
CD147 promotes glucose metabolism, invasion and metastasis via PI3K/AKT pathway in oral squamous cell carcinomas
Introduction
The incidence of oral cavity cancer has increased significantly among Asian males and females in the last few decades (1). The majority (>90%) of the oral tumors are squamous cell carcinomas, which are often not detected until the late stage of the disease (2). In addition, the 5-year survival rate for oral cancer is a dismal 50–65% due to dysplastic precancerous lesions and lymphatic metastasis (3). The aggressive nature of this malignancy and frequent metastasis calls for novel therapeutic and prognostic strategies. CD147 (Basigin, EMMPRIN), a cell surface glycoprotein, is highly expressed on the surface of malignant tumor cells, and promote their invasiveness and metastasis (4-6). Recent studies report CD147 overexpression in oral tumor tissues as well as the corresponding cell lines (7-9), with a direct role in the progression of oral squamous cell carcinoma (OSCC) (8). In addition, CD147 overexpression in OSCC tissues is associated with poor prognosis (9), indicating its potential as both a prognostic marker as well as a therapeutic target (10). Since studies have also implicated CD147 as a crucial regulator of glucose metabolism in cancer cells (11-13), we hypothesized that the oncogenic effects of CD147 are mediated via regulation of glycometabolism. To this end, we knocked down CD147 in human OSCC cell lines, and observed the effects on their in vitro migration, invasiveness and glycometabolism. Our findings provide new insights into the molecular mechanisms governing OSCC metastasis, and present CD147 as a novel prognostic marker and therapeutic target.
Methods
Cell culture
The human OSCC cell lines SCC-25 and CAL-27 were purchased from the American Type Culture Collection (ATCC). SCC-25 was cultured in Dulbecco’s Modified Eagle’s medium (DMEM)/F-12 (11330032, Thermo Fisher Scientific, USA) and CAL-27 in DMEM (11965092, Thermo Fisher Scientific, USA), both supplemented with 10% heat-inactivated FBS (16140063, Thermo Fisher Scientific, USA), at 37 °C under 5% CO2.
Plasmid construction and transfection
Three shRNAs targeting human CD147 were designed and synthesized, and the sequences were: CD147-shRNA1-5'-AATGA CAAAG GCAAG AACGT C-3', CD147-shRNA2-5'-CGTCG ATTTT TGTGA TGCTC GTCAG-3' and CD147-shRNA3-5'-GTACA AGATC ACTGA CTCT-3'. The control vector was pGLV3-GFP-puro. SCC-25 and CAL-27 cells were seeded in 6-well plates and after culturing till 60–80% confluency, transfected with 50, 100, 150 and 200 ng/mL CD147-shRNA or the control plasmid using Lipofectamine™ 2000 Transfection Reagent (11668019, Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. The transfected cells were selected with 6 µg/mL puromycin (P8230, Solarbio Life Science, China) for 1 week and with 3 µg/mL for 2 weeks. The stable transfectants were then cloned by the limiting dilution method in 96 wells, and confirmed by RT-PCR and Western blotting. For PI3K overexpression, PI3K cDNA and control vectors were transfected into SCC-25 and CAL-27 cells as described above.
Real-time qRT-PCR
Total RNA was extracted using TRIzol™ Reagent (15596026, Thermo Fisher Scientific, USA), and the concentration was measured by NanoPhotometer (P-330-31, IMPLEN, Germen). After adjusting the concentration of each paired sample, real-time quantitative RT-PCR analysis was performed using the LightCycler® 96 (Roche, USA) and One-Step SYBR PrimeScript RT-PCR Kit II (Perfect Real Time) (#RR086A, Takara Bio, Japan). Human GAPDH gene was used as the internal control. The following primer sets were used: CD147-shRNA-5'-AGGAC CGGCG AGGAA TAGGA-3' (forward) and 5'-TGCAA GCACT GGGAG TGGAC-3' (reverse) and GAPDH-5'-GCAAT GCCTC CTGCA CCACC A-3' (forward) and 5'-CCCCA GCGTC AAAGG TGGAG G-3' (reverse). The reaction mix consisted of 2.5 mL universal master mix, 0.25 mL primer and probe set, 0.33 mL cDNA and 1.92 mL H2O to a final volume of 5 mL. The RT-QPCR parameters were as follows: 50 °C for 2 minutes, 95 °C for 10 minutes, followed by 40 cycles at 95 °C for 15 seconds and 60 °C for 1 minute. Each sample was run in triplicates. The PCR products were separated on 1% agarose gels.
Western blotting
Harvested cells were washed with ice-cold PBS and lysed using RIPA lysis buffer (89900, Thermo Fisher Scientific, USA) supplemented with 1 mM PMSF (36978, Thermo Fisher Scientific, USA). The protein concentration was measured using the Pierce™ BCA Protein Assay Kit (23225, Thermo Fisher Scientific, USA), and equal amount (40 µg) of the lysates were separated by 10% SDS-PAGE. The proteins were electro-transferred to PVDF membranes, and blocked with 5% albumin bovine V (10735086001, Roche, USA) in Tris-buffered saline containing 0.5% Tween 20 (TBST buffer) at room temperature for 2 h. The membranes were then incubated overnight with the specific primary antibodies against CD147 (#13287), PI3K (#4249), p-PI3K (#4228), PDK1 (#5662), p-PDK1 (#3438), AKT (#9272), p-AKT (#4058), GLUT-1 (#12939) and GAPDH (#8884; all from Cell Signal Technology, USA). The specific protein bands were visualized using the SuperSignal™ West Pico PLUS Chemiluminescent Substrate (34577, Thermo Fisher Scientific, USA) and Tanon 5500 Chemiluminescence Detection System (Tanon, Shanghai, China).
In vitro glycolysis assessment
Cells were seeded in 6-well plates at the density of 5×105 cells/3 mL/well, and cultured in complete medium for 12 h. After replacing the old medium with fresh serum-free medium, the cells were grown for another 12 h, and the supernatants were collected. The glucose content was determined using Amplex™ Red Glucose/Glucose Oxidase Assay Kit (A22189, Thermo Fisher Scientific, USA), and lactate production using the Lactate Colorimetric/Fluorometric Assay Kit (K607, BioVision, USA) according to the manufacturers’ instructions. Absorbance was measured using GENios Plus Plate Reader (Tecan). All results were normalized on the basis of the total cellular protein content.
Transwell migration assay
Stably transfected cells were dissociated using 1X non-enzymatic cell dissociation solution (13151014, Thermo Fisher Scientific, USA), and re-suspended in the suitable media containing 1% BSA (30036727, Thermo Fisher Scientific, USA). Equal number of cells were seeded in the upper Transwell chambers (Corning Costar) onto filters coated with 20 µg/mL fibronectin (Sigma). The lower chambers were filled with complete medium, and the cells were incubated for 6 h at 37 °C under 5% CO2. The cells remaining on the upper surface of the filters were gently scrubbed with a cotton swab, and the migrated cells on the lower surface were washed with PBS, fixed with 4% paraformaldehyde, and stained with crystal violet (0.5 g dye in 20 mL methanol and 80 mL dH2O). The stained cells were observed by light microscopy, and manually counted in 15 random fields per sample (5 filters per sample ×3 fields per filter).
Statistical analysis
Data was expressed as mean ± SEM (standard error of the mean) of three independent experiments. Multiple groups were compared by one-way analysis of variance (ANOVA) followed by the Dunnett test. P values <0.05 were considered statistically significant.
Results
Silencing of CD147 expression in the OSCC lines
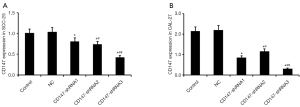
RT-PCR showed similar CD147 mRNA levels in the un-transfected and control vector-transfected OSCC cells, with significant decrease in cells transfected with CD147-shRNA1, CD147-shRNA2 and CD147-shRNA3. In addition, since CD147-shRNA3 blocked CD147 at both the mRNA and protein levels (Figure 1), it was selected for the subsequent experiments.
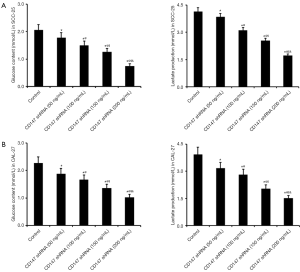
CD147 increased glucose uptake and lactate secretion in OSCC cells
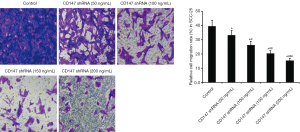
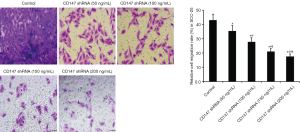
SCC-25 and CAL-27 cells were stably transfected with 50, 100, 150 and 200 ng/mL CD147 shRNA. In both cell lines, shRNA-mediated CD147 silencing significantly lowered glucose uptake and lactate secretion in a dose-dependent manner (Figure 2A,B). This indicates that CD147 promotes glycolysis in OSCC cells.
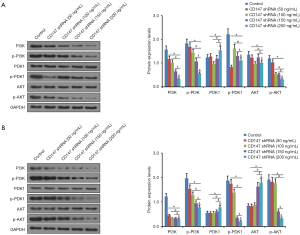
CD147 regulated glucose metabolism in the OSCC cells via the PI3K/AKT pathway
Metabolic reprogramming in cancer cells is regulated by numerous oncogenic signals, including the PI3K/AKT pathway, which mediate the increase in glucose uptake and glycolysis (14,15). Therefore, we also analyzed the effect of CD147 silencing on the key proteins of the PI3K/AKT pathway in SCC-25 and CAL-27 cells. The levels of both total and phosphorylated PI3K were decreased significantly in the CD147 knockdown cells in a dose-dependent manner (Figure 3). Activated PI3K binds to phosphorylated PDK1, thereby activating the downstream signaling molecule AKT (16,17). As shown in Figure 3, CD147 silencing down regulated both p-PDK1 and p-AKT in the SCC-25 and CAL-27 cells, indicating that CD147 regulates glucose metabolism in the OSCC cells via the PI3K/AKT pathway.
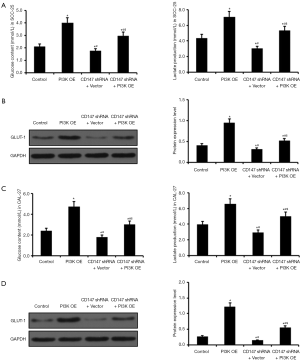
PI3K is the key factor regulating glucose metabolism in OSCC
To further validate the key role of PI3K in regulating glucose metabolism in OSCC cells, we over expressed PI3K in both wild-type and CD147 knockdown SCC-25/CAL-27 cells, and measured the glucose uptake and lactate secretion. Overexpression of PI3K resulted in more active glucose metabolism with significantly higher glucose uptake and lactate production compared to the control cells (Figure 4A). The glucose transporter-1 (GLUT-1) is often overexpressed in solid malignancies and increases glucose uptake in response to aerobic and anaerobic glycolysis (18,19). In addition, overexpression of GLUT-1 in OSCC is associated with poor prognosis (20-23). We found that PI3K overexpression significantly increased GLUT-1 levels in SCC-25 cells compared to the control cells, whereas knockdown of CD147 had the opposite effect. In addition, overexpression of PI3K reversed GLUT-1 inhibition following CD147 knockdown (Figure 4B), indicating a definite relationship between CD147 and PI3K in glucose metabolism. Likewise, CD147 knockdown significantly reduced glucose content and lactate production, as well as GLUT-1 levels in CAL-27 cells, all of which were restored by PI3K overexpression (Figure 4C,D). Taken together, CD147 regulates glucose metabolism in the OSCC cells via the PI3K/AKT pathway.
CD147 facilitated invasion and metastasis of OSCC cells via the PI3K/AKT pathway
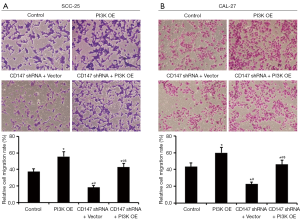
The invasiveness and distant metastasis of tumor cells are the major factors responsible for the high mortality rates of OSCC (24), and a pro-metastatic role of CD147 has been reported in previous studies (7,8,25,26). Silencing of CD147 significantly decreased the in vitro migration of OSCC cells in a dose-dependent manner (Figures 5,6). In addition, overexpressing PI3K increased cell migration, which was significantly inhibited upon simultaneously blocking CD147 (Figure 7). Therefore, CD147 facilitated invasion and metastasis of OSCC cells via the PI3K/AKT pathway.
Discussion
We report for the first time the pivotal role of CD147 in regulating glucose metabolism and migration in human OSCC cells, and identify PI3K as the key functional protein. CD147 knockdown not only decreased glucose uptake and lactate secretion, but also the migration of the OSCC cells in an shRNA dose-dependent manner. In addition, CD147 silencing in the PI3K-overexpressing cells abrogated the metabolic advantage provided by PI3K, indicating that the oncogenic functions of CD147 are mainly mediated by the PI3K/AKT pathway. Oral tumors are often discovered at an advanced stage (27,28) due to dysplastic precancerous lesions (29) and lymphatic metastasis (30-32). Therefore, metastasis is a rational therapeutic target for oral cancer (31,33). CD147, originally isolated from the plasma membranes of malignant tumor cells (34,35), is an important modulator of the anchorage-independent growth and invasiveness of tumor cells (36,37). However, the mechanistic role of CD147 in oral cancer, especially squamous cell carcinoma, is largely ambiguous. In the current study, we showed that the CD147/PI3K/AKT axis is a pro-metastatic factor, which is in accordance with a proteomics analysis on OSCC cells that positively associated CD147 with metastasis (38), and other studies that showed CD147 promoted tumorigenesis and invasion in oral cancers via degradation of the extracellular matrix (39,40). A major hallmark of cancer is metabolic reprogramming (8). Due to their higher biosynthesis rates, proliferation and migration, tumor cells require more energy compared to normal cells. Not surprisingly therefore, tumor cells often overexpress GLUT-1 for increased glucose uptake and metabolism (41). In addition, glucose metabolism is closely associated with the hypoxia inducible factor (HIF), chemokine receptor CXCR4 and monocarp boxy late transporters MCT1 and MCT4, which play important roles in tumor invasion and metastasis (42-45). We found that the transmembrane glycoprotein CD147 regulated glucose utilization and metabolism in OSCC cells by activating the PI3K/Akt pathway, which is consistent with previous reports. Research also suggested that that hypoxia-induced CD147 promoted glycolysis by upregulating HIF and SP1 in epithelial carcinoma cells (46). In addition, CD147 also promoted glucose metabolism in HCC cells by inhibiting the p53-dependent signaling pathway (13). In conclusion, we demonstrated that blocking CD147 in human OSCC cells resulted in inhibition of glucose metabolism, with lower glucose uptake and lactate secretion compared to the normal cells. Furthermore, silencing of CD147 significantly decreased OSCC cell migration in a dose-dependent manner. Mechanistically, CD147 regulated both migration and glycometabolism via the PI3K/AKT pathway. Our findings provide new insights into the molecular mechanisms governing metastasis in oral cancers, and identify CD147 as a novel prognostic marker and therapeutic target for OSCC.
Acknowledgments
Funding: The present investigation was supported by grants from
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.50). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study only involved cell experiments, not clinical or animal experiments, thus ethics approval and informed consent are not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Banoczy J. Oral cancer and precancerous lesions. Fogorv Sz 1997;90:27. [PubMed]
- Garzino-Demo P, Dell'Acqua A, Dalmasso P, et al. Clinicopathological parameters and outcome of 245 patients operated for oral squamous cell carcinoma. J Craniomaxillofac Surg 2006;34:344-50. [Crossref] [PubMed]
- Bai Y, Huang W, Ma LT, et al. Importance of N-glycosylation on CD147 for its biological functions. Int J Mol Sci 2014;15:6356-77. [Crossref] [PubMed]
- Geng JJ, Tang J, Yang XM, et al. Targeting CD147 for T to NK Lineage Reprogramming and Tumor Therapy. EBioMedicine 2017;20:98-108. [Crossref] [PubMed]
- Li J, Huang Q, Long X, et al. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARalpha pathways. J Hepatol 2015;63:1378-89. [Crossref] [PubMed]
- Lescaille G, Menashi S, Cavelier-Balloy B, et al. EMMPRIN/CD147 up-regulates urokinase-type plasminogen activator: implications in oral tumor progression. BMC Cancer 2012;12:115. [Crossref] [PubMed]
- Vigneswaran N, Beckers S, Waigel S, et al. Increased EMMPRIN (CD 147) expression during oral carcinogenesis. Exp Mol Pathol 2006;80:147-59. [Crossref] [PubMed]
- Yu YH, Morales J, Feng L, et al. CD147 and Ki-67 overexpression confers poor prognosis in squamous cell carcinoma of oral tongue: a tissue microarray study. Oral Surg Oral Med Oral Pathol Oral Radiol 2015;119:553-65. [Crossref] [PubMed]
- Huang C, Sun Z, Sun Y, et al. Association of increased ligand cyclophilin A and receptor CD147 with hypoxia, angiogenesis, metastasis and prognosis of tongue squamous cell carcinoma. Histopathology 2012;60:793-803. [Crossref] [PubMed]
- Su J, Gao T, Jiang M, et al. CD147 silencing inhibits tumor growth by suppressing glucose transport in melanoma. Oncotarget 2016;7:64778-84. [Crossref] [PubMed]
- Huang P, Mao LF, Zhang ZP, et al. Down-Regulated miR-125a-5p Promotes the Reprogramming of Glucose Metabolism and Cell Malignancy by Increasing Levels of CD147 in Thyroid Cancer. Thyroid 2018;28:613-23. [Crossref] [PubMed]
- Huang Q, Li J, Xing J, et al. CD147 promotes reprogramming of glucose metabolism and cell proliferation in HCC cells by inhibiting the p53-dependent signaling pathway. J Hepatol 2014;61:859-66. [Crossref] [PubMed]
- Fu QF, Liu Y, Fan Y, et al. Alpha-enolase promotes cell glycolysis, growth, migration, and invasion in non-small cell lung cancer through FAK-mediated PI3K/AKT pathway. J Hematol Oncol 2015;8:22. [Crossref] [PubMed]
- Moench R, Grimmig T, Kannen V, et al. Exclusive inhibition of PI3K/Akt/mTOR signaling is not sufficient to prevent PDGF-mediated effects on glycolysis and proliferation in colorectal cancer. Oncotarget 2016;7:68749-67. [Crossref] [PubMed]
- Wang XQ, Lo CM, Chen L, et al. CDK1-PDK1-PI3K/Akt signaling pathway regulates embryonic and induced pluripotency. Cell Death Differ 2017;24:38-48. [Crossref] [PubMed]
- Fresno Vara JA, Casado E, de Castro J, et al. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev 2004;30:193-204. [Crossref] [PubMed]
- Dura M, Nemejcova K, Jaksa R, et al. Expression of Glut-1 in Malignant Melanoma and Melanocytic Nevi: an Immunohistochemical Study of 400 Cases. Pathol Oncol Res 2019;25:361-8. [Crossref] [PubMed]
- Zhang TB, Zhao Y, Tong ZX, et al. Inhibition of glucose-transporter 1 (GLUT-1) expression reversed Warburg effect in gastric cancer cell MKN45. Int J Clin Exp Med 2015;8:2423-8. [PubMed]
- Harshani JM, Yeluri S, Guttikonda VR. Glut-1 as a prognostic biomarker in oral squamous cell carcinoma. J Oral Maxillofac Pathol 2014;18:372-8. [Crossref] [PubMed]
- Angadi VC, Angadi PV. GLUT-1 immunoexpression in oral epithelial dysplasia, oral squamous cell carcinoma, and verrucous carcinoma. J Oral Sci 2015;57:115-22. [Crossref] [PubMed]
- Grimm M, Munz A, Teriete P, et al. GLUT-1(+)/TKTL1(+) coexpression predicts poor outcome in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol 2014;117:743-53. [Crossref] [PubMed]
- Li CX, Sun JL, Gong ZC, et al. Prognostic value of GLUT-1 expression in oral squamous cell carcinoma: A prisma-compliant meta-analysis. Medicine (Baltimore) 2016;95:e5324. [Crossref] [PubMed]
- Yamada SI, Otsuru M, Yanamoto S, et al. Progression level of extracapsular spread and tumor budding for cervical lymph node metastasis of OSCC. Clin Oral Investig 2018;22:1311-8. [Crossref] [PubMed]
- Nasry WHS, Wang H, Jones K, et al. CD147 and Cyclooxygenase Expression in Feline Oral Squamous Cell Carcinoma. Vet Sci 2018; [Crossref] [PubMed]
- Zhang C, Man DP, Ma SM, et al. Expressions and significances of CD147, OPN and MMP-2 in oral squamous cell carcinoma. Sichuan Da Xue Xue Bao Yi Xue Ban 2012;43:683-6. [PubMed]
- Al-Maweri SA, Al-Soneidar WA, Dhaifullah E, et al. Oral Cancer: Awareness and Knowledge Among Dental Patients in Riyadh. J Cancer Educ 2017;32:308-13. [Crossref] [PubMed]
- Keshavarzi M, Darijani M, Momeni F, et al. Molecular Imaging and Oral Cancer Diagnosis and Therapy. J Cell Biochem 2017;118:3055-60. [Crossref] [PubMed]
- Wang C, Niu W, Chen H, et al. Nicotine suppresses apoptosis by regulating alpha7nAChR/Prx1 axis in oral precancerous lesions. Oncotarget 2017;8:75065-75. [PubMed]
- Chatzistefanou I, Lubek J, Markou K, et al. The role of perineural invasion in treatment decisions for oral cancer patients: A review of the literature. J Craniomaxillofac Surg 2017;45:821-5. [Crossref] [PubMed]
- Mupparapu M, Shanti RM. Evaluation and Staging of Oral Cancer. Dent Clin North Am 2018;62:47-58. [Crossref] [PubMed]
- Shanti RM, O'Malley BW Jr. Surgical Management of Oral Cancer. Dent Clin North Am 2018;62:77-86. [Crossref] [PubMed]
- Siriwardena BS, Rasnayaka RM, Masood Y, et al. Predictive model of oral cancer metastasis for different cancer sites and age groups. J Investig Clin Dent 2016;7:127-31. [Crossref] [PubMed]
- Hu X, Su J, Zhou Y, et al. Repressing CD147 is a novel therapeutic strategy for malignant melanoma. Oncotarget 2017;8:25806-13. [PubMed]
- Qiao S, Liu C, Xu W, et al. Up-regulated expression of CD147 gene in malignant bone tumor and the possible induction mechanism during osteoclast formation. Braz J Med Biol Res 2018;51:e6948. [Crossref] [PubMed]
- Nabeshima K, Iwasaki H, Koga K, et al. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol Int 2006;56:359-67. [Crossref] [PubMed]
- Yong YL, Liao CG, Wei D, et al. CD147 overexpression promotes tumorigenicity in Chinese hamster ovary cells. Cell Biol Int 2016;40:375-86. [Crossref] [PubMed]
- Kuang YH, Chen X, Su J, et al. Proteome analysis of multidrug resistance of human oral squamous carcinoma cells using CD147 silencing. J Proteome Res 2008;7:4784-91. [Crossref] [PubMed]
- Li HF, Liu YQ, Shen ZJ, et al. Downregulation of MACC1 inhibits invasion, migration and proliferation, attenuates cisplatin resistance and induces apoptosis in tongue squamous cell carcinoma. Oncol Rep 2015;33:651-60. [PubMed]
- Monteiro LS, Delgado ML, Ricardo S, et al. EMMPRIN expression in oral squamous cell carcinomas: correlation with tumor proliferation and patient survival. Biomed Res Int 2014;2014:905680. [Crossref] [PubMed]
- Cho H, Lee YS, Kim J, et al. Overexpression of glucose transporter-1 (GLUT-1) predicts poor prognosis in epithelial ovarian cancer. Cancer Invest 2013;31:607-15. [Crossref] [PubMed]
- Semenza GL. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. Embo j 2017;36:252-9. [Crossref] [PubMed]
- Wang M, Wang W, Wang J, et al. MiR-182 promotes glucose metabolism by upregulating hypoxia-inducible factor 1alpha in NSCLC cells. Biochem Biophys Res Commun 2018;504:400-5. [Crossref] [PubMed]
- Lan X, Cheng K, Chandel N, et al. High glucose enhances HIV entry into T cells through upregulation of CXCR4. J Leukoc Biol 2013;94:769-77. [Crossref] [PubMed]
- Jie W, Wang X, Zhang Y, et al. SDF-1alpha/CXCR4 axis is involved in glucose-potentiated proliferation and chemotaxis in rat vascular smooth muscle cells. Int J Exp Pathol 2010;91:436-44. [Crossref] [PubMed]
- Ke X, Fei F, Chen Y, et al. Hypoxia upregulates CD147 through a combined effect of HIF-1alpha and Sp1 to promote glycolysis and tumor progression in epithelial solid tumors. Carcinogenesis 2012;33:1598-607. [Crossref] [PubMed]


