Detection of circulating stage III–IV gastric cancer tumor cells based on isolation by size of epithelial tumor: using the circulating tumor cell biopsy technology
Introduction
Gastric cancer is one of the most common malignant tumors, and in China the morbidity and mortality from GC rank second among all tumor types (1). According to the 7th Edition of the AJCC Cancer Staging Manual, the prognosis for stage III–IV gastric cancer patients is poor, with the 5-year survival rate ranging from 26.9% to 8.4% (2), and adjuvant chemotherapy or palliative chemotherapy are the main treatments for these patients. Currently, the evaluation of chemotherapy efficacy mainly relies on end points such as progress free survival (PFS), time to progression (TTP), and overall survival (OS), which cannot monitor the efficacy in real time. Studies have shown that in patients with advanced gastric cancer the prognosis of the patients with a good chemotherapy response is better than those with a poor response (3). Even patients with metastasis may also achieve longer-term survival through effective chemotherapy (4,5). But more than 40% of the patients show primary or secondary resistance to it (6). Studies have revealed that pre-treatment detection of the biomarkers related to treatment sensitivity can help screen tumor chemosensitivity (7-9). For patients with advanced gastric cancer the impossibility of repeating tumor tissue harvesting limits the capability of tumor tissue biomarkers being a tool for continuous real-time monitoring of clinical efficacy (10,11). Consequently, the detection of circulating tumor cells (CTCs) could serve as a marker to predict prognosis and efficacy.
The term CTCs refers to tumor cells that are shed from primary or metastatic lesions and released into the peripheral blood circulation during the formation and development of solid tumors (12,13). In recent years, CTCs have attracted attention and have been investigated in various tumor studies (14-16). From the year 2004 to 2008, the United States FDA approved the usage of Johnson & Johnson’s CellSearch technology for the clinical diagnosis of metastatic breast cancer, prostate cancer, and colorectal cancer (16-18), but for gastric cancer, this technology was not further developed because of a high false negative rate and low detection rate (19-21).
In 2000, Vona et al. (22) proposed a membrane isolation method to isolate tumor cells based on size and morphological differences between tumor cells and normal cells, namely, the isolation by size of epithelial tumor cells (ISET) technology. This technology preserves biological characteristics of the cells, allows direct and routine pathological staining, and the observation of CTCs under a light microscope. It can avoid the false negatives caused by the epithelial to mesenchymal transition (EMT) process and can improve the detection rate of CTCs, and it is applicable to all types of tumor cells.
CTCBIOPSY® is a novel one-stop ISET device developed by Wuhan Youzhiyou Medical Technology Co. Ltd, which can automatically isolate and identify CTCs. And it had been approved by the CFDA for clinical application in cancer management. It mainly utilizes the physical differences between CTCs and normal blood cells in cell size (the vast majority of CTCs have a larger diameter than normal blood cells), cell deformability (CTCs are hard to deform but easily trapped on the microporous membranes due to the complex structure formed by keratin and other substances), and cell chromatophilia. CTCBIOPSY® uses a micro-sieve nano-microporous membrane filter to effectively separate the CTCs from the vast majority of blood cells; retaining most of the CTCs. CTCs do not depend on specific surface markers, and preserves the morphological integrity of the cells.
In this study, we detect CTCs from 42 stage III–IV gastric cancer patients using the ISET technology and analyze the clinical significance of CTCs and its relationship with peripheral blood tumor markers associated with gastric cancer. We then discuss the significance of the CTC threshold number predicting response to treatment in a cohort of III–IV gastric cancer patients receiving chemotherapy.
Methods
Research design
Forty two patients with gastric cancer that had been admitted for treatment to the Union Hospital affiliated to Fujian Medical University from September, 2015 to April, 2016, were incorporated into this study. The study was approved by the hospital’s ethics committee and informed consent was obtained from patients before they entered the study. The inclusion criteria included: diagnosed stage III–IV gastric cancer (according to the 7th Edition of the AJCC Cancer TNM Staging Manual, 2010), adenocarcinoma as the pathological type, ECOG PS ≤ score 2, all patients receive routine courses of postoperative adjuvant chemotherapy or palliative chemotherapy. Patients with other malignant tumors at the time of admission or in the past were excluded.
One to three days before starting chemotherapy 5ml of peripheral venous blood was extracted and processed by the CTCBIOPSY® cell staining and isolation apparatus, and CTCs were observed and enumerated under a light microscope. Meanwhile, CA724, CA199, and CEA were detected, and information, including patients’ basic clinical features, their chemotherapy regimens, and imaging examinations after chemotherapy, were collected in baseline and every 2 to 3 chemotherapy cycles. The evaluation of the efficacy of therapy was fulfilled according to the Response Evaluation Criteria in Solid Tumor 1.1 (RECIST1.1).
CTCs isolation and staining
Samples were transferred onto CTCBIOPSY® cell isolation filters, followed by immediately adding 200µl of 4% paraformaldehyde (PFA) solution. The sample and the solution were fully mixed by gentle pipetting with a Pasteur pipette. The sample was then fixed for 10 min at room temperature before CTCs isolation and automatic Diff-Quick staining with the CTCBIOPSY® apparatus. When isolation and staining was complete the filter was removed. First the upper plug of the filter was opened then the lower one was opened. The filter membrane was taken out using a membrane extractor and placed face up on the slide, which was then dried in the air-dry oven at 50 to 60 °C for 30 to 60 min. Mounting medium was added dropwise onto the membrane surface, which was then sealed under a cover glass and solidified in an air-dry oven for 30 to 60 min. When completely prepared, the slide was checked for staining and observed under a light microscope.
Result reading
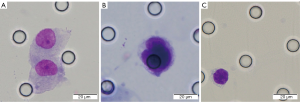
In order to reduce errors, two experienced pathologists reviewed all the counting results. The morphological evaluation parameters for CTCs should meet at least four of the following criterion: (I) nuclear heteromorphism; (II) nucleo-cytoplasmic ratio greater than 0.8; (III) cell length greater than 15 µm; (IV) hyperchromatic and unevenly stained nucleus; (V) thickened nuclear membrane with pits or folds, making it serrated; (VI) nuclear marginal chromatin, or large nucleoli, or abnormal nuclear division (Figure 1).
Statistical analysis
All data were analyzed using IBM SPSS version 19.0. Patients’ clinical data descriptions are presented as percentages or median values, and the measurement data expressed as mean values ± standard deviations. A t-test, variance analysis, or a chi-square (χ2) test was used to compare the differences between the groups and a Mann-Whitney U-test was used to compare the differences in the tumor marker values between the two groups. GraphPad Prism 7 was used for graph generation analysis. All the data tests were two-tailed, and it was considered statistically significant with a P value is less than 0.05 (P<0.05).
Results
CTC threshold number
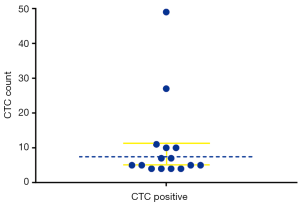
A CTCs count of ≥4 per 5 mL was defined as CTC high threshold numbers (23). In 15 out of the 42 cases the patients were positive for CTCs, with the detection rate being 35.7% (Figure 2). The number of CTCs detected ranged from 0 to 49 per 5 mL of blood, with a median value of 1 per 5 mL, and the total number of CTCs detected was 173, with a mean value of 4.12±8.60 per 5 mL.
The correlation between CTCs detection and clinical features
The correlations between CTCs detection and clinical factors are shown in Table 1. Of the 25 cases of stage IV gastric cancer patients, 12 presented with CTCs ≥4, accounting for 48%, while in the 17 stage III patients CTCs were found in only 17.6%. The threshold number of CTCs is significantly associated with different clinical stags (P=0.044).
Table 1
| Basic clinical feature | CTC threshold numbers | χ2 or t | P value | |
|---|---|---|---|---|
| ≥4 CTC/mL (N=15) (%) | <4 CTC/mL (N=27) (%) | |||
| Gender | 1.310 | 0.252 | ||
| Male | 12 (41.4) | 17 (58.6) | ||
| Female | 3 (23.1) | 10 (76.9) | ||
| Tumor site | 0.036 | 0.982 | ||
| Fundus/cardia | 3 (37.5) | 5 (62.5) | ||
| Antrum pylorus | 9 (34.6) | 17 (65.4) | ||
| Body | 3 (37.5) | 5 (62.5) | ||
| Age | 59.33±12.40 | 59.41±15.61 | 0.016 | 0.987 |
| Grade of differentiation | 3.389 | 0.066 | ||
| Moderate or well differentiated | 2 (15.4) | 11 (84.6) | ||
| Poorly differentiated | 13 (44. 8) | 16 (55.2) | ||
| Clinical stage | 4.061 | 0.044 | ||
| Stage III | 3 (17.6) | 14 (82.4) | ||
| Stage IV | 12 (48.0) | 13 (52.0) | ||
| Tumor depth | 0.084 | 0.359 | ||
| T3 | 3 (25.0) | 9 (75.0) | ||
| T4 | 12 (40.0) | 18 (60.0) | ||
| Lymph node metastasis | 0.104 | 0.747 | ||
| N1–N2 | 8 (38.1) | 13 (61.9) | ||
| N3 | 7 (33.3) | 14 (66.7) | ||
| Distant metastasis | 1. 847 | 0.174 | ||
| No | 4 (23.5) | 13 (76.5) | ||
| Yes | 11 (44.0) | 14 (56.0) | ||
| Vessel carcinoma embolus | 0.089 | 0.956 | ||
| Negative | 5 (38.5) | 8 (61.5) | ||
| Not detected | 4 (36.4) | 7 (63.6) | ||
| Positive | 6 (33.3) | 12 (66.7) | ||
| Surgery | 2.586 | 0.274 | ||
| Radical surgery | 5 (25.0) | 15 (75.0) | ||
| Palliative surgery | 5 (55.6) | 4 (44.4) | ||
| No surgery | 5 (38.5) | 8 (61.5) | ||
CTC, circulating tumor cell.
The correlation between CTC rate and the tumor markers in patients with advanced gastric cancer
For the 42 patients with advanced gastric cancer, there is a statistically significant difference in the expression of the tumor marker CA724 between the patients with CTCs ≥4 and those with CTCs <4 (P=0.004), while no differences are observed in the expression of either CEA or CA199 (Table 2).
Table 2
| Gastric cancer related tumor markers | CTC threshold numbers | Z | P value | |
|---|---|---|---|---|
| ≥4 CTC/mL | <4 CTC/mL | |||
| CA724 (U/mL) | 28.72±37.14 | 10.63±16.22 | 2.849 | 0.004 |
| CEA (ng/mL) | 13.11±18.24 | 34.06±70.86 | 0.578 | 0.563 |
| CA199 (U/mL) | 126.42±282.79 | 130.19±259.13 | 0.433 | 0.665 |
CTC, circulating tumor cell.
The correlation between CTC rate in peripheral blood of patients with advanced gastric cancer and their chemotherapy regimens and efficacy
The 42 patients were divided into two groups according to the chemotherapy efficacy evaluation standard, namely the disease control rate (DCR) group and the progressive disease (PD) group. DCR was defined as complete response (CR), partial response (PR) and stable disease (SD) of those with prior progression. There were 22 cases in the DCR group, and 4 of them had CTCs ≥4 (18.2%). A total of 20 cases were in the PD group, and 11 of them presented with CTCs ≥4 (55.0%). The difference in the CTCs threshold number between the two groups was statistically significant (P=0.023) (Table 3).
Table 3
| Variable | CTC threshold numbers | χ2 | P value | |
|---|---|---|---|---|
| ≥4 CTC/mL (N=15) (%) | <4 CTC/mL (N=27) (%) | |||
| Chemotherapy response | ||||
| DCR | 4 (18.2) | 18 (81.8) | 6.185 | 0.023 |
| PD | 11 (55.0) | 9 (45.0) | ||
CTC, circulating tumor cell; DCR, disease control rate; PD, progressive disease.
Discussion
In our study, CTCs were detected in stage III–IV gastric cancer by using ISET technology, and were found to be associated with Clinical stage, and was positively correlated with the value in U/mL of CA724. The high threshold number of CTCs in peripheral blood indicates poor tumor response to chemotherapy in patients with gastric cancer. CTC counts can serve as an indicator in predicting efficacy of chemotherapy for stage III–IV gastric cancer.
In this study, CTCs were enriched and identified by ISET combined with cytology. The CTCs were detected in 17 out of 42 patients with stage III–IV gastric cancer (38.6%), which was more sensitive than other approaches previously reported. Okabe et al. (24) used the CellSearch system to evaluate 140 patients with advanced gastric cancer in 2016, with CTCs ≥2 per 7.5 mL being their positive standard, and only 25 (18.4%) were positive for CTC. Matsusaka et al. (23) assayed for CTCs in the peripheral blood of 52 patients with advanced gastric cancer, and the CTC positive rates in the PD and the non-PD groups were 13.4% and 19.2% respectively, when CTCs ≥4 per 7.5 mL was considered positive. Khoja et al. (25) was also observed that compared with the Celltracks AutoPrep system, ISET has the greater sensitivity. The ISET rules out the interference of the false negative results caused by tumor cells that show no expression or incomplete expression of the EpCAM antigen, especially the ones undergoing EMT, and it detects more CTCs with a smaller amount of blood (5 mL), greatly boosting the detection sensitivity.
This study has also found that the incidence of CTC positive was closely correlated to the clinical staging of the tumor. This may be due to the fact that stage IV gastric cancer patients possess more aggressive tumor cells that more easily break through their surrounding blood vessels and enter the peripheral blood circulation. There have been several meta-analyses pointing out that stage III–IV gastric cancer patients compared with stage I–II gastric cancer patients have a markedly increased incidence of CTC positive results (26,27). Zheng et al. (28) used the ISET method to detect CTCs and have shown that stage IV gastric cancer patients have much higher numbers of CTCs detected than do stage I, II, III patients. Therefore, detection of the CTCs in patients’ peripheral blood may be a supplement to the TNM staging system, and the number of CTCs may be an important prognostic indicator. Although in this study, between the CTC positive group and the CTC negative group, the number of cases and percentage differences in the degree of differentiation, depth of invasion, and distant metastasis were relatively large, no statistically significant difference was found (P>0.05). This may be associated with the small number of cases and further research is needed to confirm our findings.
In this study, it was found that there was a statistically significant difference in the presence of the tumor marker CA724 between the CTC positive and the CTC negative group. At present, CA724 detection is attracting increasing attention due to its high sensitivity and specificity in the diagnosis of gastric cancer (29). A meta-analysis has demonstrated that serum CA724 is the most relevant gastric cancer tumor marker, and its correlation with gastric cancer is significantly superior to CEA, CA199, CA242, CA125, or CA153 (30). Moreover, CA724 is also related to the prognosis of gastric cancer patients and the efficacy of chemotherapy. The probability of metastasis of diffuse gastric cancer is as high as 36%, and some studies have shown that CA724 can be used to detect lymph node metastasis of gastric cancer (31). Zou et al. (32) also found that the serum CA724 level was related to the chemotherapy efficacy on advanced gastric cancer. The number of patients with PD + stable disease chemotherapy results in their CA724 >8.2 U/mL group was significantly higher than that in the CA724 <8.2 U/mL group. Our study found that the presence of CTCs is very well correlated with CA724 and, to a certain extent, to the chemotherapy efficacy of patients with advanced gastric cancer. It can potentially be part of a combined index to evaluate the tumor sensitivity of chemotherapy drugs and the efficacy of future chemotherapy, and whether a patient has recurrence or metastasis. However, the value of this combination still needs to be confirmed by large-sample clinical trials and in-depth and standardized long-term clinical follow-ups.
It can be perceived from the statistical analyses in this experiment that the PD group had a significantly higher CTC threshold number than the DCR group. As for advanced gastric cancer, there are very few articles on clinical detection of CTCs to evaluate the efficacy of chemotherapy in patients. In one study, CellSearch was used to detect the number of CTCs in blood from 95 patients with metastatic gastric cancer before chemotherapy, and it was found that the probability that patients were evaluated as PD after chemotherapy in the CTCs ≥5 per 7.5 mL group was much higher than in the CTCs <5 per 7.5 mL group (60% vs. 23.4%) (33). Another study in Japan that involved 52 patients with advanced gastric cancer found that two weeks and four weeks after chemotherapy, the number of OS and PFS patients with a high number of CTCs is significantly lower than for patients with a low number of CTCs. This suggests a patient with a high CTCs count may have stronger drug resistance to chemotherapy and poorer efficacy (23). The above results are consistent with the conclusions of our study. Tumor cells in primary and metastatic lesions in cancer patients are heterogeneous (34). Some chemotherapy regimens or drugs are effective for primary lesions but ineffective for metastatic lesions, making it difficult to achieve an accurate judgment with conventional examinations. CTCs is closely related to cancer metastasis, so detection of CTCs can indirectly reflect the response of tumor cells in metastatic lesions to chemotherapy drugs, so as to evaluate chemotherapy regimens or drug efficacy (35). The CTCs test may also imply efficacy of chemotherapy earlier than imaging examinations, and evaluate in real time without the interference of radiation side effects (36). Studies have found that in the process of EMT the CTCs can acquire stem cell properties, which can result in drug resistance in tumor patients and resistance to chemotherapy (37). The more mesenchymal cells there are among the tumor cells in patients, the stronger the drug resistance may be (38). In our study we detected CTCs by means of ISET, excluding the interference from EMT, so the reliability and the sensitivity were well guaranteed.
Since the sample size was small in the study, and the CTCs tests were only run before the treatment, enlarging the sample size and dynamic monitoring of patients will be warranted to further study the significance of the CTCs detected by ISET technology in the treatment of gastric cancer.
Conclusions
This study utilized the ISET technology to monitor CTCs in peripheral blood with high sensitivity, and found that patients with higher CTCs counts might be more likely to be prone to drug resistance and have a poor prognosis, and that the detection of the number of CTCs could predict the chemotherapy efficacy. Our study also revealed that the level of CA724 was positively correlated with CTCs expression in the peripheral blood of advanced gastric cancer patients. Combined detection of these two indicators has the potential to be a predictive index for chemotherapy efficacy in patients with advanced gastric cancer.
Acknowledgments
Funding: The work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.32). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approved by the Ethics Committee of Fujian Medical University Union Hospital, and written informed consent was obtained from all patients. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or have followed the principles outlined in the Declaration of Helsinki (as revised in 2013) for all human experimental investigations.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Fang WL, Huang KH, Chen JH, et al. Comparison of the survival difference between AJCC 6th and 7th editions for gastric cancer patients. World J Surg 2011;35:2723-9.
- Satoh S, Hasegawa S, Ozaki N, et al. Retrospective analysis of 45 consecutive patients with advanced gastric cancer treated with neoadjuvant chemotherapy using an S-1/CDDP combination. Gastric Cancer 2006;9:129-35. [Crossref] [PubMed]
- Okabe H, Ueda S, Obama K, et al. Induction chemotherapy with S-1 plus cisplatin followed by surgery for treatment of gastric cancer with peritoneal dissemination. Ann Surg Oncol 2009;16:3227-36. [Crossref] [PubMed]
- Satoh S, Okabe H, Teramukai S, et al. Phase II trial of combined treatment consisting of preoperative S-1 plus cisplatin followed by gastrectomy and postoperative S-1 for stage IV gastric cancer. Gastric Cancer 2012;15:61-9. [Crossref] [PubMed]
- Hartgrink HH. Improving outcome for scirrhous gastric cancer. Gastric Cancer 2009;12:3-5. [Crossref] [PubMed]
- Scartozzi M, Bittoni A, Pistelli M, et al. Toward molecularly selected chemotherapy for advanced gastric cancer: state of the art and future perspectives. Cancer Treat Rev 2009;35:451-62. [Crossref] [PubMed]
- Wang M, Li Y, Gao J, et al. p16 Methylation is associated with chemosensitivity to fluorouracil in patients with advanced gastric cancer. Med Oncol 2014;31:988. [Crossref] [PubMed]
- Liang H, Kim YH. Identifying molecular drivers of gastric cancer through next-generation sequencing. Cancer Lett 2013;340:241-6. [Crossref] [PubMed]
- Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov 2014;4:650-61. [Crossref] [PubMed]
- Alix-Panabieres C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem 2013;59:110-8. [Crossref] [PubMed]
- Nelson NJ. Circulating tumor cells: will they be clinically useful? J Natl Cancer Inst 2010;102:146-8. [Crossref] [PubMed]
- Masuda T, Hayashi N, Iguchi T, et al. Clinical and biological significance of circulating tumor cells in cancer. Mol Oncol 2016;10:408-17. [Crossref] [PubMed]
- Gaforio JJ, Serrano MJ, Sanchez-Rovira P, et al. Detection of breast cancer cells in the peripheral blood is positively correlated with estrogen-receptor status and predicts for poor prognosis. Int J Cancer 2003;107:984-90. [Crossref] [PubMed]
- Witzig TE, Bossy B, Kimlinger T, et al. Detection of circulating cytokeratin-positive cells in the blood of breast cancer patients using immunomagnetic enrichment and digital microscopy. Clin Cancer Res 2002;8:1085-91. [PubMed]
- Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007;450:1235-9. [Crossref] [PubMed]
- Ross AA, Cooper BW, Lazarus HM, et al. Detection and viability of tumor cells in peripheral blood stem cell collections from breast cancer patients using immunocytochemical and clonogenic assay techniques. Blood 1993;82:2605-10. [PubMed]
- Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 2006;12:4218-24. [Crossref] [PubMed]
- Galletti G, Sung MS, Vahdat LT, et al. Isolation of breast cancer and gastric cancer circulating tumor cells by use of an anti HER2-based microfluidic device. Lab Chip 2014;14:147-56. [Crossref] [PubMed]
- Dardaei L, Shahsavani R, Ghavamzadeh A, et al. The detection of disseminated tumor cells in bone marrow and peripheral blood of gastric cancer patients by multimarker (CEA, CK20, TFF1 and MUC2) quantitative real-time PCR. Clin Biochem 2011;44:325-30. [Crossref] [PubMed]
- Uen YH, Lin SR, Wu CH, et al. Clinical significance of MUC1 and c-Met RT-PCR detection of circulating tumor cells in patients with gastric carcinoma. Clin Chim Acta 2006;367:55-61. [Crossref] [PubMed]
- Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol 2000;156:57-63. [Crossref] [PubMed]
- Matsusaka S, Chin K, Ogura M, et al. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in patients with advanced gastric cancer. Cancer Sci 2010;101:1067-71. [Crossref] [PubMed]
- Okabe H, Tsunoda S, Hosogi H, et al. Circulating Tumor Cells as an Independent Predictor of Survival in Advanced Gastric Cancer. Ann Surg Oncol 2015;22:3954-61. [Crossref] [PubMed]
- Khoja L, Backen A, Sloane R, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer 2012;106:508-16. [Crossref] [PubMed]
- Huang X, Gao P, Sun J, et al. Clinicopathological and prognostic significance of circulating tumor cells in patients with gastric cancer: a meta-analysis. Int J Cancer 2015;136:21-33. [Crossref] [PubMed]
- Wang HY, Wei J, Zou ZY, et al. Circulating tumour cells predict survival in gastric cancer patients: a meta-analysis. Contemp Oncol (Pozn) 2015;19:451-7. [Crossref] [PubMed]
- Zheng L, Zou K, Yang C, et al. Inflammation-based indexes and clinicopathologic features are strong predictive values of preoperative circulating tumor cell detection in gastric cancer patients. Clin Transl Oncol 2017;19:1125-32. [Crossref] [PubMed]
- Gaspar MJ, Arribas I, Coca MC, et al. Prognostic value of carcinoembryonic antigen, CA 19-9 and CA 72-4 in gastric carcinoma. Tumour Biol 2001;22:318-22. [Crossref] [PubMed]
- Chen XZ, Zhang WK, Yang K, et al. Correlation between serum CA724 and gastric cancer: multiple analyses based on Chinese population. Mol Biol Rep 2012;39:9031-9. [Crossref] [PubMed]
- Ucar E, Semerci E, Ustun H, et al. Prognostic value of preoperative CEA, CA 19-9, CA 72-4, and AFP levels in gastric cancer. Adv Ther 2008;25:1075-84. [Crossref] [PubMed]
- Zou L, Qian J. Decline of serum CA724 as a probable predictive factor for tumor response during chemotherapy of advanced gastric carcinoma. Chin J Cancer Res 2014;26:404-9. [PubMed]
- Lee SJ, Lee J, Kim ST, et al. Circulating tumor cells are predictive of poor response to chemotherapy in metastatic gastric cancer. Int J Biol Markers 2015;30:e382-6. [Crossref] [PubMed]
- Bonnomet A, Syne L, Brysse A, et al. A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene 2012;31:3741-53. [Crossref] [PubMed]
- Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 2012;30:525-32. [Crossref] [PubMed]
- Liu MC, Shields PG, Warren RD, et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol 2009;27:5153-9. [Crossref] [PubMed]
- Barriere G, Fici P, Gallerani G, et al. Circulating tumor cells and epithelial, mesenchymal and stemness markers: characterization of cell subpopulations. Ann Transl Med 2014;2:109. [PubMed]
- Basu D, Nguyen TT, Montone KT, et al. Evidence for mesenchymal-like sub-populations within squamous cell carcinomas possessing chemoresistance and phenotypic plasticity. Oncogene 2010;29:4170-82. [Crossref] [PubMed]