
FAM46B suppresses proliferation, migration and invasion of non-small cell lung cancer via β-catenin/MMP7 signaling
Introduction
Lung cancer, which is generally divided into non-small cell lung cancer (NSCLC, about 85% cases) and small cell lung cancer (SCLC, about 15% cases), is the leading cause of cancer-related mortality worldwide (1). The major histological subtypes of NSCLC are adenocarcinoma and squamous cell carcinoma (2). When diagnosed at an earlier stage (stage I), surgical therapy of NSCLC possesses a good prognosis for localized and small tumor patients with a 5-year survival rate of 70–90% (3). Unfortunately, most patients (75%) are diagnosed at later stages (4) and although prominent progress in the therapy of advanced stage lung cancer (stage III/IV) has been made in recent years, the survival rate remains poor. Data from the UK Office of National Statistics revealed that the one-year survival rate for stage IV patients is only 15–19% (5).
Chemotherapy is a central strategy for both surgical and inoperable cancer patients, while platinum is mild in the treatment of advanced NSCLC (6). Drug resistance and adverse drug reactions, however, greatly weaken the therapeutic effects of these strategies. In recent years, the therapeutic strategy for advanced NSCLC has transformed from histopathology-based traditional chemotherapy into carcinogenic factor-based individualized precision treatment (7). Previous study revealed that MTA1 might be an important biomarker in the diagnosis of NSCLC (8). Hyperactivation of Wnt/β-catenin and transforming growth factor-β (TGF-β) signaling has been implicated in the metastasis of NSCLC (9). Other studies showed that phosphatase and tensin homolog (PTEN) deficiency (10) and over-expression of insulin-like growth factor-1R (IGF-1R) (11)were closely associated with poor prognosis in NSCLC patients. Although therapeutic targets and biomarkers have, to some degree, contributed to improving NSCLC diagnosis and therapy, the complex pathogenesis of NSCLC remains a challenge and thus necessitates additional genetic information to develop better medical treatment (12).
FAM46 proteins, which belong to the nucleotidyltransferase (NTase) fold superfamily (13) of proteins, contain four human paralogs (FAM46A, FAM46B, FAM46C, FAM46D). FAM46A has been reported as a positional candidate for human retinal diseases and might associate with NSCLC (14,15). FAM46B is described as a potential marker for refractory lupus nephritis (16), while FAM46C is considered as a type I interferon-stimulated gene (ISG), which promotes replication of certain viruses (17). Results from gene set enrichment analysis (GSEA) revealed that lower FAM46B expression is correlated with the metastasis of lung cancer and involves Wnt signaling pathway (Supplementary file). However, only a few reports focus on the relationship between FAM46B and NSCLC. Hence, the present study examined the expression of FAM46B in 5 NSCLC cell lines, investigated the effects of FAM46B on NSCLC invasiveness, and probed the involvement of downstream β-catenin signaling in this process.
Methods
Chemicals and reagents
The cell counting kit-8 (CCK-8) assay kit was obtained from Signal way& Antibody (SAB, MA, USA). Trizol reagent and reverse transcription kits were purchased from Thermo Fisher (MA, USA). Crystal violet was obtained from Solarbio (Shanghai, China). FAM46B and vascular endothelial growth factor (VEGF) antibodies were purchased from Proteintech (IL, USA). β-catenin and matrix metalloproteinase 7 (MMP7) antibodies were obtained from Abcam Biotech (Cambridge, MA, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody was purchased from Cell Signaling Technology (MA, USA).
Patients
Human cancer tissues and corresponding paracancerous normal tissues were collected from lung cancer patients [37 patients with a mean ± standard error of mean (SEM) age of 55±10 years] who received pulmonary resection. This study was conducted with approval from the Ethics Committee of our hospital (YS-2019-065) and in accordance with the Declaration of Helsinki. Written informed consent was obtained from patients. Relative mRNA expression of FAM46B and protein levels of FAM46B and β-catenin were detected in the paired tissues.
Cells
NSCLC cell lines (A549, H1299, H1975, H292 and H358) and human bronchial epithelial cells (16HBE) were obtained from cell bank of the Chinese Academy of Sciences (Shanghai, China). These cells were cultured with Dulbecco’s modified Eagle medium (DMEM) medium (Hyclone, Logan, UT, USA) supplemented with 100 U/mL penicillin and 10% fetal bovine serum (FBS) (Gibco Company, USA) at 37 °C (5% CO2). Cells at logarithmic phase were used for follow-up experiments.
Construction of lentiviral vector
FAM46B (NM_052943.3) shRNA and over-expression lentiviral vectors were designed (shown in Table 1), constructed and verified by JRDun Biotechnology (Shanghai, China). Viral supernatants were diluted in the medium at appropriate concentrations and added to monolayer cells in follow-up experiments.
Table 1
| Description | Sequences or primers (5'-3') |
|---|---|
| FM46B (NM_052943.3) shRNA | (762–780) |
| Forward | GCAAGAACGUGGAGCUCAAUU |
| Group 1-reversed | UUGAGCUCCACGUUCUUGCUU |
| FM46B (NM_052943.3) OE | (173–1450) |
| Forward | CGGAATTCATGATGCCGTCGGAGAGC |
| Reversed | CGGGATCCTCAGTTACAAGGCAGCCAGG |
OE, over-expression.
Cell proliferation, migration and invasion
Viral supernatants were diluted in the medium at appropriate concentrations and added to monolayer cells. Cells were then cultured with DMEM medium supplemented with 10% FBS at 37 °C (5% CO2) for 48 h. Cells were harvested at 0, 24 and 48 h after treatment. Cell proliferation was assessed using CCK-8 assay kit following manufacturer’s instructions.
For transwell migration assay, a transwell chamber (corning-costar) was used according to manufacturer’s instructions. Briefly, cell culture inserts (aperture 6.5 mm) were rehydrated with serum-free DMEM at 37 °C for 2 h, and then 1×105/mL cells (in 500 µL serum-free DMEM) were seeded onto the upper chamber with 0.1% dimethyl sulfoxide (DMSO) control or viral supernatants/test drug. Meanwhile, 750 µL DMEM with 10% FBS was added into the lower chamber. After 24 h, cells on the upper surface of the membrane were wiped off by gentle swabbing. The cells that had migrated through the pores were fixed. After standard staining and microscopic examination, five random images per chamber were obtained at 200× magnification. Relative migration was calculated by counting cells and normalized to the negative control cells.
Invasion of cells into matrigel was determined similar to the migration assay. Briefly, after pre-hydration, 80 µL matrigel was added into the invasion chambers following incubation at 37 °C for 30 min. 2×105/mL cells (in 500 µL serum-free DMEM) were seeded onto the upper chamber with 0.1% DMSO control or viral supernatants/test drug. Meanwhile, 750 µL DMEM with 10% FBS was added into the lower chamber and cells were incubated at 37 °C with 5% CO2 for 24 h. Non-migratory cells from the upper filter surface were removed by a cotton swab, and the cells that had migrated through the pores were fixed, stained and counted. The calculation method of relative invasion was the same as the migration assay described above.
Quantitative real-time polymerase chain reaction (PCR)
Total mRNA (2 µg) of cultured NSCLC cells were extracted using Trizol reagent, quantified by the Qubit®2.0 quantitative machine using Qubit RNA HS Assay Kit (Life, MA, USA), and then reverse transcribed into cDNA using the first-strand cDNA synthesis kit. Then, reverse transcription-PCR (RT-PCR) analysis was performed on a quantitative reverse transcription-PCR (qRT-PCR) machine (ABI-7500, USA) using SYBR Green reagents and the following primers: pri-FAM46B-sense (S) 5'-ACAAGAGCGGCAAGAACG-3', pri-FAM46B-antisense (AS) 5'-CAGACATGGGAGTGGACGAG-3', pri-GAPDH-sense (S) 5'-AATCCCATCACCATCTTC, pri-GAPDH-antisense (AS) 5'-AGGCTGTTGTCATACTTC. Data were calculated by the 2−ΔΔCq method (18). GAPDH was used as a reference gene.
Western blot analysis
The total protein of each cell sample was isolated and detected by a bicinchoninic acid (BCA) protein assay kit following manufacturer’s instructions. Protein (35 µg) was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 5% skimmed milk at 25 °C for 1 h. Subsequently, relative membranes were incubated with the following primary antibodies: FAM46B (1:1,000), β-catenin (1:5,000), MMP7 (1:1,000), VEGF (1:1,000) and GAPDH (1:1,000). Membranes were then washed with phosphate buffered saline (PBS) and subsequently incubated with horseradish peroxidase-conjugated (HRP-conjugated) goat anti-rabbit IgG secondary antibody (Beyotime Biotech, Shanghai, China) for 1 h. Finally, protein bands were detected by an enhanced chemiluminescence (ECL) detection kit (Beyotime Biotech, Shanghai, China). Blots against GAPDH served as loading controls.
Statistics analysis
The data were presented as mean ± standard deviation (SD). Statistical analysis was performed using SPSS 19.0 software (SPSS, Inc., Chicago, USA). Analysis of variance (ANOVA) and paired Student’s t-test, along with the Dunnett’s test and post-hoc tests least significant difference test, were used for comparison between groups. P<0.05 was considered as statistically significant.
Results
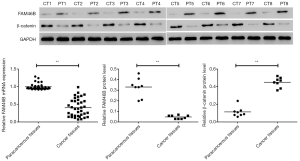
Differentially expressed FAM46B and β-catenin in paracancerous and cancer tissues of lung cancer patients
The present results revealed that relative mRNA expression of FAM46B was lower in lung cancer tissues compared to paired paracancerous tissues (Figure 1). Likewise, the protein level of FAM46B displayed a similar trend as its mRNA expression. In contrast, the protein level of β-catenin was higher in lung cancer tissues compared to paired paracancerous tissues.
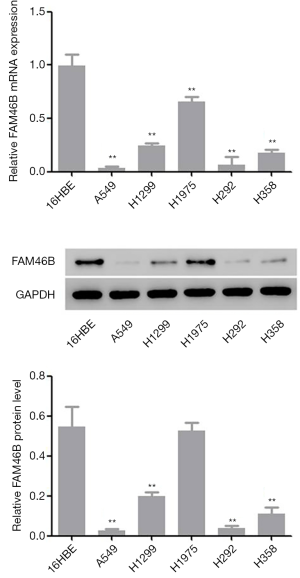
Screening of cell lines A549 and H1975
Results from GSEA revealed a correlation between lung cancer metastasis and reduced FAM46B expression (Supplementary file). In the present study, 5 NSCLC cell lines (A549, H1299, H1975, H292 and H358) and 1 human bronchial epithelial cell line (16HBE) were used to investigate the mRNA and protein levels of FAM46B. The results indicated that FAM46B displayed lower expressions in A549 and H292 cells but higher expressions in H1975 cells compared with 16HBE cells (Figure 2). These three cell lines (A549, H292 and H1975) were subsequently used in follow-up experiments.
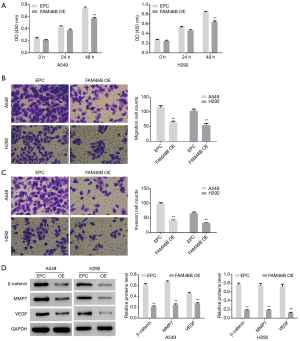
Effects of FAM46B over-expression on A549 and H292 cells
Our earlier findings indicated that lower FAM46B expression was associated with the metastasis of lung cancer and suggested a molecular mechanism involving Wnt signaling (Supplementary file). In the present study, we investigated the effects of FAM46B over-expression on the proliferation, migration and invasion of H292 and A549 cells, as well as on β-catenin, MMP7 and VEGF protein levels. As shown in Figure 3, proliferation, migration and invasion of H292 and A549 cells were suppressed by FAM46B over-expression. Moreover, the protein levels of β-catenin, MMP7 and VEGF were decreased by FAM46B over-expression both in A549 and H292 cells.
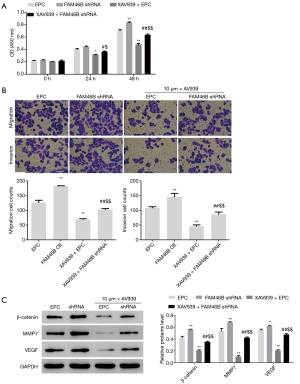
FAM46B RNAi enhances proliferation, migration and invasion of H1975 cells via Wnt/β-catenin signaling
Of the 5 NSCLC cell lines, H1975 cells displayed the highest FAM46B expression (Figure 2) and were used for follow-up experiments. We investigated the effects of FAM46B RNAi on the proliferation, migration and invasion of H1975 cells, as well as the effects of blocking Wnt/β-catenin signaling in H1975 cells. Interestingly, we found that FAM46B knockdown by shRNA markedly enhanced proliferation, migration and invasion of H1975 cells (Figure 4). Moreover, these effects were ameliorated by treatment with the Wnt/β-catenin inhibitor, XAV939 (19). Furthermore, β-catenin, MMP7 and VEGF protein levels were increased by FAM46B knockdown but decreased by co-treatment with XAV939 (Figure 4).
Discussion
Lung cancer is the leading cause of cancer-related mortality worldwide (1). Air contamination and cigarette smoke are primary causes of lung cancer (20,21). Traditional strategies for lung cancer treatment frequently lead to toxic side effects and are unavailable for some patients (22). Compared with histopathology-based traditional chemotherapy, molecular-targeted therapy shows reduced toxicity to normal tissues, high selectivity and better promise for eradicating the tumor (7,23,24). Our present study suggested that FAM46B acts as a tumor suppressor in NSCLC. Its expression is markedly reduced in lung cancer tissues compared to paired paracancerous tissues. Furthermore, over-expression of FAM46B weakened the proliferation, migration and invasion of H292 and A549 cells, while inhibiting β-catenin/MMP7 signaling.
β-catenin is the major component of Wnt signaling and has been shown to influence epithelial mesenchymal transition (EMT) (25,26). Hyperactivation of β-catenin is known to enhance cell migration and invasion, as well as induce EMT in NSCLC (27). MMP7 is a downstream target of β-catenin and involved in tumor progression by promoting EMT, cell invasion and metastasis (28). A previous report indicated that phospholipase Cδ1 suppresses cell migration and invasion of breast cancer cells via down-regulation of β-catenin/MMP7 signaling pathway (29). VEGF is known as a key regulatory factor involved in the regulation of tumor growth by activating host vascular endothelial cells, stimulating tumor angiogenesis, and enhancing malignant tumor progression (30). The present study revealed that FAM46B over-expression significantly reduced the protein levels of β-catenin, MMP7 and VEGF in both A549 and H292 cells. Conversely, FAM46B knockdown elevated the protein levels of β-catenin, MMP7 and VEGF, and stimulated proliferation, migration and invasion of H1975 cells. These effects were weakened by co-treatment with the Wnt/β-catenin signaling inhibitor XAV939, suggesting that β-catenin signaling is likely to mediate the functions of FAM46B in NSCLC.
Taken together, our study demonstrated that proliferation, migration and invasion of NSCLC cells are inhibited by FAM46B over-expression and stimulated by FAM46B knockdown. Hence, FAM46B may function as a tumor suppressor in NSCLC by regulating β-catenin, MMP7 and VEGF.
Supplementary
| Dataset |
| Lung cancer TCGA.FAM46B_profile_in_Lung_cancer_TCGA.cls#FAM46B |
| Phenotype |
| FAM46B_profile_in_Lung_cancer_TCGA.cls#FAM46B |
| Upregulated in class |
| FAM46B_neg |
| Gene set |
| GRADE_METASTASIS_DN |
| ES |
| –0.536809 |
| NES |
| –2.1690097 |
| Nominal P value |
| 0.0 |
| FDR Q value |
| 1.8023093E–4 |
| FWER P value |
| 0.002 |
ES, enrichment score; NES, normalized enrichment score; FDR, false discovery rate; FWER, familywise error rate.
| Dataset |
| Lung cancer TCGA.FAM46B_profile_in_Lung_cancer_TCGA.cls#FAM46B |
| Phenotype |
| FAM46B_profile_in_Lung_cancer_TCGA.cls#FAM46B |
| Upregulated in class |
| FAM46B_neg |
| Gene set |
| REACTOME_SIGNALING_BY_WNT |
| ES |
| –0.47817597 |
| NES |
| –2.014252 |
| Nominal P value |
| 0.0 |
| FDR Q value |
| 6.2330585E–4 |
| FWER P value |
| 0.011 |
ES, enrichment score; NES, normalized enrichment score; FDR, false discovery rate; FWER, familywise error rate.
Acknowledgments
We greatly appreciate the language help from YiBio Editing Inc.
Funding: This work was funded by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.27). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of the Sixth People’s Hospital affiliated to Shanghai University of Medicine & Health Sciences and conducted in accordance with the Declaration of Helsinki 2013 (YS-2019-065). Signed written informed consents were obtained from the participants and/or guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax 2013;68:551-64. [Crossref] [PubMed]
- Cancer Research UK. Lung cancer survival statistics 2015.
- Song W, Tang Z, Li M, et al. Polypeptide-based combination of paclitaxel and cisplatin for enhanced chemotherapy efficacy and reduced side-effects. Acta Biomater 2014;10:1392-402. [Crossref] [PubMed]
- Jin Y, Chen Y, Yu X, et al. A real-world study of treatment patterns and survival outcome in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. Oncol Lett 2018;15:8703-10. [PubMed]
- Zhu W, Li G, Guo H, et al. Clinicopathological significance of MTA 1 expression in patients with non-small cell lung cancer: a meta-analysis. Asian Pac J Cancer Prev 2017;18:2903-09. [PubMed]
- Cai J, Fang L, Huang Y, et al. Simultaneous overactivation of Wnt/β-catenin and TGFβ signalling by miR-128-3p confers chemoresistance-associated metastasis in NSCLC. Nat Commun 2017;8:15870. [Crossref] [PubMed]
- Zhao Y, Zheng R, Li J, et al. Loss of phosphatase and tensin homolog expression correlates with clinicopathological features of non-small cell lung cancer patients and its impact on survival: a systematic review and meta-analysis. Thorac Cancer 2017;8:203-13. [Crossref] [PubMed]
- Zhao J, Shi X, Wang T, et al. The prognostic and clinicopathological significance of IGF-1R in NSCLC: a meta-analysis. Cell Physiol Biochem 2017;43:697-704. [Crossref] [PubMed]
- Riess JW, Gandara DR, Frampton GM, et al. Diverse EGFR exon 20 insertions and co-occurring molecular alterations identified by comprehensive genomic profiling of NSCLC. J Thorac Oncol 2018;13:1560-8. [Crossref] [PubMed]
- Kuchta K, Muszewska A, Knizewski L, et al. FAM46 proteins are novel eukaryotic non-canonical poly(A) polymerases. Nucleic Acids Res 2016;44:3534-48. [Crossref] [PubMed]
- Barragán I, Borrego S, Abd El-Aziz MM, et al. Genetic analysis of FAM46A in Spanish families with autosomal recessive retinitis pigmentosa: characterisation of novel VNTRs. Ann Hum Genet 2008;72:26-34. [PubMed]
- Etokebe GE, Bulat-Kardum L, Munthe LA, et al. Association of variable number of tandem repeats in the coding region of the FAM46A gene, FAM46A rs11040 SNP and BAG6 rs3117582 SNP with susceptibility to tuberculosis. PLoS One 2014;9:e91385. [Crossref] [PubMed]
- Benjachat T, Tongyoo P, Tantivitayakul P, et al. Biomarkers for refractory lupus nephritis: a microarray study of kidney tissue. Int J Mol Sci 2015;16:14276-90. [Crossref] [PubMed]
- Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature 2011;471:467-72. [Crossref] [PubMed]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402-8. [Crossref] [PubMed]
- Tian XH, Hou WJ, Fang Y, et al. XAV939, a tankyrase 1 inhibitior, promotes cell apoptosis in neuroblastoma cell lines by inhibiting Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res 2013;32:100. [Crossref] [PubMed]
- Fenton-Ambrose L, Kazerooni EA. Preventative care: lung-cancer screens now worth the cost. Nature 2014;514:35. [Crossref] [PubMed]
- Lee PN, Forey BA, Coombs KJ. Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer 2012;12:385. [Crossref] [PubMed]
- Hui D, Elsayem A, Li Z, et al. Antineoplastic therapy use in patients with advanced cancer admitted to an acute palliative care unit at a comprehensive cancer center: a simultaneous care model. Cancer 2010;116:2036-43. [Crossref] [PubMed]
- Beck JT. Potential role for mammalian target of rapamycin inhibitors as first-line therapy in hormone receptor-positive advanced breast cancer. Onco Targets Ther 2015;8:3629-38. [Crossref] [PubMed]
- Oda K. Recent process of molecular target therapies in ovarian serous and endometrioid carcinomas on basis of intergrated genomic characterization. Gan To Kagaku Ryoho 2015;42:169-73. [PubMed]
- Masszi A, Fan L, Rosivall L, et al. Integrity of cell-cell contacts is a critical regulator of TGF-beta 1-induced epithelial-to-myofibroblast transition: role for beta-catenin. Am J Pathol 2004;165:1955-67. [Crossref] [PubMed]
- Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal 2014;7:re8. [Crossref] [PubMed]
- Zhang J, Zhang X, Zhao X, et al. DKK1 promotes migration and invasion of non-small cell lung cancer via β-catenin signaling pathway. Tumour Biol 2017;39:1010428317703820. [Crossref] [PubMed]
- Sizemore ST, Keri RA. The forkhead box transcription factor FOXC1 promotes breast cancer invasion by inducing matrix metalloprotease 7 (MMP7) expression. J Biol Chem 2012;287:24631-40. [Crossref] [PubMed]
- Shao Q, Luo X, Yang D, et al. Phospholipase Cδ1 suppresses cell migration and invasion of breast cancer cells by modulating KIF3A-mediated ERK1/2/β- catenin/MMP7 signalling. Oncotarget 2017;8:29056-66. [Crossref] [PubMed]
- Harmey JH, Bouchier-Hayes D. Vascular endothelial growth factor (VEGF), a survival factor for tumour cells: implications for anti-angiogenic therapy. Bioessays 2002;24:280-3. [Crossref] [PubMed]


