Spontaneous tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: a case report
Introduction
Tumor lysis syndrome (TLS) is an oncologic emergency that usually occurs after the initial treatment of a malignant tumor. Chemotherapy often causes lysis of large quantities of tumor cells, leading to the release of intracellular contents into the blood which causes hyperuricaemia, hyperkalaemia, hyperphosphataemia and hypocalcaemia. These electrolyte and metabolic abnormalities ultimately result in symptoms such as acute kidney failure, seizures, cardiac arrhythmias, and even sudden death (1-3).
TLS commonly occurs in hematologic cancers in view of their high cell turnover, rapid proliferation rates and increased chemosensitivity (4). While the incidence of TLS has been increasingly reported in solid tumors (5-9), TLS in gastric cancer is uncommon (10). TLS usually occurs within a week of initiation of cytotoxic therapy and may occur spontaneously in certain circumstances (11-13). Here, we report a rare case of spontaneous TLS in a patient with advanced gastric cancer.
Case presentation
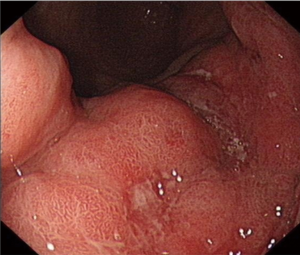
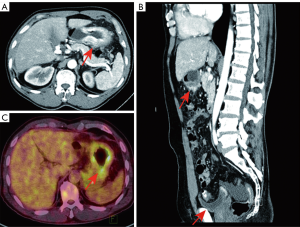
A 62-year-old gentleman was admitted in the Second Affiliated Hospital of Zhejiang University, School of Medicine, with a 20-day history of worsening weakness and poor appetite. This was associated with discomfort of the right flank, hard stools and a loss of weight of 5 kg over one month. No nausea, vomiting or fever were reported. The patient had no history of renal disease. Physical examination on admission revealed BMI of 24.2 (a height of 172 cm and a weight of 71.6 kg). Left cervical lymph nodes were not palpable. Mild tenderness was present in the epigastric region. Gastroscopy showed a huge annular irregular mass on the wall of lower two thirds of the gastric body with peripheral mucosa edema (Figure 1). Biopsy confirmed the presence of a low-grade adenocarcinoma. Abdominal enhanced CT scan revealed a large-scale thickness (size of 9 cm) of the gastric wall with serosa invasion, infiltration of the left lower ureter, multiple enlarged perigastric lymph nodes (lymph nodes No. 2, 4, 5, 6), suspicious metastatic nodes in greater omentum, and a small amount of pelvic effusion (Figure 2). Furthermore, 18F-fluorodeoxyglucose (FDG) PET/CT scan showed that diffuse enhancement in the stomach (SUVmax =6.72), perigastric lymph nodes (SUVmax =4.22), a blurry omentum and a small amount of pelvic effusion (Figure 2).
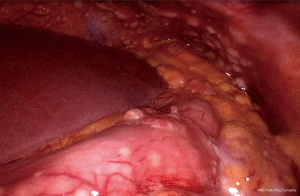
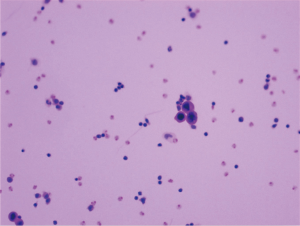
Laboratory findings at admission are as follows: white blood cells: 13.8×109/L (normal range, 4.0–10.0×109/L), hemoglobin: 143 g/L (normal range, 131–172 g/L), sodium:137.4 mmol/L (normal range, 135–145 mmol/L), potassium: 5.32 mmol/L (normal range, 3.50–5.50 mmol/L), calcium: 2.37 mmol/L (normal range, 2.08–2.60 mmol/L), phosphorus: 2.20 mmol/L (normal range, 0.81–1.45 mmol/L), serum creatinine (Cr): 437 µmol/L (normal range, 40–106 µmol/L), uric acid: 8.74 mg/dL (normal range, 3.50–7.20 mg/dL), urea nitrogen: 23.76 mmol/L (normal range, 2.80–7.20 mmol/L), lactic dehydrogenase (LDH): 353 U/L (normal range, <248 U/L), tumor marker CA-199: 926.9 U/mL (normal range, <37 U/mL) and tumor marker CA-242: 20.4 U/mL (normal range, <20 U/mL). Laparoscopic exploration was performed on the ninth day of admission, revealing numerous white nodules in the peritoneum, ligamentum teres hepatis, and pelvic cavity (Figure 3). Intraoperative frozen section one nodule revealed metastatic low-grade adenocarcinoma. Numerous free tumor cells were observed upon microscopic inspection of the peritoneal lavage fluid (Figure 4).
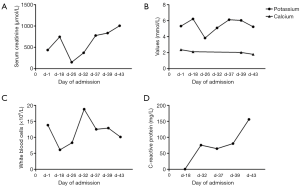
An intraabdominal catheter was inserted in preparation of subsequent intraperitoneal chemotherapy. Surprisingly, the patient’s renal function deteriorated immediately after surgery, as evidenced by a serum creatinine level of 746 µmol/L. On the nineteenth day of admission, bilateral ureteral stents were inserted in order to alleviate ureteral obstruction. Subsequently, serum creatinine declined to 150 µmol/L. Nevertheless, this decrease was short-lived as serum creatinine began to rise gradually again. Potassium and phosphorus levels were slightly increased, while calcium levels remained low during the entire course of treatment. Inflammatory biomarkers such as white blood cells and C-reactive protein (CRP) were relatively high despite anti-infection therapy (Table 1) (Figure 5). The patient was diagnosed as having TLS during multi-disciplinary team (MDT) discussion of the case. Subsequently, the patient was treated with volume expansion, diuretics, sodium bicarbonate, anti-infective therapy, with the aim to improve his electrolyte imbalances. Despite these interventions, serum creatinine continued rising, reaching a peak of 1,005 µmol/L. The patient refused hemodialysis and eventually succumbed to renal failure a month after his initial surgery.
Table 1
| Date | Serum creatinine (μmol/L) | Uric acid (mg/dL) | Potassium (mmol/L) | Phosphorus (mmol/L) | Calcium (mmol/L) | White blood cells (×109/L) | CRP (mg/L) | Urine output per day (mL/kg) |
|---|---|---|---|---|---|---|---|---|
| Day 1 | 437 | 8.74 | 5.32 | 2.20 | 2.37 | 13.8 | – | – |
| Day 18 | 746 | – | 6.22 | – | 2.12 | 6.1 | 0.5 | 48.6 |
| Day 26 | 150 | – | 3.83 | – | – | 8.3 | – | 41.9 |
| Day 32 | 372 | – | 5.10 | – | – | 18.8 | 75.5 | 38.2 |
| Day 37 | 777 | – | 6.12 | – | – | 12.5 | 64.6 | 23.5 |
| Day 39 | 834 | – | 6.04 | 1.95 | 2.03 | 12.9 | 80.4 | 36.2 |
| Day 43 | 1,005 | 6.17 | 5.24 | 2.25 | 1.79 | 10.1 | 156.1 | 8.3 |
CRP, C-reactive protein.
Discussion
TLS is frequently reported during initiation of therapy against malignant tumors. It comprises of a series of metabolic abnormalities caused by massive release of intracellular contents into the blood that exceeds the ability of renal clearance. It is manifested by hyperuricaemia, hyperkalaemia, hyperphosphataemia and hypocalcaemia (1-3). While there is currently no universal definition of TLS, the most accepted TLS classification was proposed by Cairo and Bishop, which was modified from works of Hande and Garrow (14). Based on the Cairo and Bishop classification, TLS is stratified into laboratory TLS (L-TLS) and clinical TLS (C-TLS). L-TLS is defined as changes in any two or more measurable values, including uric acid of 8 mg/dL or greater, potassium 6 mmol/L or greater, phosphorus 2.1 mmol/L or greater, calcium 1.75 mmol/L or less, or a 25% change from baseline in any of these electrolytes. C-TLS is defined as presence of L-TLS and at least one clinical manifestation including renal insufficiency, arrhythmia, seizure or sudden death (15). The patient in our case suffered from hyperuricemia (8.74 mg/dL) and hyperphosphataemia (2.20 mmol/L) at baseline, and experienced a decrease in calcium at almost 25% from baseline as well as along with renal insufficiency (1,005 µmol/L). These findings fulfill the criteria of both L-TLS and C-TLS.
TLS is usually associated with haematological malignancies in view of the large cell turnover, higher proliferation rates and increased chemosensitivity of these cells (4,14,16,17). Despite the increasing reports of TLS in solid tumors, the incidence of TLS in gastric adenocarcinoma is very rare (10). To the best of our knowledge, there were only 5 reports of TLS in gastric adenocarcinoma (10,18-21). Three of them were chemotherapy-induced while another two developed spontaneous TLS. However, the patient in one of the case reports of spontaneous TLS was noted to have received chemotherapy 2 months prior to the development of TLS, while the other case report of a patient with spontaneous TLS was diagnosed at time of presentation. While the risk factors for the development of spontaneous TLS has yet to be identified, previous studies have revealed several risk factors that may be associated with spontaneous TLS in solid tumor, such as tumor extension, a large initial tumor burden, bulky tumors, extensive metastasis, extrinsic compression of the genitourinary tract by the tumor, tumor cells with high proliferative rate, and abnormal pretreatment laboratory findings such as elevated LDH, serum creatinine, and uric acid. Patient-related factors such as preexisting nephropathy, hypotension, and obstructive uropathy are also risk factors for developing TLS (10). In this report, the patient was diagnosed with metastatic gastric cancer, an indication of a large tumor burden. Furthermore, this patient was found to have elevated LDH, serum creatinine, and uric acid levels at the point of presentation. Ureteral obstruction was also present in this patient. All these factors placed this patient at a high risk of developing TLS. Additionally, surgical procedures and severe infection may have further increased the risk of TLS in this patient as operative procedures have the potential to trigger tumor cell death or to stimulate tumor cell growth.
The metabolic abnormalities in TLS, if untreated, will lead to life-threatening complications such as acute kidney failure, seizures, cardiac arrhythmias, and even death. A study by Vodopivec et al. estimates the mortality of TLS in solid tumors to be approximately 41% (10). Early recognition and prevention are especially crucial for patients at high risk. Treatment of TLS focuses on correction of electrolyte disturbances and preservation of renal function. A typical TLS treatment regimen involves volume expansion, urinary alkalinisation, allopurinol, rasburicase, or even dialytic modalities if necessary (1,22). Our patient received volume expansion, urinary alkalinisation and correction of electrolyte disturbances as soon as he was diagnosed as TLS. Nevertheless, his renal function continued to deteriorate, and without the initiation of life-saving haemodialysis, this patient eventually succumbed to his disease.
Acknowledgments
We acknowledge Liang Han for his revision of English grammar. We acknowledge Lin Chen for providing PET-CT imaging.
Funding: This study was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.53). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the relatives of the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis 2014;21:18-26. [Crossref] [PubMed]
- Davidson MB, Thakkar S, Hix JK, et al. Pathophysiology, clinical consequences, and treatment of tumor lysis syndrome. Am J Med 2004;116:546-54. [Crossref] [PubMed]
- Sobota J, Püsküllüoglu M, Zygulska AL. Oncological emergencies: tumor lysis syndrome. Przegl Lek 2014;71:210-4. [PubMed]
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med 2011;364:1844-54. [Crossref] [PubMed]
- Kim JO, Jun DW, Tae HJ, et al. Low-dose steroid-induced tumor lysis syndrome in a hepatocellular carcinoma patient. Clin Mol Hepatol 2015;21:85-8. [Crossref] [PubMed]
- Taira F, Horimoto Y, Saito M. Tumor lysis syndrome following trastuzumab for breast cancer: a case report and review of the literature. Breast Cancer 2015;22:664-8. [Crossref] [PubMed]
- Noyes AM, Lonial K, Siegel RD. Tumor lysis syndrome in a nonsmall cell lung cancer. Conn Med 2014;78:421-3. [PubMed]
- Norberg SM, Oros M, Birkenbach M, et al. Spontaneous tumor lysis syndrome in renal cell carcinoma: a case report. Clin Genitourin Cancer 2014;12:e225-7. [Crossref] [PubMed]
- Takeuchi N, Miyazawa S, Ohno Z, et al. A Case of Spontaneous Tumor Lysis Syndrome in Malignant Melanoma. World J Oncol 2016;7:40-4. [Crossref] [PubMed]
- Vodopivec DM, Rubio JE, Fornoni A, et al. An unusual presentation of tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: case report and literature review. Case Rep Med 2012;2012:468452. [Crossref] [PubMed]
- Jasek AM, Day HJ. Acute spontaneous tumor lysis syndrome. Am J Hematol 1994;47:129-31. [Crossref] [PubMed]
- Agarwala R, Batta A, Suryadevera V, et al. Spontaneous tumour lysis syndrome in hepatocellular carcinoma presenting with hypocalcemic tetany: An unusual case and systematic literature review. Clin Res Hepatol Gastroenterol 2017;41:e29-31. [Crossref] [PubMed]
- Weerasinghe C, Zaarour M, Arnaout S, et al. Spontaneous Tumor Lysis Syndrome in Small-Cell Lung Cancer: A Rare Complication. World J Oncol 2015;6:464-71. [Crossref] [PubMed]
- Hande KR, Garrow GC. Acute tumor lysis syndrome in patients with high-grade non-Hodgkin's lymphoma. Am J Med 1993;94:133-9. [Crossref] [PubMed]
- Burns RA, Topoz I, Reynolds SL. Tumor lysis syndrome: risk factors, diagnosis, and management. Pediatr Emerg Care 2014;30:571-6; quiz 577-9. [Crossref] [PubMed]
- Belay Y, Yirdaw K, Enawgaw B. Tumor Lysis Syndrome in Patients with Hematological Malignancies. J Oncol 2017;2017:9684909. [Crossref] [PubMed]
- Busakhala W, Joshi MD, Abinya NO, et al. Incidence of chemotherapy-related tumour lysis syndrome at Kenyatta National Hospital, Nairobi. East Afr Med J 2007;84:100-9. [PubMed]
- Woo IS, Kim JS, Park MJ, et al. Spontaneous acute tumor lysis syndrome with advanced gastric cancer. J Korean Med Sci 2001;16:115-8. [Crossref] [PubMed]
- Goyal H, Sawhney H, Bekara S, et al. Spontaneous acute tumour lysis syndrome in gastric adenocarcinoma: a case report and literature review. J Gastrointest Cancer 2014;45:208-11. [Crossref] [PubMed]
- Han HS, Park SR, Kim SY, et al. Tumor lysis syndrome after capecitabine plus cisplatin treatment in advanced gastric cancer. J Clin Oncol 2008;26:1006-8. [Crossref] [PubMed]
- Kobayashi T, Kuwai T, Yamamoto S, et al. Acute tumor lysis syndrome in the setting of advanced gastric cancer. Nihon Shokakibyo Gakkai Zasshi 2012;109:1372-8. [PubMed]
- Mika D, Ahmad S, Guruvayoorappan C. Tumour lysis syndrome: implications for cancer therapy. Asian Pac J Cancer Prev 2012;13:3555-60. [Crossref] [PubMed]