
The expression and role of trefoil factors in human tumors
Introduction
The trefoil factor (TFF) family consists of three members: TFF1, TFF2, and TFF3. They are also called breast cancer-associated peptide (pS2), spasmolytic peptide (SP), and intestinal trefoil factors (ITF). The gene encoding the human TFF family is located on chromosome 21q22.3 (1). This family is made of small cysteine-rich polypeptide whose secondary structure consists of 38–39 amino acids formed by 6 highly conserved cysteine residues to form three intramolecular disulfide bonds folded into a “trefoil” structure. The “trefoil domain” is also called the P domain. One such motif is found in TFF1 and TFF3, whereas TFF2 possesses two trefoil domains. The trefoil domain is very stable; such that TFFs are strongly resistant to acid, protease hydrolysis and heat. Therefore, TFFs can maintain their biological activity in the complex physiological environment of the gastrointestinal tract, with the function of mucosal protection and epithelial restitution (2).
In normal tissues, TFFs are mainly expressed in mucosal epithelium, co-secreted with mucins in the gastrointestinal tract and respiratory tract to form protective barriers, as well as expressed in the central nervous system, eyes, and other tissues (3). Among the TFF family, TFF1 is mainly expressed in the mucosal epithelial cells of the gastric body and gastric antrum, followed by low-level expression in the empty ileum, colon, salivary glands, pancreas and other mucous epithelial tissues (4). TFF2 is mainly expressed in the mucous cells of the gastric body and gastric antrum and the lower duodenum (5). TFF3 is mainly expressed in the small intestine and colon goblet cells, as well as in the uterus, breast, hypothalamus, pituitary, salivary gland, respiratory tract, ocular conjunctiva, nasal mucosa, gastric juice and duodenum ampulla (6).
TFFs function to protect mucosa, promote epithelial repair, inhibit inflammation, regulate cell proliferation, apoptosis and migration. Rapid increase in expression of TFF1 and TFF2 at the margins of digestive tract ulcers promotes migration of epithelial cells (7). TFF2 promotes the repair of gastric mucosal injury by increasing the migration of human colonic epithelial cells and by inhibiting the occurrence of acute gastric injury (8). TFF3 plays a pivotal role in maintaining the integrity of the gastrointestinal mucosa and promoting repair (9). In addition, TFF3 expression increases in corneal diseases and promotes corneal repair (10).
Over the past few decades, a large number of studies have found abnormal expression of TFFs in many types of tumors, where they were involved in tumorigenesis and tumor development, which are closely related to the malignancy and prognosis of tumors. However, contradictory results were also found in some tumors.
The expression of TFFs in tumors
As shown in Table 1, studies have found increased expression of TFF members in multiple tumors. In addition to the high expression of TFF1 in gastric cancer, TFF1 and TFF3 in 97 patients with colon cancer were significantly higher than those in 79 healthy people (32). Using an immunohistochemical method, researchers found that the expression of TFF3 in thyroid papillary carcinoma was higher than in adjacent precarcinomatous tissue in 31 cases of thyroid papillary carcinoma (13). A clinical study showed that the positive expression rate of TFF3 increased from normal gastric tissues to paracancerous tissues and gastric cancer tissues, which were 5.0%, 40.0% and 58.6% respectively. The expression of TFF3 in gastric cancer tissues was related to the stage of local lymph node metastasis and prognosis (19). Another study found that the serum TFF3 concentration in patients with gastric cancer was significantly higher than that in the healthy counterparts (20). The increased expression of TFF3 protein in breast cancer was positively correlated with tumor size, lymph node metastasis, poor survival and prognosis (26). In addition, a two-step immunohistochemical method was used to detect the expression of TFF3 in 90 pairs of hepatocellular carcinomas (HCC) tumor tissues and non-cancerous adjacent tissues. The results showed that the positive expression rate of TFF3 was 62.1% in tumor tissues and 33.8% in adjacent tissues. The difference between the two groups was statistically significant (17).
Table 1
| Tumor type | Expression change of TFFs | |||
|---|---|---|---|---|
| Tumor vs. normal tissues or positive ratio in tumor tissues | Ref. | Serum of patients vs. healthy people | Ref. | |
| Retinal neuroblastoma | TFF1 ↑ | (11) | – | – |
| Oral carcinoma | TFF2 ↓, TFF3 ↓ | (12) | – | – |
| Thyroid papillary carcinoma | 83.87% TFF3 ↑ | (13) | – | – |
| Lung cancer | – | – | TFF3 ↑ | (14) |
| Cholangiocarcinoma | 79.17% TFF3 ↑ | (15) | – | – |
| TFF1, TFF2 ↑ | (16) | – | – | |
| HCC | 62.1% TFF3 ↑ | (17) | – | – |
| 93% TFF3 ↑ | (18) | – | – | |
| Gastric cancer | 58.6% TFF3 ↑ | (19) | TFF3 ↑ | (20) |
| TFF2 ↓ | (21) | – | – | |
| 67.5% TFF1 ↓ | (22) | – | – | |
| 53.8% TFF1 ↓; 44.2% TFF3 ↑ | (23) | – | – | |
| 58.3% TFF1 ↓; 83.3% TFF2 ↓; 41.7% TFF3 ↑ | (24) | – | – | |
| 36.9% TFF2 ↑; 44.1% TFF3 ↑ | (25) | – | – | |
| Mammary cancer | 60.4% TFF3 ↑ | (26) | 34.6% TFF1 ↑; 46.2% TFF3 ↑ | (27) |
| TFF1 ↑ | (28) | TFF3 ↑ | (29) | |
| Endometrial carcinoma | TFF3 ↑ | (9) | – | – |
| TFF1 ↓ | (30) | – | – | |
| Colorectal cancer | TFF1 ↑ | (31) | TFF1, TFF3 ↑ | (32) |
| TFF3 ↑ | (33) | |||
| Prostate cancer | TFF1, TFF3 ↑ | (34) | TFF1, TFF3 ↑ | (35) |
HCC, hepatocellular carcinoma; TFF, trefoil factor.
Contrary to the aforementioned studies, the expression of TFF1 and TFF2 in normal gastric mucosa, paracancerous tissues and gastric cancer tissues decreased gradually, suggesting that TFF1 and TFF2 proteins may be specific inhibitors of gastric cancer and important protective factors for the digestive tract mucosa (21). There are two explanations for the deletion of TFF1 expression in gastric cancer. The first is a somatic mutation, since the TFF1 gene locus 21q22 is frequently mutated and allelic deletion is common (36). The second explanation is the hypermethylation of the promoter region of the TFF1 gene in gastric cancer and intestinal metaplasia, resulting in transcriptional silencing of the TFF1 gene (37). Buache et al. (38) found that the incidence rate, number and size of breast tumors induced by dimethylbenzanthracene (DMBA) in TFF1-KO mice were higher than those in wild-type mice. TFF1 can act as a tumor suppressor and inhibit the development of tumors in breast epithelial cells. This is consistent with the clinical findings of primary breast cancer, in which patients with TFF1 positive expression received better prognosis. In addition, in type I endometrial carcinoma, TFF3 up-regulation was found to be positively correlated to the outcome of patients (9).
The role and mechanism of TFFs
In vitro and in vivo experiments showed that TFFs can affect tumor development mainly by affecting tumor cell apoptosis, cell proliferation, metastasis and angiogenesis, which are summarized in Figure 1.
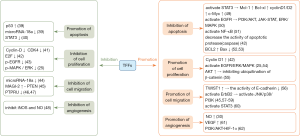
Apoptosis
TFF1 can not only protect epithelial cells from apoptosis induced by chemical toxic reagents, but can also prevent gastric cancer cells from apoptosis induced by etoposide (63). TFF2 can inhibit the apoptosis of breast cancer cells such as; MCF-7, and colon cancer cells and can promote cell survival (64). The addition of exogenous TFF3 could reduce the apoptosis induced by starvation or drugs in the colon cancer cell line, HCT116. Consistent with previous studies, our group found that TFF3 can inhibit apoptosis induced by palmitic acid (PA). When TFF3 was inhibited, apoptosis of gastric cancer cells was up-regulated by chemotherapeutic agents, which increased their chemosensitivity (65).
TFF members may affect apoptosis through different signal transduction pathways. Signal transducers and activators of transcription (STATs) family can inhibit cell apoptosis and promote cell proliferation, and STAT3 is especially active. TFF3 can activate STAT3 signaling by up-regulating the expression and phosphorylation of apoptosis suppressor genes (Mcl-1, Bcl-xl) and cell cycle regulators (cyclinD1/D2, c-Myc), thereby, inhibiting apoptosis (49). TFF3 can induce phosphorylation and activation of epidermal growth factor receptor (EGFR) in HT-29 cells, and further activates anti-apoptotic signaling pathways such as; PI3K/AKT, JAK-STAT and ERK/MAPK (50). Chen et al. (66) found that TFF3 could induce the activation of NF-κB to inhibit apoptosis of gastric cancer cells. NF-κB inhibited apoptosis of gastric cancer cells through three pathways: mitochondrial pathway, endoplasmic reticulum pathway and extracellular death receptor pathway. The expression of NF-κB p65 was positively correlated with the abnormal expression of TFF3 in gastric cancer (51). TFF1 can protect gastric and intestinal cancer cells by decreasing the activity of apoptotic protease (caspase), and improve their survival rate (42). TFF3 could increase the expression of the anti-apoptotic protein Bcl-2 (52) and decrease the expression of Bax of the Bcl-2 family (53), thus inhibiting apoptosis.
However, other authors have found that TFFs can promote tumor cell apoptosis. Overexpression of TFF1 has been found to significantly increase the apoptosis level of retinoblastoma (RB) cells, and decrease cell viability, proliferation and growth. The inhibitory effect of TFF1 on RB cells is partly mediated by the activation of p53 and down-regulation of miR-18a. The activated tumor suppressor gene p53 itself can induce apoptosis and cell cycle arrest (39). The pro-apoptosis effect of TFF2 was stronger than the anti-apoptosis role in isolated mouse retina. The recombinant TFF2-mediated apoptosis is dependent on caspase proteins. TFF2 administration could down-regulate STAT3 activation, thus decreasing the expression of apoptosis suppressor genes and cell cycle regulators, and promoting apoptosis of mouse retina cells (40).
Proliferation
Cell proliferation is the decisive factor in tumorigenesis. The experimental results of our group proved that TFF3 can promote growth and proliferation in HepG2 and Huh-7. TFF1 can increase the expression of cyclin D1 in cells, thereby promoting cell proliferation to accelerate tumor formation (42). TFF2 as a proliferating factor can promote growth and proliferation in HCT116 and the breast cancer cell line, MCF-7. TFF2 promotes cell proliferation through the activation of epidermal growth factor receptor (EGFR) and intracellular MAPK signaling pathway (16). As a growth factor-like small molecule peptide, TFF3 can activate ERK1/2, which is an important component of MAPK signaling pathway, promoting tumor cell proliferation (54). Up-regulation of TFF3 expression significantly increased the proliferation and activity of breast and prostate cancer cells (67). AKT activated by TFF3 through EGFR phosphorylation, inhibits ubiquitination of β-catenin which leads to its nuclear translocation and promotes the proliferation of colorectal carcinoma cells (55).
However, some authors reported that TFFs can inhibit tumor cell proliferation. TFF1 could inhibit the activity of G1/S- specific cyclin-D1 and cyclin dependent protein kinase (CDK) 4 complex, which retards the initiation of S phase during the cell cycle and arrests the cells at the G1 stage, preventing cell proliferation (41). Transcription factor E2F is the main regulatory factor for cells entering S phase. TFF1 mediates the increased level of CDK inhibitors to decrease the transcriptional activity of E2F, preventing cells from entering S phase (42). Uchino et al. (43) found that the overexpression of TFF3 inhibited EGFR phosphorylation and cell proliferation in human colon cancer cell lines. Mitogen-activated protein kinase (MAPK) is a type of serine/threonine protein kinase in cells. Studies have shown that the signaling pathway of MAPKs exists in most cells, transducing extracellular stimulation signals into cells and nuclei, and inducing biological responses such as; cell proliferation, differentiation, transformation and apoptosis. Extracellular regulated protein kinase (ERK) is key to transmitting signals from surface receptors to the nucleus. Kosriwong et al. (16) found that TFF3 reduced the phosphorylation of MAPK/ERK and thus, significantly suppressed cell proliferation and growth, differentiation and apoptosis.
Migration
Cell migration leads to tumor metastasis and recurrence during tumor progression. Most published work showed that the migration of tumor cells could be promoted by TFFs, which was closely related to the prognosis of tumor. TFF2 and TFF3 can promote cell dispersion, and all three TFF members can induce invasion and metastasis of renal and intestinal cancer cells (68). TFF1 was also found to promote the migration and invasion of breast cancer cells (69). High expression of TFF2 and TFF3 in gastric and intestinal cancer cells is positively associated with cell migration and invasion (70). Up-regulated TFF3 in non-metastatic intestinal cancer cells enhanced the ability of cell migration and malignancy. Additionally, the ability of invasion and migration was inhibited after a TFF3 knock-down (71). Supporting this finding, our group showed that the overexpression of TFF3 or using recombinant human TFF3 (rhTFF3) can promote liver cancer cell migration.
Migration is also essential for epithelial restitution, during which the first step is the reduction of cell-cell contact. TFF3 can reduce the activity of E-cadherin by up-regulating the expression of TWIST1 (56), which destroys the intercellular adhesion and makes tumor cells separate. TFF3 can activate ErbB2 and then activates c-Jun N-terminal kinase (JNK), p38 and the phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB) pathway. JNK may promote migration through focal adhesion disassembly. Increased MAP kinase-activated protein kinase 2 (MAPKAPK2) phosphorylation in the p38 pathway may indirectly promote migration (45). Moreover, TFF3 can activate the PI3K/AKT pathway (57). AKT stimulates metastasis through Twist, Snail, Slug, Zeb1, and Zeb2 (58,59). It was reported that TFF3 produces a migratory and invasive phenotype in human colon carcinoma cells which was dependent on the activity of the transcription factor signal transducer and activator of transcription 3 (STAT3) (60). What’s more, TFF1 stimulates the migration and invasion of human gastric carcinoma cells in a PI3K-dependent manner (63).
The inhibitory effect of TFF on migration has been rarely reported. MicroRNA-18a down-regulated by TFF1 results in reduced migration and invasion of gastric cancer cells (44). It was found that the overexpression of TFF1 can induce the increase expression of tumor suppressor gene scaffold protein membrane-associated guanylate kinase inverted-2 (MAGI-2) (45). This subsequently inhibits the migration and proliferation of human hepatoma cells through the tumor suppressor gene, PTEN (72). The overexpression of TFF1 can down-regulate the expression of protein tyrosine phosphatase receptor U (PTPRU) gene in glioma cells (46). PTPRU is required for the growth, migration, invasion and adhesion of glioma and gastric cancer cells (46,47).
Angiogenesis
Angiogenesis is a process necessary for tumor growth, invasion and metastasis. TFF3 plays a role in promoting angiogenesis, both in vivo and in vitro. TFF3 can promote angiogenesis of the chorioallantoic membrane and can induce the formation of microvascular structures (73). The methylation of the CpG island in the abnormal starting area may regulate the expression of TFF3. Liver cancer tissue may promote TFF3 expressing, thus, affecting angiogenesis and promoting the formation of the tumor (17). Nitric oxide (NO) is an important angiogenic factor in tumor angiogenesis, and is stimulated by TFF3 inducing NO synthetase. The occurrence of tumor angiogenesis in gastric cancer is always associated with the enhancement of TFF3 (25). TFF3 can also promote the generation of NO by combining with MUC2 (74). Some carcinogenic factors can induce cyclooxygenase-2 (COX-2) expression, increase the expression of vascular endothelial growth factor (VEGF), and promote tumor angiogenesis (61). Previous studies have found that TFF3 can activate the PI3K/AKT pathway (57). Angiogenesis is mediated by PI3K/AKT signaling through Hypoxia induced factor 1 alpha (HIF-1α) and the angiogenic factors ANG-2 (angiopoietin 2), plasminogen activator inhibitor type 1 (PAI-1),Thrombospondin1 (THBS1) and vascular endothelial growth factor (VEGF) (62).
There are few reports on the anti-angiogenic function of TFFs. It has been shown that TFF2 can suppress iNOS and NO to inhibit angiogenesis (48).
Conclusions
As an important cytokine in mucosal epithelium, TFF family plays an important role in gastrointestinal mucosal injury, repair, and malignancy. Clinical studies during the past few decades have found the abnormal expression of TFFs in many types of tumors; some related to the degree of tumor malignancy, and some might be involved in the occurrence and metastasis of tumors. It should be noted that TFFs may confer invasive potential and resistance to therapy, but their expression does not always result in a more aggressive cancer phenotype. Moreover, although most studies have shown that TFFs play a promotional role in tumors, the results of its anti-tumor effects should not be ignored. A large number of studies in vivo or in vitro demonstrated that TFFs bear the ability of promoting migration and anti-apoptosis of tumor cells, but a few other studies suggested the contrary. There were some contradictory reports and divergences on the role in promoting or suppressing tumor proliferation and growth. This might be due to different factors such as; tumor cell type and the specific microenvironment. Furthermore, TFFs’ impact also seems to be dependent on the system studied. Therefore, it needs to be clarified by further randomized controlled clinical studies and molecular biological mechanism studies to determine whether TFFs could act as a potential molecular marker or therapeutic target of a certain type of tumor.
Acknowledgments
We would like to acknowledge Sara EI-Sahli (Department of Biochemistry, Microbiology and Immunology, Faculty of Medicine, University of Ottawa, Canada) for critical reading and modification of the manuscript.
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.48). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chinery R, Williamson J, Poulsom R. The gene encoding human intestinal trefoil factor (TFF3) is located on chromosome 21q22.3 clustered with other members of the trefoil peptide family. Genomics 1996;32:281-4. [Crossref] [PubMed]
- Wong WM, Poulsom R, Wright NA. Trefoil peptides. Gut 1999;44:890-5. [Crossref] [PubMed]
- Busch M, Dunker N. Trefoil factor family peptides--friends or foes? Biomol Concepts 2015;6:343-59. [Crossref] [PubMed]
- Masiakowski P, Breathnach R, Bloch J, et al. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res 1982;10:7895-903. [Crossref] [PubMed]
- Jørgensen KH, Thim L, Jacobsen HE. Pancreatic spasmolytic polypeptide (PSP): I. Preparation and initial chemical characterization of a new polypeptide from porcine pancreas. Regul Pept 1982;3:207-19. [Crossref] [PubMed]
- Suemori S, Lynch-Devaney K, Podolsky DK. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci U S A 1991;88:11017-21. [Crossref] [PubMed]
- Wright NA, Poulsom R, Stamp G, et al. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology 1993;104:12-20. [Crossref] [PubMed]
- Playford RJ, Marchbank T, Chinery R, et al. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology 1995;108:108-16. [Crossref] [PubMed]
- Mhawech-Fauceglia P, Wang D, Samrao D, et al. Trefoil factor family 3 (TFF3) expression and its interaction with estrogen receptor (ER) in endometrial adenocarcinoma. Gynecol Oncol 2013;130:174-80. [Crossref] [PubMed]
- Paulsen FP, Woon CW, Varoga D, et al. Intestinal trefoil factor/TFF3 promotes re-epithelialization of corneal wounds. J Biol Chem 2008;283:13418-27. [Crossref] [PubMed]
- Busch M, Metz K, Beier M, et al. Trefoil factor family 1 expression correlates with clinical outcome in patients with retinoblastoma. Retina 2018;38:2422-8. [PubMed]
- Chaiyarit P, Utrawichian A, Leelayuwat C, et al. Investigation of trefoil factor expression in saliva and oral mucosal tissues of patients with oral squamous cell carcinoma. Clin Oral Investig 2012;16:1549-56. [Crossref] [PubMed]
- Zhang CL, Wu JF, Wan JX, et al. Clinical significance of STAT3, pSTAT3 and TFF3 in human papillary thyroid carcinoma. Modern Preventive Medicine 2016;143:182-6.
- Qu Y, Yang Y, Ma D, et al. Increased trefoil factor 3 levels in the serum of patients with three major histological subtypes of lung cancer. Oncol Rep 2012;27:1277-83. [Crossref] [PubMed]
- Ailawadhi S, Nagase H, Khoury T, et al. Intestinal trefoil factor (TFF-3) and extracellular signal-regulated kinase (ERK) in cholangiocarcinoma. Hepatogastroenterology 2007;54:1339-44. [PubMed]
- Kosriwong K, Menheniott TR, Giraud AS, et al. Trefoil factors: tumor progression markers and mitogens via EGFR/MAPK activation in cholangiocarcinoma. World J Gastroenterol 2011;17:1631-41. [Crossref] [PubMed]
- Shang YLQ. Y.B. Clinical significance of expression of trefoil factor 3 in hepatocellular carcinoma World Chinese Journal of Digestology 2014;22:1141-5. [Crossref]
- Khoury T, Chadha K, Javle M, et al. Expression of intestinal trefoil factor (TFF-3) in hepatocellular carcinoma. Int J Gastrointest Cancer 2005;35:171-7. [Crossref] [PubMed]
- Fan LF, Liu HM. Expression of TFF3 and VEGF in Gastric Cancer and Their Clinicopathological Significance. Journal of Chinese Oncology 2017;22:125-9.
- Zhang X, Song YF, Zhang XS, et al. Clinical Research of Serum Trefoil Factor 3 Expression in Gastric Cancer. Chinese Journal of Gastroenterology 2016;21:93-7.
- Shi L, Liang ZH, Liu SQ, et al. Correlation between TFF2 expression and tumor angiogenesis in gastric carcinoma, adjacent carcinoma and normal gastric mucosa. World Chin J Dig 2011;19:246-50.
- Cobler L, Mejias-Luque R, Garrido M, et al. Activation of the NF-kB pathway downregulates TFF-1 in gastric carcinogenesis. Virchows Arch 2013;463:497-507. [Crossref] [PubMed]
- Im S, Yoo C, Jung JH, et al. Reduced expression of TFF1 and increased expression of TFF3 in gastric cancer: correlation with clinicopathological parameters and prognosis. Int J Med Sci 2013;10:133-40. [Crossref] [PubMed]
- Kirikoshi H, Katoh M. Expression of TFF1, TFF2 and TFF3 in gastric cancer. Int J Oncol 2002;21:655-9. [PubMed]
- Dhar DK, Wang TC, Tabara H, et al. Expression of trefoil factor family members correlates with patient prognosis and neoangiogenesis. Clin Cancer Res 2005;11:6472-8. [Crossref] [PubMed]
- Pandey V, Wu ZS, Zhang M, et al. Trefoil factor 3 promotes metastatic seeding and predicts poor survival outcome of patients with mammary carcinoma. Breast Cancer Res 2014;16:429. [Crossref] [PubMed]
- Elnagdy MH, Farouk O, Seleem AK, et al. TFF1 and TFF3 mRNAs Are Higher in Blood from Breast Cancer Patients with Metastatic Disease than Those without. J Oncol 2018;2018:4793498. [Crossref] [PubMed]
- Tjensvoll K, Gilje B, Oltedal S, et al. A small subgroup of operable breast cancer patients with poor prognosis identified by quantitative real-time RT-PCR detection of mammaglobin A and trefoil factor 1 mRNA expression in bone marrow. Breast Cancer Res Treat 2009;116:329-38. [Crossref] [PubMed]
- Neubert D, Ondrova D, Hambalek J, et al. Elevated levels of TFF3 in endometrial cancer patients. Ceska Gynekol 2018;83:109-14. [PubMed]
- Zhang HM, Fan TT, Li W, et al. Expressions and significances of TTF-1 and PTEN in early endometrial cancer. Eur Rev Med Pharmacol Sci 2017;21:20-6. [PubMed]
- Huang YG, Li YF, Pan BL, et al. Trefoil factor 1 gene alternations and expression in colorectal carcinomas. Tumori 2013;99:702-7. [Crossref] [PubMed]
- Vocka M, Langer D, Petrtyl J, et al. Trefoil factor family (TFF) proteins as potential serum biomarkers in patients with metastatic colorectal cancer. Neoplasma 2015;62:470-7. [Crossref] [PubMed]
- Morito K, Nakamura J, Kitajima Y, et al. The value of trefoil factor 3 expression in predicting the longterm outcome and early recurrence of colorectal cancer. Int J Oncol 2015;46:563-8. [Crossref] [PubMed]
- Vestergaard EM, Nexo E, Torring N, et al. Promoter hypomethylation and upregulation of trefoil factors in prostate cancer. Int J Cancer 2010;127:1857-65. [Crossref] [PubMed]
- Vestergaard EM, Borre M, Poulsen SS, et al. Plasma levels of trefoil factors are increased in patients with advanced prostate cancer. Clin Cancer Res 2006;12:807-12. [Crossref] [PubMed]
- Park WS, Oh RR, Park JY, et al. Somatic mutations of the trefoil factor family 1 gene in gastric cancer. Gastroenterology 2000;119:691-8. [Crossref] [PubMed]
- Fujimoto J, Yasui W, Tahara H, et al. DNA hypermethylation at the pS2 promoter region is associated with early stage of stomach carcinogenesis. Cancer Lett 2000;149:125-34. [Crossref] [PubMed]
- Buache E, Etique N, Alpy F, et al. Deficiency in trefoil factor 1 (TFF1) increases tumorigenicity of human breast cancer cells and mammary tumor development in TFF1-knockout mice. Oncogene 2011;30:3261-73. [Crossref] [PubMed]
- Busch M, Grosse-Kreul J, Wirtz JJ, et al. Reduction of the tumorigenic potential of human retinoblastoma cell lines by TFF1 overexpression involves p53/caspase signaling and miR-18a regulation. Int J Cancer 2017;141:549-60. [Crossref] [PubMed]
- Paunel-Görgülü AN, Franke AG, Paulsen FP, et al. Trefoil factor family peptide 2 acts pro-proliferative and pro-apoptotic in the murine retina. Histochem Cell Biol 2011;135:461-73. [Crossref] [PubMed]
- Du TY, Zhang Y, Zhang Y. Trefoil Factor: from Laboratory to Clinic. Zoological Research 2010;31:17-26. [Crossref] [PubMed]
- Bossenmeyer-Pourié C, Kannan R, Ribieras S, et al. The trefoil factor 1 participates in gastrointestinal cell differentiation by delaying G1-S phase transition and reducing apoptosis. J Cell Biol 2002;157:761-70. [Crossref] [PubMed]
- Uchino H, Kataoka H, Itoh H, et al. Overexpression of intestinal trefoil factor in human colon carcinoma cells reduces cellular growth in vitro and in vivo. Gastroenterology 2000;118:60-9. [Crossref] [PubMed]
- Chen YJ, Wu H, Zhu JM, et al. MicroRNA-18a modulates P53 expression by targeting IRF2 in gastric cancer patients. J Gastroenterol Hepatol 2016;31:155-63. [Crossref] [PubMed]
- Dürer U, Hartig R, Bang S, et al. TFF3 and EGF induce different migration patterns of intestinal epithelial cells in vitro and trigger increased internalization of E-cadherin. Cell Physiol Biochem 2007;20:329-46. [Crossref] [PubMed]
- Zhu Z, Liu Y, Li K, et al. Protein tyrosine phosphatase receptor U (PTPRU) is required for glioma growth and motility. Carcinogenesis 2014;35:1901-10. [Crossref] [PubMed]
- Liu Y, Zhu Z, Xiong Z, et al. Knockdown of protein tyrosine phosphatase receptor U inhibits growth and motility of gastric cancer cells. Int J Clin Exp Pathol 2014;7:5750-61. [PubMed]
- Giraud AS, Pereira PM, Lars T, et al. TFF-2 inhibits iNOS/NO in monocytes, and nitrated protein in healing colon after colitis. Peptides 2004;25:803-9. [Crossref] [PubMed]
- Rivat C, Rodrigues S, Bruyneel E, et al. Implication of STAT3 signaling in human colonic cancer cells during intestinal trefoil factor 3 (TFF3) -- and vascular endothelial growth factor-mediated cellular invasion and tumor growth. Cancer Res 2005;65:195-202. [PubMed]
- Uribe P, Gonzalez S. Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: molecular bases for EGFR-targeted therapy. Pathol Res Pract 2011;207:337-42. [Crossref] [PubMed]
- Zhang R, Yan ZH, Zhang ZY. Expressions of TFF3, NF-κB and their implications in Helicobacter pylori associated-gastric ulcerand gastric cancer. Chinese Journal of Gastroenterology and Hepatology 2013;22:130-2.
- Kannan N, Kang J, Kong X, et al. Trefoil factor 3 is oncogenic and mediates anti-estrogen resistance in human mammary carcinoma. Neoplasia 2010;12:1041-53. [Crossref] [PubMed]
- Czabotar PE, Colman PM, Huang DC. Bax activation by Bim? Cell Death Differ 2009;16:1187-91. [Crossref] [PubMed]
- Storesund T, Schenck K, Osmundsen H, et al. Signal transduction and gene transcription induced by TFF3 in oral keratinocytes. Eur J Oral Sci 2009;117:511-7. [Crossref] [PubMed]
- Raja SB, Murali MR, Devaraj H, et al. Differential expression of gastric MUC5AC in colonic epithelial cells: TFF3-wired IL1 β/Akt crosstalk-induced mucosal immune response against Shigella dysenteriae infection. J Cell Sci 2012;125:703-13. [Crossref] [PubMed]
- Zhu YQ, Tan XD. TFF3 modulates NF-κB and a novel negative regulatory molecule of NF-κB in intestinal epithelial cells via a mechanism distinct from TNF-α. Am J Physiol Cell Physiol 2005;289:C1085-93. [Crossref] [PubMed]
- Zhaorui S, Hongmei L, Zhizhou Y, et al. Intestinal trefoil factor activates the PI3K/Akt signaling pathway to protect gastric mucosal epithelium from damage. Int J Oncol 2014;45:1123-32. [Crossref] [PubMed]
- Ramsdale R, Jorissen RN, Li FZ, et al. The transcription cofactor c-JUN mediates phenotype switching and BRAF inhibitor resistance in melanoma. Sci Signal 2015;8:ra82. [Crossref] [PubMed]
- Lv B, Yang X, Lv S, et al. CXCR4 Signaling Induced Epithelial-Mesenchymal Transition by PI3K/AKT and ERK Pathways in Glioblastoma. Mol Neurobiol 2015;52:1263-8. [Crossref] [PubMed]
- Christine R, Rivat C, Sylvie R, et al. Implication of STAT3 signaling in human colonic cancer cells during intestinal trefoil factor 3 (TFF3) -- and vascular endothelial growth factor-mediated cellular invasion and tumor growth. Cancer Res 2005;65:195. [PubMed]
- Hua Q, Wang BH, Xu LC. Advances in the relationship between cyclooxygenase-2 and galactose lectin-1 and gastric cancer. Int J Dig Dis 2016;36:37-9.
- Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res 2009;102:19-65. [Crossref] [PubMed]
- Yio X, Diamond M, Zhang JY, et al. Trefoil factor family-1 mutations enhance gastric cancer cell invasion through distinct signaling pathways. Gastroenterology 2006;130:1696-706. [Crossref] [PubMed]
- Lalani EN, Williams R, Jayaram Y, et al. Trefoil factor-2, human spasmolytic polypeptide, promotes branching morphogenesis in MCF-7 cells. Lab Invest 1999;79:537-46. [PubMed]
- Taupin DR, Kinoshita K, Podolsky DK. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci U S A 2000;97:799-804. [Crossref] [PubMed]
- Chen YH, Lu Y, De Plaen IG, et al. Transcription factor NF-kappaB signals antianoikic function of trefoil factor 3 on intestinal epithelial cells. Biochem Biophys Res Commun 2000;274:576-82. [Crossref] [PubMed]
- Perera O, Evans A, Pertziger M, et al. Trefoil factor 3 (TFF3) enhances the oncogenic characteristics of prostate carcinoma cells and reduces sensitivity to ionising radiation. Cancer Lett 2015;361:104-11. [Crossref] [PubMed]
- Emami S, Le Floch N, Bruyneel E, et al. Induction of scattering and cellular invasion by trefoil peptides in src- and RhoA-transformed kidney and colonic epithelial cells. FASEB J 2001;15:351-61. [Crossref] [PubMed]
- Prest SJ, May FE, Westley BR. The estrogen-regulated protein, TFF1, stimulates migration of human breast cancer cells. FASEB J 2002;16:592-4. [Crossref] [PubMed]
- Tu SP, Chi AL, Ai W, et al. p53 inhibition of AP1-dependent TFF2 expression induces apoptosis and inhibits cell migration in gastric cancer cells. Am J Physiol Gastrointest Liver Physiol 2009;297:G385-96. [Crossref] [PubMed]
- Babyatsky M, Lin J, Yio X, et al. Trefoil factor-3 expression in human colon cancer liver metastasis. Clin Exp Metastasis 2009;26:143-51. [Crossref] [PubMed]
- Hu Y, Li Z, Guo L, et al. MAGI-2 Inhibits cell migration and proliferation via PTEN in human hepatocarcinoma cells. Arch Biochem Biophys 2007;467:1-9. [Crossref] [PubMed]
- Rodrigues S, Van Aken E, Van Bocxlaer S, et al. Trefoil peptides as proangiogenic factors in vivo and in vitro: implication of cyclooxygenase-2 and EGF receptor signaling. FASEB J 2003;17:7-16. [Crossref] [PubMed]
- Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 2010;12:319-30. [Crossref] [PubMed]


