed]
- Seely S. Possible reasons for the high resistance of muscle to cancer. Med Hypotheses 1980;6:133-7. [Crossref] [PubMed]
- Djaldetti M, Sredni B, Zigelman R, et al. Muscle cells produce a low molecular weight factor with anti-cancer activity. Clin Exp Metastasis 1996;14:189-96. [PubMed]
- Caramella E, Bruneton JN, Roux P, et al. Metastases of the digestive tract. Report of 77 cases and review of the literature. Eur J Radiol 1983;3:331-8. [PubMed]
- Puglisi M, Varaldo E, Assalino M, et al. Anal metastasis from recurrent breast lobular carcinoma: a case report. World J Gastroenterol 2009;15:1388-90. [Crossref] [PubMed]
- Van Trappen P, Serreyn R, Elewaut AE, et al. Abdominal pain with anorexia in patients with breast carcinoma. Ann Oncol 1998;9:1243-5. [Crossref] [PubMed]
- Nazareno J, Taves D, Preiksaitis HG. Metastatic breast cancer to the gastrointestinal tract: a case series and review of the literature. World J Gastroenterol 2006;12:6219-24. [Crossref] [PubMed]
- Pectasides D, Psyrri A, Pliarchopoulou K, et al. Gastric metastases originating from breast cancer: report of 8 cases and review of the literature. Anticancer Res 2009;29:4759-63. [PubMed]
- Ambroggi M, Stroppa EM, Mordenti P, et al. Metastatic breast cancer to the gastrointestinal tract: report of five cases and review of the literature. Int J Breast Cancer 2012;2012:439023. [PubMed]
- Peters IT, van Zwet EW, Smit VT, et al. Prevalence and Risk Factors of Ovarian Metastases in Breast Cancer Patients <41 Years of Age in the Netherlands: A Nationwide Retrospective Cohort Study. PLoS One 2017;12:e0168277. [Crossref] [PubMed]
- Tian W, Zhou Y, Wu M, et al. Ovarian metastasis from breast cancer: a comprehensive review. Clin Transl Oncol 2019;21:819-27. [Crossref] [PubMed]
- Abdalla AS, Lazarevska A, Omer MM, et al. Metastatic Breast Cancer to the Cervix Presenting with Abnormal Vaginal Bleeding During Chemotherapy: A Case Report and Literature Review. Chirurgia (Bucur) 2018;113:564-70. [Crossref] [PubMed]
- Zannoni GF, Vellone VG, Petrillo M, et al. Secondary malignancies of the uterine cervix: a potential diagnostic pitfall. Virchows Arch 2013;463:23-9. [Crossref] [PubMed]
- Ayhan A, Guvenal T, Salman MC, et al. The role of cytoreductive surgery in nongenital cancers metastatic to the ovaries. Gynecol Oncol 2005;98:235-41. [Crossref] [PubMed]
- Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery 1993;114:637-41; discussion 641-2. [PubMed]
- Harris M, Howell A, Chrissohou M, et al. A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating duct carcinoma of the breast. Br J Cancer 1984;50:23-30. [Crossref] [PubMed]
- Dixon AR, Ellis IO, Elston CW, et al. A comparison of the clinical metastatic patterns of invasive lobular and ductal carcinomas of the breast. Br J Cancer 1991;63:634-5. [Crossref] [PubMed]
- Lamovec J, Bracko M. Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol 1991;48:28-33. [Crossref] [PubMed]
- Jain S, Fisher C, Smith P, et al. Patterns of metastatic breast cancer in relation to histological type. Eur J Cancer 1993;29A:2155-7. [Crossref] [PubMed]