
A scoring model combining serum alpha-fetoprotein and tumor size and number predicts prognosis in hepatitis B virus-related hepatocellular carcinoma patients after curative hepatectomy
Introduction
Primary hepatocellular cancer (HCC) is common worldwide and is one of the most common causes of cancer mortality (1-4). Currently, surgical treatment is available to improve outcomes in some selected patients (5). However, primary lesion, tumor metastasis, and concomitant underlying diseases (such as cirrhosis) should also be taken into consideration for surgical resection.
Various cancer stage systems have been proposed to stratify HCC patients to help physicians decide on surgical treatment. Some common stage systems are tumor-node-metastasis (TNM) stage, Okuda staging, Cancer of the liver Italian Program (CLIP) score, Barcelona Clinic Liver Cancer (BCLC) stage, Chinese University Prognostic Index (CUPI), and Japanese Integrated Scoring (JIS) (6-10). The European Association for Study of Liver (EASL) in 2012 and the American Associations for Study of Liver Diseases (AASLD) in 2017 published guidelines for HCC treatment reporting well-defined hepatectomy indications for HCC: patients with a single HCC <5 cm or up to 3 tumors all less than 3 cm with completely preserved liver function and no portal hypertension (11,12). However, the above guidelines excluded some potentially resectable HCC patients to some extent.
The use of neoadjuvant therapies for successful curative interventions in down-staging HCC has seen a significant rise owing to the recent advances in oncology. Adjuvant therapies, however, were already in use to control local recurrence or distant metastasis before acceptance for use in HCC. HCC patients with portal vein hypertension have a more dismal outcome in contrast to compensated patients. The effects of cirrhosis on surgery are unclear, given the existence of different clinical stages in the histological variability of cirrhosis. So it is worth noting that some cirrhosis patients without portal vein hypertension might have curative hepatectomy beyond limitation on anatomical information (tumor size and number). Nevertheless, There is a need for a new accurate prediction system for surgical resection among HCC patients.
Serum AFP is a widely used cancerous biomarker for hepatocarcinogenesis and is a prognostic indicator for recurrence (13,14). Higher AFP levels reportedly are associated with nodular size, microvascular invasion (MVI), and other factors (15-18). A French study group subsequently combined blood AFP level with nodules number and size, considering the simplicity and feasibility, and the result showed the effect of integration on prognosis after liver transplantation (16,19,20). However, whether the AFP score model could predict the prognosis of HCC patients after liver resection needs further research.
To that effect, we proposed the AFP score model based on the preoperative AFP levels combined with nodule number and size to predict the prognosis of HCC patients after liver resection in the present study.
Methods
Patients and clinicopathological information
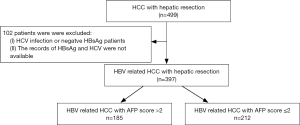
We recruited 397 patients with HCC from the Prince of Wales Hospital, Hong Kong, China, from November 2001 to November 2013. The criteria for patient inclusion were: (I) the presence of positive HBsAg; (II) liver function tests showing Child-Pugh grade A and clearance of indocyanine green less than 15% at 15 minutes (ICG-R15); (III) absence of distant metastasis; (IV) patients who accepted curative liver resection; (V) patients without autoimmune liver diseases or serious heart, lung, kidney, or blood diseases. The criteria for patient exclusion were: (I) the presence of other types of malignant tumors; (II) accompanied by portal vein hypertension; (III) the presence of other types of hepatic viral infections. Chart flow for patient selection is shown in Figure 1. All patients or their legal representatives gave written informed consent for all investigations and inquiries that were conducted during this study. The hospital ethical committee approved the design of the study.
Serum AFP concentration was measured using the electrochemiluminescence immunoassay (E170 Analytics; Roche Diagnostics Corp., Indianapolis, IN) (21). The AFP data included in this analysis are preoperative measurements obtained before the date of resection. The data on other biochemical markers, including albumin, alanine aminotransferase (ALT), and bilirubin, were retrieved from the medical records of patients. The degree of fibrosis (including cirrhosis) was diagnosed by imaging examination (ultrasonography, CT, or MRI) or pathology. Microvascular invasiveness was diagnosed in liver specimens by pathology. Following resection, all liver specimens were examined by a single, dedicated liver pathologist who was blinded to all patients’ identities and clinical outcomes.
AFP score model
The AFP score is calculated by adding individual points for each obtained variable, as shown in Table 1 and was calculated for each patient enrolled in the study (Table 1).
Table 1
| Variables | Point |
|---|---|
| Largest diameter (cm) | |
| ≤3 | 0 |
| 3–6 | 1 |
| >6 | 4 |
| Number of nodules | |
| 1–3 | 0 |
| 4 and more | 2 |
| AFP level (μg/L) | |
| ≤100 | 0 |
| 100–1,000 | 2 |
| >1,000 | 3 |
AFP, alpha-fetoprotein.
Follow-up evaluations
Follow up evaluation were performed in outpatients every three months during the first postoperative year, every four months of the second postoperative year, and every six months after that. Images with CT or MRI were obtained during postoperative follow-up examinations. Tumor recurrence was diagnosed using CT or MRI scans. OS was defined as the time from operation to death or 8 November 2018; DFS as the time from curative hepatectomy to the first occurrence of either intra- or extra-hepatocellular metastasis.
Statistical analysis
All statistical analyses were performed by SPSS 20. Continuous variables are shown as the means ± standard deviation. The One-way analysis of variance (ANOVA) was used to identify significant differences. Categorical variables by the chi-squared test were used to identify significant differences between groups. The ROC curve was generated to identify the AFP score to categorize survived patients. A Kaplan-Meier survival curve was used to estimate recurrence and survival. The univariable Cox regression was carried out to evaluate the effects of various clinicopathological variables on survival. Only results with a P value <0.05 were considered statistically significant.
Results
Patients characteristics
A total of 397 patients were eligible for the present study. All the patients received curative resection for primary tumors, as well as laparoscopic hepatectomy (LH) (n=161) and open hepatectomy (OH) (n=236, including patients who reverted from LH to OH). There were 302 patients with clear information about blood loss. The mean blood loss volume of the patients was 223.54±48.91 mL. The demography, antiviral treatment history, neoadjuvant or adjuvant treatment, information about the operation, and the biochemical and clinical characteristics of the included patients are presented in Table 2. Two hundred and forty patients had no antiviral treatment history, while 119 patients had received antiviral treatment before hepatic resection. The other 38 patients were not sure if they had had antiviral treatment or not. In the retrospective study, 128 patients had postoperative treatment history, and 43 patients had received neoadjuvant treatment.
Table 2
| Clinical character | All patients |
|---|---|
| Age (years) | 56.42±9.96 [27–81] |
| Gender (female/male) | 53/344 |
| Differentiation (well/moderate/poor) | 46/308/43 |
| AJCC stages (1/2/3) | 248/101/48 |
| Biology pre-operation | |
| ALT (U/L) | 51.88±38.39 [10–283] |
| Albumin (g/L) | 40.52±4.93 [24–81] |
| Bilirubin (μmol/L) | 11.18±6.27 [3–83] |
| Vascular invasion (negative/positive) | 302/95 |
| Degree of fibrosis (normal/fibrosis/cirrhosis) | 38/123/236 |
| HCC features | |
| Tumor diameter (cm) | 4.73±3.33 [1–24] |
| Tumor number | 1.29±0.77 [1–7] |
| AFP (μg/L) | 2,220.36±7,405.23 [1–69,980] |
| Neoadjuvant treatment (yes/no) | 43/354 |
| Adjuvant treatment (yes/no) | 128/269 |
| Anti-virology treatment (yes/no/unclear) | 119/240/38 |
| Operation modality (LH/OH) | 161/236 |
| Blood loss (mL) | 223.54±48.91 |
ALT, alanine aminotransferase; LH, laparoscopic hepatectomy; OH, open hepatectomy.
During the followed-up period, the mean OS and DFS were 66.38±49.03 and 48.68±48.10 m, respectively. Furthermore, the number of patients with AFP ≤100, 100 µg/L < AFP <1,000 µg/L and ≥1,000 µg/L were 241, 83, and 73, respectively. The difference in the rate of OS between the three groups was significant (P=0.003). There were 167, 138, and 92 patients with tumor sizes ≤3, ≤6, and >3 and >6 cm, respectively. The difference in the rate of OS and DFS between the three groups was significant (P=0.000). Although the patients with tumor size >6 cm and AFP level >1,000 µg/L are fewer than the tumor sizes ≤6 cm and AFP levels ≤1,000 µg/L, the proportion of the patients cannot be ignored.
Determination of cut-off values for AFP score model
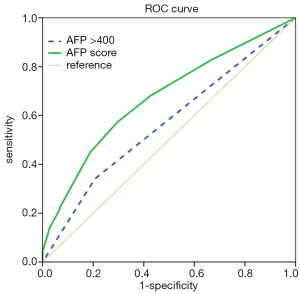
We used ROC curve analyses to test for the AFP score model’s capacity as a prognostic factor. As the results in Figure 2 show, the AUC of the AFP score model and AFP >400 µg/L were 0.673 [95% confidence interval (CI) =0.619–0.726] and 0.567 (95% CI =0.510–0.624), respectively, for predicting survival. Furthermore, we performed ROC curve analyses to identify the optimal cut-off value, and the value we obtained for AFP score model cutoff was 2.
Impact of the AFP score model on overall survival (OS) and disease-free survival (DFS)
Of the 397 patients selected for our studies, 185 patients were put in the AFP score >2 group, and 212 in the AFP score ≤2 group. As shown in Table 3, the patients with an AFP score >2 had significant differences in age (P=0.035), tumor differentiation (P=0.037), tumor size (P=0.000), tumor number (P=0.005), vascular invasion (P=0.000), cirrhosis (P=0.039), albumin (P=0.000), white blood cells (WBCs) (P=0.013), platelets (PLT) (P=0.000), neoadjuvant treatment (P=0.012), adjuvant treatment (P=0.003), antiviral treatment (P=0.004), and operation choice (P=0.000). However, the two groups had no significant difference in some factors (such as gender, ALT, bilirubin, RBC, HBV DNA level, and blood loss) between them.
Table 3
| Clinical character | Subgroup | P value | |
|---|---|---|---|
| AFP score >2 | AFP score ≤2 | ||
| Age (years) | 55.20±10.74 | 57.32±9.24 | 0.035 |
| Gender (female/male) | 21/148 | 32/196 | 0.658 |
| Differentiation (well/moderate/poor) | 12/135/22 | 34/173/21 | 0.037 |
| ALT (U/L) | 54.10±38.71 | 50.24±38.16 | 0.323 |
| Albumin (g/L) | 39.48±5.68 | 41.29±4.14 | 0.000 |
| Bilirubin (μmol/L) | 11.16±8.02 | 11.22±4.59 | 0.953 |
| RBC (×1012/L) | 13.67±1.84 | 14.69±9.41 | 0.166 |
| WBC (×109/L) | 6.60±1.86 | 5.83±1.80 | 0.013 |
| PLT (×1012/L) | 192.95±76.23 | 161.63±71.88 | 0.000 |
| HBV DNA (copies/mL) | 12,627,910.55±40,195,981.53 | 18,218,314.12±47,020,864.95 | 0.577 |
| LH/OH | 87/82 | 74/154 | 0.000 |
| Blood loss (mL) | 231.87±36.70 | 212.67±42.27 | 0.732 |
| Antiviral treatment (yes/no) | 36/133 | 83/145 | 0.004 |
| Neoadjuvant treatment (yes/no) | 26/143 | 17/211 | 0.012 |
| Adjuvant treatment (yes/no) | 68/101 | 60/168 | 0.003 |
| MVI (negative/positive) | 103/66 | 199/29 | 0.000 |
| Cirrhosis (negative/positive) | 79/90 | 82/146 | 0.039 |
| Differentiation (well/moderate/poor) | 12/135/22 | 34/173/21 | 0.037 |
| Tumor diameter | 7.03±3.82 | 3.02±1.31 | 0.000 |
| Tumor number | 1.43±1.007 | 1.20±0.558 | 0.005 |
| AFP (μg/L) | 5,063.23±10,737.89 | 125.62±455.5 | 0.000 |
ALT, alanine aminotransferase; RBC, red blood cell; WBC, white blood cell; PLT, platelets; HBV, hepatitis B virus; LH, laparoscopic hepatectomy; OH, open hepatectomy; MVI, microvascular invasion; AFP, alpha-fetoprotein.
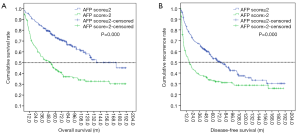
We also estimated the cumulative OS and PFS using the Kaplan-Meier method (Figure 3). The results showed that the 1-, 3-, and 5-year OS incidences were 95.6%, 85.0%, and 79.6%, respectively, in the AFP score ≤2 group, while the 1-, 3-, and 5-year OS incidences were 94.1%, 79.7%, and 65.9%, respectively, in the AFP score >2 group. The OS rate in the AFP score >2 group was significantly lower than that in the AFP score ≤2 group (37.3% vs. 65.40%, P=0.000). Similarly, the recurrence rate in the AFP score >2 group was significantly higher than that in the AFP score ≤2 group (65.1% vs. 51.8%, P=0.010). Furthermore, the results showed that there were significant differences in the median OS and DFS between the two groups. The median OS in the AFP score ≤2 and AFP score >2 groups were 173.4±1.00 and 50.30±8.67 m, respectively, with a significant difference (P=0.000). In the same way, the median PFS in the AFP score >2 group was shorter than that in the AFP score ≤2 group (17.20±3.66 vs. 73.7±10.39 m, P=0.000).
Prognostic risk factors for OS and DFS
Some factors without differences in the two groups did not undergo univariate and multivariate analyses. The important clinical factors and prognostic factors that were compared by univariate and multivariate analyses to identify potential risk factors are shown in Table 4. The univariate analysis revealed that AFP score (HR =3.173, 95% CI: 2.097–4.802, P=0.000), MVI (HR =2.427, 95% CI: 1.509–3.906, P=0.000), and cirrhosis (HR =1.980, 95% CI: 1.314–2.984, P=0.001) were risk factors for OS. Ultimately, the multivariate Cox proportional hazards model confirmed that AFP score (HR =0.563, 95% CI: 0.398–0.798, P=0.001), MVI (HR =0.653, 95% CI: 0.441–0.967, P=0.033), and cirrhosis (HR =0.358, 95% CI: 0.185–0.696, P=0.002) were indeed risk factors of OS.
Table 4
| Variable | OS | DFS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||||
| Gender (female/male) | 1.521 | 0.839–2.756 | 0.165 | – | – | – | 1.354 | 0.758–2.417 | 0.305 | – | – | – | |||
| Differentiation (well/moderate-poor) | 1.153 | 0.621–2.143 | 0.652 | – | – | – | 1.043 | 0.561–1.939 | 0.895 | – | – | – | |||
| Antiviral treatment (no/yes) | 0.731 | 0.473–1.128 | 0.156 | – | – | – | 1.215 | 0.809–1.825 | 0.348 | – | – | – | |||
| Neoadjuvant treatment (no/yes) | 0.724 | 0.379–1.381 | 0.325 | – | – | – | 1.437 | 0.742–2.785 | 0.280 | – | – | – | |||
| Adjuvant treatment (no/yes) | 1.281 | 0.840–1.953 | 0.249 | – | – | – | 1.125 | 0.734–1.725 | 0.589 | – | – | – | |||
| LH/OH | 1.086 | 0.727–1.623 | 0.686 | – | – | – | – | – | – | – | – | – | |||
| MVI (negative/positive) | 2.427 | 1.509–3.906 | 0.000 | 0.653 | 0.441–0.967 | 0.033 | 2.387 | 1.441–3.953 | 0.001 | 1.589 | 1.496–2.854 | 0.003 | |||
| Cirrhosis (negative/positive) | 1.980 | 1.314–2.984 | 0.001 | 0.358 | 0.185–0.696 | 0.002 | 1.435 | 0.957–2.151 | 0.080 | – | – | – | |||
| AFP score model (AFP score ≤2/AFP score >2) | 3.173 | 2.097–4.802 | 0.000 | 0.563 | 0.398–0.798 | 0.001 | 1.738 | 1.154–2.617 | 0.008 | 0.876 | 0.404–0.925 | 0.040 | |||
OS, overall survival; DFS, disease-free survival; LH, laparoscopic hepatectomy; OH, open hepatectomy; MVI, microvascular invasion; AFP, alpha-fetoprotein.
Univariate analysis, likewise, showed (Table 4) that AFP score (HR =1.738, 95% CI: 1.154–2.617, P=0.008) and MVI (HR =2.387, 95% CI: 1.441–3.953, P=0.001) were risk factors for DFS, an outcome predictably backed by the multivariate Cox proportional hazards model with findings of MVI (HR =1.589, 95% CI: 1.496–2.854, P=0.003) and AFP score (HR =0.876, 95% CI: 0.404–0.925, P=0.040).
The prognostic significance of MVI or cirrhosis as stratified by the AFP score model on OS
We explored the prospect of prognostic significance on OS when stratified by the AFP score model combined with MVI or cirrhosis. According to stratification by the AFP score with MVI (Figure 4A), the mean OS in the AFP score >2 combined with the MVI group compared with no MVI group were 65.58±9.18 and 94.21±8.25 m, respectively, with significant difference (P=0.024). Equally, the mean OS in the AFP score ≤2 group combined with the MVI group compared with no MVI group were 87.76±13.14 and 129.66±5.89 m, respectively, (P=0.064). Also shown in Figure 4A are the 1-, 3-, and 5-year OS rates in the AFP score >2 group combined with the MVI group compared with the score >2 without the MVI group at 74.2%, 43.9%, and 31% and 80.3%, 63.3%, and 52.6%, respectively. The 1-, 3-, and 5-year OS rates in the AFP score ≤2 group combined with MVI group compared with AFP score ≤2 without the MVI group were 91.3%, 73.9%, and 64.1% and 95.1%, 84.3%, and 74.7%, respectively. From these data, the performance of the AFP score >2 model combined with MVI in predicting OS was the most remarkable one. ROC curve analyses (Figure 4B) also showed that the AUC of the AFP score combined with MVI, AFP score alone, and MVI alone were 0.658 (P=0.000, 95% CI: 0.604–0.712), 0.638 (P=0.000, 95% CI: 0.583–0.693), and 0.580 (P=0.006, 95% CI: 0.523–0.636), respectively. The findings suggest that the predictability of the AFP score alone or with MVI is more sensitive to survival, and both were superior to that of MVI alone.
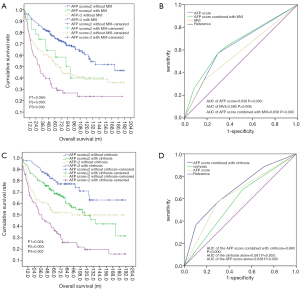
We next analyzed the impact of combining the AFP score with cirrhosis on OS. As shown in Figure 4C, compared with the mean OS (145.31±8.38 m) in the AFP score ≤2 group without cirrhosis, the mean OS in the AFP score ≤2 group combined with the cirrhosis group, AFP score >2 group without the cirrhosis group, and the AFP score >2 group combined with the cirrhosis group were 114.71±7.30, 106.41±9.61, and 64.08±7.38 m, respectively, with significance differences (P1=0.024, P2=0.000, and P3=0.000). Figure 4C also shows that the 1-, 3-, and 5-year OS rates in the AFP score >2 group combined with the cirrhosis group compared to AFP score >2 without cirrhosis were 76.5%, 50.4%, and 37.6% and 79.8%, 62.0%, and 52.0%, respectively. The 1-, 3-, and 5-year OS rates in the AFP score ≤2 group combined with the cirrhosis group and in the AFP score ≤2 without cirrhosis were 92.4%, 79.9%, and 69.6% and 97.6%, 88.9%, and 80.5%, respectively. From the data above, the performance of AFP score >2 combined with cirrhosis in predicting OS was the most remarkable one. ROC curve (Figure 4D) also showed that the AUC of the AFP score combined with cirrhosis, AFP score alone, and cirrhosis alone were 0.683 (P=0.000, 95% CI: 0.631–0.736), 0.638 (P=0.000, 95% CI: 0.583–0.693), and 0.581 (P=0.005, 95% CI: 0.525–0.637), respectively.
Discussion
This study explores the value of the AFP score model in the prognosis of HBV-related HCC patients after liver resection. Based on the Kaplan-Meier analysis, patients with an AFP score >2 displayed worse outcomes. Also, we demonstrated that an AFP score >2 with MVI and cirrhosis could further highlight patients with very poor prognosis.
Previous studies have highlighted the interaction between AFP levels and other biological and pathophysiological roles in cancer (17,18). Our group reported previously that preoperative serum AFP levels >400 µg/L predict poor overall and recurrence-free survival after hepatectomy (22). In the present study ROC curves showed further that the AFP score model could predict outcomes effectively after HCC resection when the AFP score value is 2. Notably, applying the ROC curve method, the present study showed that an AFP score >2 has a better sensitivity or specificity in predicting survival than AFP >400 µg/L alone. The AFP score >2 exhibited superior categorization of HCC prognoses after resection, which could be used to stratify patients and guide follow-up. The result is similar to previous reports that the AFP score model has a prognostic value in HCC recurrence after liver transplantation (20). The above data suggest that the AFP score model might be a powerful prognostic marker for HBV-related HCC patients after radical resection.
Combining several other factors with the AFP score model would provide comprehensive and individualized risk assessments. It is accepted generally that MVI and cirrhosis are strong prognostic factors for poor outcome after liver resection (23,24). Our past data had also confirmed the effects of cirrhosis alone on prognosis in HCC patients after resection (25). In the present study, we combined the AFP score with MVI or cirrhosis to stratify patients according to responses to therapy. Data obtained from these inquisitions showed that an AFP score >2 with or without MVI decreased the risk of survival significantly. The ROC curve results indicate that the new combination of higher AFP scores with MVI at an initial visit predicted more poor survivals, which may help guide clinicians to predict disease prognosis. On the other hand, AFP ≤2 with MVI induced no significant difference in OS.
A previous study reported that MVI is common in HCC patients with large size and multiple nodules (10). Nevertheless, the effect of AFP on angiogenesis in HCC remains unclear to date. So whether an AFP score ≤2 is related to MVI or not needs further research. We added more confidence in the ability to predict clinical prognosis by combining the AFP score and cirrhosis rather than go with cirrhosis alone. A chronic viral infection reportedly induces inflammation, which progresses eventually to cirrhosis. Chronic viral inflammation and the degree of fibrosis play very important roles in determining postoperative survival and DFS in patients with HCC (26-28). However, we did not, at this time, compare the degree of cirrhosis in predicting the prognosis of HCC. We expect to investigate these claims in subsequent studies.
It is accepted widely that proper treatment affects the progression and prognosis of HCC, with the outcome of antiviral therapy on HBV-related HCC recurrence after hepatic resection an example of positivity (29). However, we did not observe a similar significant improvement in survival among the 119 patients with clear antiviral treatment before hepatic resection. More research should stratify patients according to the type of antiviral treatment, HBV DNA level, and immune status before surgical resection.
It is worth noting that surgical resection offers a curative option and a better OS. Some meta-analyses have revealed significantly improved outcomes following LH in patients with HCC and underlying cirrhosis (30,31). In the real world, patients receive surgery and perioperative anti-treatment to improve OS and DFS. Adjuvant and neoadjuvant treatments have gained popularity according to the therapeutic algorithms for CC. Previous research has shown that local ablation, TACE, and sorafenib provide significant survival benefits (32-35). Yet, in the present study, LH and OH provided a similar impact on survival or recurrence. Although neoadjuvant/adjuvant therapy was used to downstage and control distant metastasis, no improvements were observed in survival and recurrence in our patients. As a matter of fact, in this present research, the relatively relaxed included criteria and insufficient data might have led to negative results. New clinical trials to determine the effects of anticancer therapy in HCC patients are needed.
Some limitations to the present study should be noted. First, this is a retrospective study from a single center. Second, the selection criteria for HCC patients were very restrictive, with patients who did not have blood AFP data, patients who presented incomplete medical records, those with relatively worse liver function, or those who failed to be followed up excluded. So, the study sample size was relatively small, especially for the subgroup analysis. Therefore, comprehensive, randomized multicenter studies are required to recruit high homogeneous patients and analyze patients with complete data.
In conclusion, our data suggest that the AFP model categorizes HCC patients with relatively good liver functions after radical resection into low and high-risk prognoses. This important finding suggests that the adoption of the AFP score model combined with MVI or cirrhosis is a powerful prognostic predictor for HBV-related HCC. Comparisons of pathological features and liver disease etiology between the high and low AFP score groups warrants further research.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.49). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The hospital ethical committee approved the design of the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Are C, Meyer B, Stack A, et al. Global trends in the burden of liver cancer. J Surg Oncol 2017;115:591-602. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians 2015;65:87-108. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Zamora-Valdes D, Taner T, Nagorney DM. Surgical Treatment of Hepatocellular Carcinoma. Cancer Control 2017;24:1073274817729258. [Crossref] [PubMed]
- Faria SC, Szklaruk J, Kaseb AO, et al. TNM/Okuda/Barcelona/UNOS/CLIP International Multidisciplinary Classification of Hepatocellular Carcinoma: concepts, perspectives, and radiologic implications. Abdom Imaging 2014;39:1070-87. [Crossref] [PubMed]
- Matsukuma S, Sakamoto K, Tokuhisa Y, et al. Outcomes following liver resection for multinodular Barcelona Clinic Liver Cancer-B hepatocellular carcinoma. Oncol Lett 2018;16:6383-92. [PubMed]
- Zhang Y, Chen SW, Liu LL, et al. A model combining TNM stage and tumor size shows utility in predicting recurrence among patients with hepatocellular carcinoma after resection. Cancer Manag Res 2018;10:3707-15. [Crossref] [PubMed]
- Chan EE, Chow PK. A review of prognostic scores after liver resection in hepatocellular carcinoma: the MSKCC, SLICER and SSCLIP scores. Jpn J Clin Oncol 2017;47:287-93. [PubMed]
- Zhang X, Li J, Shen F, et al. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol 2018;33:347-54. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- European Association For The Study Of The Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Ahmed Mohammed HF, Roberts LR. Should AFP (or any biomarkers) be used for HCC surveillance? Curr Hepatol Rep 2017;16:137-45. [Crossref] [PubMed]
- Lersritwimanmaen P, Nimanong S. HCC surveillance benefit of serum AFP in real world practice. Euroasian J Hepatogastroenterol 2018;8:83-7. [Crossref] [PubMed]
- Zhao H, Hua Y, Lu Z, et al. prognostic value and preoperative predictors of MVI in solitary HCC less than 5cm without macrovascular invasion. Oncotarget 2017;8:61203-14. [PubMed]
- McHugh PP, Gilbert J, Vera S, et al. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB (Oxford) 2010;12:56-61. [Crossref] [PubMed]
- Lu S, Ma Y, Sun T, et al. Expression of alpha-fetoprotein in gastric cancer AGS cells contributes to invasion and metastasis by influencing anoikis sensitivity. Oncol Rep 2016;35:2984-90. [Crossref] [PubMed]
- Li P, Wang SS, Liu H, et al. Elevated serum alpha fetoprotein levels promote pathological progression of hepatocellular carcinoma. World J Gastroenterol 2011;17:4563-71. [Crossref] [PubMed]
- Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-94.e3; quiz e14-5.
- Notarpaolo A, Layese R, Magistri P, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol 2017;66:552-9. [Crossref] [PubMed]
- Chan SL, Mo F, Johnson PJ, et al. Performance of serum alpha-fetoprotein levels in the diagnosis of hepatocellular carcinoma in patients with a hepatic mass. HPB (Oxford) 2014;16:366-72. [Crossref] [PubMed]
- Yang SL, Liu LP, Yang S, et al. Preoperative serum α-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg 2016;103:716-24. [Crossref] [PubMed]
- Huang JL, Fu YP, Jing CY, et al. A novel and validated prognostic nomogram based on liver fibrosis and tumor burden for patients with hepatocellular carcinoma after curative resection. J Surg Oncol 2018;117:625-33. [Crossref] [PubMed]
- O'Rourke JM, Sagar VM, Shah T, et al. Carcinogenesis on the background of liver fibrosis: Implications for the management of hepatocellular cancer. World J Gastroenterol 2018;24:4436-47. [Crossref] [PubMed]
- Yang SL, Liu LP, Sun YF, et al. Distinguished prognosis after hepatectomy of HBV-related hepatocellular carcinoma with or without cirrhosis: a long-term follow-up analysis. J Gastroenterol 2016;51:722-32. [Crossref] [PubMed]
- Kamarajah SK. Fibrosis score impacts survival following resection for hepatocellular carcinoma (HCC): A Surveillance, End Results and Epidemiology (SEER) database analysis. Asian J Surg 2018;41:551-61. [Crossref] [PubMed]
- Lee DH, Lee JM, Chang W, et al. Prognostic Role of Liver Stiffness Measurements Using Magnetic Resonance Elastography in Patients with Compensated Chronic Liver Disease. Eur Radiol 2018;28:3513-21. [Crossref] [PubMed]
- Yang JD, Mannalithara A, Piscitello AJ, et al. Impact of surveillance for hepatocellular carcinoma on survival in patients with compensated cirrhosis. Hepatology 2018;68:78-88. [Crossref] [PubMed]
- Wong JS, Wong GL, Tsoi KK, et al. Meta-analysis: the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther 2011;33:1104-12. [Crossref] [PubMed]
- Goh EL, Chidambaram S, Ma S. Laparoscopic vs open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: A meta-analysis of the long-term survival outcomes. Int J Surg 2018;50:35-42. [Crossref] [PubMed]
- Twaij A, Pucher PH, Sodergren MH, et al. Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: systematic review and meta-analysis. World J Gastroenterol 2014;20:8274-81. [Crossref] [PubMed]
- Thandassery RB, Goenka U, Goenka MK. Role of local ablative therapy for hepatocellular carcinoma. J Clin Exp Hepatol 2014;4:S104-11. [Crossref] [PubMed]
- Katsanos K, Kitrou P, Spiliopoulos S, et al. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. PLoS One 2017;12:e0184597. [Crossref] [PubMed]
- Kim HC. Radioembolization for the treatment of hepatocellular carcinoma. Clin Mol Hepatol 2017;23:109-14. [Crossref] [PubMed]
- Ziogas IA, Tsoulfas G. Evolving role of Sorafenib in the management of hepatocellular carcinoma. World J Clin Oncol 2017;8:203-13. [Crossref] [PubMed]


