
HSP27 promotes epithelial-mesenchymal transition through activation of the β-catenin/MMP3 pathway in pancreatic ductal adenocarcinoma cells
Introduction
As one of the common digestive system neoplasms, pancreatic ductal adenocarcinoma (PDAC) is featured by high degree of malignancy, rapid tumor progression, difficult for early diagnosis, poor prognosis and easy recurrence, for which, surgery is still a potential curative method (1,2). Statistical data in the United States in 2018 from National Center for Health Statistics showed that the new morbidity and mortality of PDAC accounted for 3% and 7% of all tumors in the year, respectively, which was basically equal to data in 2017 (3). While the statistical result from Chinese Center for Cancer Control and Prevention showed that the morbidity and mortality of PDAC in China between 2009 and 2011 accounted for 2.1% and 2.8% of all tumors, respectively (4). Of late years, the technical means and therapeutic measures of clinical diagnosis for PDAC had made great progress, while the short-term survival and early postoperative recurrence are not affected significantly. Method of early diagnosis and treatment for PDAC is still a difficult problem to be solved in clinical practice.
Heat shock proteins (HSPs) are highly conserved key protein molecules to regulate cell homeostasis in nature, which can act as molecular chaperones to assist in correct folding, aggregation and migration during protein synthesis. In the case of stress stimulation, the expression of HSPs can be up-regulated to prevent excessive aggregation and regulate the correct folding of damage proteins. HSPs shows much higher expression during tumor growth and maintain the microenvironment for tumor progression through a variety of receptor-mediated signaling pathway for promoting tumor growth, proliferation and metastasis (5). HSP27 is a molecular chaperone of the HSPs with a molecular weight of 27KD, its expression will be induced when cells are exposed to different stress stimulation (6). As a kind of protein in the HSPs, HSP27 can regulate the cell homeostasis in the condition of normal oxidative stress and also involve in the development and growth of tumor cells. Studies showed that HSP27 had high expression in multiple tumors including liver cancer, lung cancer and intestinal cancer, and indicated poor prognosis in cancer patients (7-9). But, the correlation between the expression level of HSP27 and the postoperative survival time and prognosis of patients with PDAC had remains to be elucidated, of which the underlying mechanism in PDAC is unknown.
In this study, we firstly explored the expression level of HSP27 in PDAC tissues, also, we would explore whether and how the HSP27 regulating the invasion and migration of PDAC cells. We found that HSP27 was highly expressed in PDAC tissues, which is positively correlated with tumor differentiation, TNM staging and poor prognosis of PDAC patients. After silencing HSP27, the invasion and migration ability of PDAC cells were significantly depressed. In mechanism, we have found that HSP27 can enhance the epithelial mesenchymal transition (EMT) so as to promote the invasion and migration of PDAC cells by activating the β-catenin/MMP3 Pathway.
Methods
Experimental reagents and antibodies
Transfected liposome and total RNA extraction reagent (TRIzol) are purchased from Invitrogen (Grand Island, NY, USA). HSP27 (ab155987, 1:500), MMP-9 (ab38898, 1:300), MMP-3 (ab52915, 1:300) antibodies are purchased from Abcam (Cambridge, UK). E-cadherin (#14472, 1:200), β-catenin (#8480, 1:200), N-cadherin (#13116, 1:200), and Vimentin (#5741, 1:300) antibodies are bought from Cell Signaling Technology (Massachusetts, USA). Other reagents are bought from Sigma (St. Louis, MO, USA).
Patients specimens
A total of 50 pairs of PDAC tumors and corresponding para-carcinoma tissue paraffin specimens are randomly selected in this study. Specimens and clinical data are randomly collected from patients with PDAC who underwent hepatobiliary and pancreatic surgery and were clinically confirmed in Jiangxi provincial people’s hospital from 2009 to 2017. Clinical data has been examined and approved by the Ethics Committee of Jiangxi provincial people's hospital with the consent of patients. Clinicopathologic data include gender, age, tumor site, tumor size and diameter, pathological type, and PDAC tumors’ types are confirmed by TNM staging according to the TNM staging method revised by the Union for International Cancer Control (UICC) in 2002. 50 patients are followed up to July 2018. The Clinical Study Agreement is approved by the Ethics Committee of Jiangxi provincial people’s hospital with patients being informed to sign the Informed Consent prepared in accordance with the Declaration of Helsinki.
Immunohistochemistry
Resected PDAC and adjacent tissues are immersed in 4% paraformaldehyde overnight and cut into sections with 4 µm thick after embedding in paraffin. Immunohistochemical staining is then performed in the light of the Envision two-step method (10). Divided the results into four levels according to the degree of immunohistochemical staining: 0: <10% positive staining for PDAC cell; 1+: positive staining for 11–25% PDAC cell; 2+: positive staining for 26–50% PDAC cell; 3+: positive staining for >50% PDAC cell.
Cell culture and cell transfection
Four PDAC cells (PANC-1, BXPC-3, ASPC-1, and CFPAC-1) are purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). Thereinto, PANC-1 and BXPC-3 use Dulbecco’s Modified Eagle’s medium (DMEM) (Life technologies, South America) containing 10% fetal bovine serum (Life technologies, South America), and ASPC-1 use Roswell Park Memorial Institute-1640 (RPMI-1640) medium (Life technologies, South America) containing 10% fetal bovine serum (Life technologies, South America). CFPAC-1 use Iscove’s Modified Dulbecco Medium (IMDM) (Life technologies, South America) containing 10% fetal bovine serum (Life technologies, South America). Cells are cultured in 37 °C incubator with air conditions of 5% CO2/95% air, and resuscitate cells every 3 to 4 months. Preparation and transfection of HSP27-siRNA are conducted as described previously (Table S1) (11).
Western blotting
Add the collected cells to the lysis buffer containing protease inhibitor and the concentration of the total protein extracted is determined by a BCA (bicinchoninic acid) method (12); 40 µg of protein lysate is separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis system (SDS-PAGE), detected with specific antibody and peroxidase-labeled and targeted secondary antibody, and the ECL chemiluminescence is used as described above for showing the label (13).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Add collected cells and tissues to TRIzol (Invitrogen, Carlsbad, CA, USA) to extract total RNA according to the RNAiso TM Plus (Takara, Japan) kit instruction, and the concentration of RNA is measured by a spectrophotometer (NanoDrop, Thermo Fisher Scientific, USA). Use FastQuant cDNA First Chain Synthesis Kit (Tiangen Biotech, Beijing, China) to conduct the reverse transcription, prepare the reaction system and expand (Applied Biosystems, Thermo Fisher Scientific, USA) according to the qRT-PCR kit Fast Start Universal SYBR Green Master Mix (Rox) (Roche Diagnostics GmbH Mannheim, Germany) instructions and analyze results.
Scratch assay
Inoculate the prepared cell suspension into a 6-pore plate at 5×105 cells/pore and gently shake to ensure uniform distribution of cells in the 6-pore plate. and then observe the cells growth density in the 6-pore plate under the microscope during 1–2 days. When the cells fill the 6-pore plate, draw straight lines at 1/3 bottom of the 6-pore plate with 10 µL pipette tip, three lines in total. Wash cells for three times with PBS, remove the cells scratched, add serum-free culture medium and put in a 37 °C incubator with 5% CO2 for further cultivation. finally, the cell’s center aggregation condition was observed with microscope and recorded it by photographing hourly at 0 and 24 h.
Invasion and migration assays
Place transwell chamber (8 µm, BD Biosciences, USA) (chamber bottom with matrigel is used for invasion assay, and chamber bottom without matrigel is used for migration assay) on 24-pore plate, add about 600 µL culture medium containing 10% FBS at the chamber bottom (14), inoculate the cell suspension into well prepared based on 1,000 cells/hole in transwell chamber. After 1–2 d, observe the cell penetration situation in the chamber with a microscope, and after the defined amount of cells penetrate chamber pores, use 4% paraformaldehyde for fixing, and use 1% crystal violet (G1062, Solarbio, Japan) for staining and photographing.
Statistic analysis
Analyze the various experiment results through SPSS18.0 statistical software (SPSS Inc., Chicago, IL, USA). chi-square test (χ2) was used to evaluate the relationship between HSP27 tissue expression level and clinical pathology data, and match t-test to evaluate quantitative experiment data. Kaplan-Meier method was used to evaluate HSP27 expression and survival rate and poor prognosis of patients with PDAC, and compare situation of survivorship curve between groups through Log-Rank Test. Experiment of every group are repeated for three times, and the statistical result P<0.05 is used as the basis for being determined as statistic difference.
Cell proliferation assay
PDAC Cells were seeded in a 96-well plate (100 µL/well), with cell density of 3×103 cells/well. After incubation for a certain time, 10 µL of cell counting kit-8 (CCK-8) solution was added. Then cells were cultured at 37 °C under 5% CO2/95% air again with 1–4 h, the absorbance at 450 nm was measured using a microplate reader (Molecular Devices, Thermo Fisher Scientific).
Results
HSP27 is highly expressed in PDAC tissues, and positively correlated with tumor differentiation
We randomly select 50 pairs of PDAC and para-carcinoma tissue paraffin specimens sections for immunohistochemical analysis (Figure 1A). The result shows that the cell ratio with positive HSP27 staining in PDAC tissue is higher than that of para-carcinoma tissue (Figure 1B). According to the method in the “immunohistochemical” part of the material method, we divided HSP27 expression level in PDAC into high expression group (n=30) and low expression group (n=20), and further analyze the correlation of HSP27 expression level with clinical prognosis of the patients with PDAC. The statistical result shows that HSP27 expression level are significantly related with TNM stage (P=0.006) and vascular metastasis (P=0.001), but not related to other clinical pathology characteristics, such as age, gender, tumor diameter, etc. (Table 1). The above result prompts that HSP27 is in the highly-expressed state in PDAC tissues, and its high expression is positively correlated with tumor differentiation.
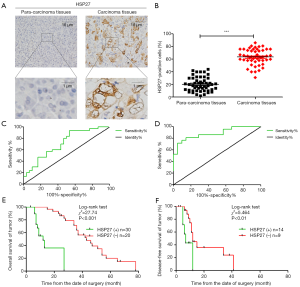
Table 1
| Variables | HSP27 expression, n [%] | Total | P | |
|---|---|---|---|---|
| Low (n=20) | High (n=30) | |||
| Age, years | 0.365 | |||
| <40 | 8 (33) | 16 (67) | 24 | |
| ≥40 | 12 (46) | 14 (54) | 26 | |
| Gender | 0.429 | |||
| Male | 11 (46) | 13 (54) | 24 | |
| Female | 9 (35) | 17 (65) | 26 | |
| Tumor diameter | 0.634 | |||
| <2 | 14 (42) | 19 (58) | 33 | |
| ≥2 | 6 (35) | 11 (65) | 17 | |
| Tumor differentiation | 0.053 | |||
| Well | 3 (23) | 10 (77) | 13 | |
| Moderate | 7 (35) | 13 (65) | 20 | |
| Poor | 10 (59) | 7 (41) | 17 | |
| TNM stage | 0.006* | |||
| I–II | 7 (24) | 22 (76) | 29 | |
| III–IV | 13 (62) | 8 (38) | 21 | |
| Vascular metastasis | 0.001* | |||
| No | 9 (26) | 26 (74) | 35 | |
| Yes | 11 (73) | 4 (27) | 15 | |
*, P<0.05.
High expression of HSP27 in PDAC tissues is positively correlated with poor prognosis of PDAC patients
We take a follow-up visit to the survival rate of the patients with PDAC in 5 years after surgery in real time. Through analyzing ROC curve, the expression degree of HSP27 has a statistical significance on determination of overall survival (OS) (AUC =0.711, 95% CI: 0.587–0.837, P=0.003) and recurrence-free survival (RFS) (AUC =0.859, 95% CI: 0.734–0.984, P=0.001) (Figure 1C,D). We further analyze correlation of HSP27 expression and the clinical prognosis of patients with PDAC through Kaplan-Meier curve. The result shows that the median overall survival time of the patients with PDAC in HSP27 high expression group is significantly lower than that of HSP27 low expression group (median, 12 vs. 43 months; P<0.001) (Figure 1E), while recurrence-free survival time is also significantly lower than that of HSP27 low expression group (median, 7 vs. 12 months; P<0.01) (Figure 1F). The multi-factor Cox regression analysis result shows that HSP27 high expression (relative risk =4.561; P=0.021) is an independent predictive factor of overall survival prognosis of the patients with PDAC (Table 2). These results fully show that HSP27 high expression in PDAC tissue is closely related to poor prognosis of the patients with PDAC.
Table 2
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | P | RR | 95% CI | P | ||
| Age (years) (<40 vs. >40) | 0.431 | 0.360–0.617 | 0.480 | 0.537 | 0.410–0.364 | 0.109 | |
| Gender (male vs. female) | 1.375 | 1.213–1.986 | 0.652 | 0.822 | 0.631–1.027 | 0.073 | |
| Tumor diameter (cm) (<2 vs. >2) | 1.387 | 1.0.2–1.653 | 0.436 | 2.983 | 2.762–5.120 | 0.104 | |
| Tumor differentiation (well vs. poor) | 1.327 | 0.703–2.127 | 0.471 | 0.652 | 0.387–0.913 | 0.071 | |
| TNM stage (I–II vs. III–IV) | 0.920 | 0.673–1.826 | 0.037 a | 0.533 | 0.376–0.727 | 0.015* | |
| Vascular metastasis (no vs. yes) | 6.735 | 5.471–8.932 | 0.023 a | 6.270 | 5.829 –8.697 | 0.016* | |
| HSP27 expression (high vs. low) | 3.983 | 3.286–6.732 | 0.041a | 4.561 | 3.106–6.328 | 0.021* | |
*, P<0.05. RR, relative risk; CI, confidence interval.
HSP27 can promote invasion and migration of PDAC cells
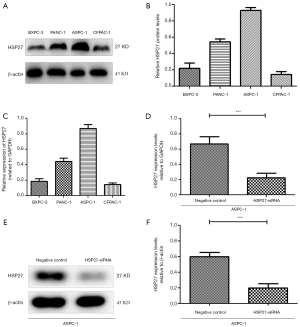
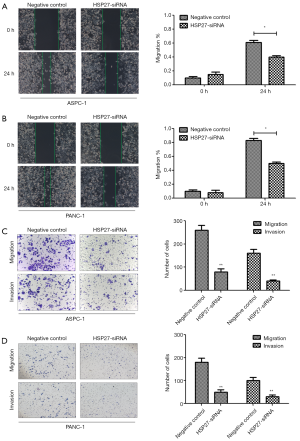
We select 4 PDAC cell lines (ASPC-1, BXPC-3, CFPAC-1, PANC-1), and verify HSP27 expression herein. Western blotting and qRT-PCR detection results show a significant difference of HSP27 protein and mRNA expression level in four types of PDAC cell lines (P<0.05) (Figure 2A,B,C). Subsequently, we select highly-expressed ASPC-1 and PANC-1 for subsequent experiments. We further construct HSP27-siRNA transfection to silence HSP27, and use western blotting and qRT-PCR detection to screen out siRNA sequences (Figure 2D,E,F) with best silencing effect. After that, by the scratch assay, we found that the migration abilities of ASPC-1 and PANC-1 cells weaken significantly after silencing HSP27 (Figure 3A,B). Meanwhile, we used invasion and migration assays with transwell chamber and found that invasions and migration abilities of ASPC-1 and PANC-1 cells also depressed significantly after silencing HSP27 (Figure 3C,D).
HSP27 can promote proliferation of PDAC cells
After siRNA-mediated silencing of HSP27 in ASPC-1 and PANC-1 cells, tumor cell proliferation was evaluated in CCK-8 assays. We can find that siRNA-mediated HSP27 silencing significantly reduced ASPC-1 and PANC-1 cell proliferation (Figure S1) compared with the negative control groups.
HSP27 promote EMT of PDAC cells via β-catenin/MMP3 Pathway
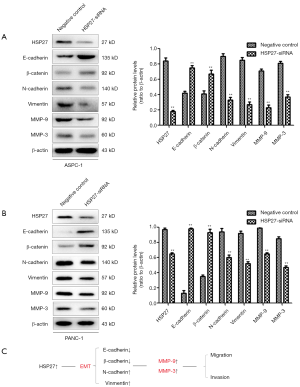
We then down-regulated the expression of HSP27 on PDAC cells, and detected the expression of epithelioma and mesenchyme-related protein through western blotting. It shows that the epithelioma-related proteins have increased (E-cadherin, β-catenin) after down-regulation of HSP27, but mesenchyme-related proteins (N-cadherin, Vimentin) decreased, while MMP-9 and MMP-3 proteins also have significantly decreased (Figure 4A,B). The above results show that HSP27 can regulate EMT, meanwhile, up-regulate MMP-9 and MMP-3 expression on PDAC cells, so as to promote invasion and migration of PDAC cells (Figure 4C).
Discussion
As a type of small molecular protein in HSPs, HSP27 is regulated by HSF1 in heat shock and other stress states (15,16), and widely regulates tumorigenesis such as proliferation and distant metastasis (5,17). But the researches on its role in the regulation of PDAC and its underlying mechanisms remains to be elucidated. As a tumor of alimentary tract with high malignancy, PDAC has a trend of increasing incidence year by year, and discovering the tumor biomarkers to diagnose of PDAC in the early period is always a problem to be solved. Our research shows that HSP27 is highly expressed in PDAC tissue compared with normal tissues and is positively correlated with tumor differentiation and poor prognosis of patients with PDAC. Moreover, HSP27 is also an independent predictive factor of prognosis in PDAC patients.
The current research result prompts that EMT is one of the key factors for distant metastasis of tumors. EMT plays a characteristic driving role in many aspects such as tumor stem cells, tumor cell drug resistance and tumor recurrence (18), and a very important regulatory role in the development of PDAC tumors (19). The previous researches prompt that HSP27 can regulate EGF-induced AKT and GSK3b phosphorylation, inhibit β-catenin ubiquitinoylation and degradation, and then prevent combination of β-catenin nuclear translocation with Slug promoter, thus promoting EMT to drive tumor development (20). Meanwhile, HSP27 inhibits activation of pro-apoptotic protein caspase-3 and active proteins Lamin B in BCL2 by regulating EMT, so as to promote tumor occurrence (21). Our research shows that after silencing HSP27, the expressions of PDAC cell epithelium related proteins E-cadherin and β-catenin increase, while the mesenchyme-related proteins V-cadherin and vimentin decrease, and mesenchymal epithelial transformation (MET) occurs in PDAC cells, as a result of that, the invasion and migration abilities of PDAC cells are weakened significantly. As a type of matrix metalloproteinase, MMPs participates in invasion and migration of PDAC cells through degrading cellular matrix (22). Meanwhile, our experiment result shows that expression of MMP-9, MMP-3 proteins decreases significantly after silencing HSP27. Therefore, we infer that PEA15 can promote increase in expression of MMP-9, MMP-3 proteins through down-regulation of expression of epithelium-related proteins E-cadherin and β-catenin and up-regulation of mesenchyme-related proteins, thus promoting occurrence of invasion and migration of PDAC cells.
Conclusions
In conclusion, we found that HSP27 is high expressed in PDAC tissues, which is positively correlated with the degree of tumor differentiation of PDAC, TNM staging and poor prognosis. Meanwhile, the invasion and migration ability of PDAC cells were decrease while down-regulating of HSP27, which showed that HSP27 can promote the invasion and migration abilities of PDAC cells. In mechanism, we have proved that HSP27 can regulate the expression of EMT-related proteins in PDAC cells, thus enhancing the invasion and migration abilities of PDAC cells. In summary, we deduced that HSP27 can be used as a tumor biomarker for diagnosis of PDAC, and HSP27 can enhanced the EMT so as to promote the invasion and migration of PDAC cells by activating the β-catenin/MMP3 Pathway. Therefore, inhibition of HSP27 has therapeutic potential for the treatment of PDAC.

Table S1
| Genes | Sequences 5'-3' | |
|---|---|---|
| Forward | Reverse | |
| HSP27-siRNA | CAAGUUUCCUCCUCCCUGUTT | ACAGGGAGGAGGAAACUUGTT |
| Negative control | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
Acknowledgments
We wish to particularly acknowledge the patients enrolled in this study for their participation, and the Department of Pathology and Physiopathology, Jiangxi Provincial People’s Hospital, for its collaboration in providing the human samples and the clinical information used in this project with appropriate ethics approval.
Funding: The study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.07.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Clinical Study Agreement is approved by the Ethics Committee of Jiangxi provincial people’s hospital with patients being informed to sign the Informed Consent prepared in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Froeling F, Tuveson D. Pancreatic cancer foiled by a switch of tumour subtype. Nature 2018;557:500-501. [Crossref] [PubMed]
- Perez K, Clancy TE, Mancias JD, et al. When, What, and Why of Perioperative Treatment of Potentially Curable Pancreatic Adenocarcinoma. J Clin Oncol 2017;35:485-9. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. Ca Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Ca Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Calderwood SK, Gong J. Heat Shock Proteins Promote Cancer: It's a Protection Racket. Trends Biochem Sci 2016;41:311-23. [Crossref] [PubMed]
- Xu Y, Diao Y, Qi S, et al. Phosphorylated Hsp27 activates ATM-dependent p53 signaling and mediates the resistance of MCF-7 cells to doxorubicin-induced apoptosis. Cell Signal 2013;25:1176-85. [Crossref] [PubMed]
- Wang RC, Huang CY, Pan TL, et al. Proteomic Characterization of Annexin l (ANX1) and Heat Shock Protein 27 (HSP27) as Biomarkers for Invasive Hepatocellular Carcinoma Cells. PLoS One 2015;10:e0139232. [Crossref] [PubMed]
- Ankersmit HJ, Lambers C, Zimmermann M, Hacker S, Moser B. Serendipity and technical considerations for the measurement of serum heat shock protein HSP27 in patients with COPD and lung cancer. Cell Stress Chaperones 2015;20:727-8. [Crossref] [PubMed]
- Tweedle EM, Khattak I, Ang CW, et al. Low molecular weight heat shock protein HSP27 is a prognostic indicator in rectal cancer but not colon cancer. Gut 2010;59:1501-10. [Crossref] [PubMed]
- Pinato DJ, Tan TM, Toussi ST, et al. An expression signature of the angiogenic response in gastrointestinal neuroendocrine tumours: correlation with tumour phenotype and survival outcomes. Br J Cancer 2014;110:115-22. [Crossref] [PubMed]
- Li F, Mahato RI. RNA interference for improving the outcome of islet transplantation. Adv Drug Deliv Rev 2011;63:47-68. [Crossref] [PubMed]
- Olson BJ. Assays for Determination of Protein Concentration. Curr Protoc Pharmacol 2016;73:A.3A.1-A.3A.32.
- Hu GB, Xiong CY, Liang WB, et al. Highly Stable Mesoporous Luminescence-Functionalized MOF with Excellent Electrochemiluminescence Property for Ultrasensitive Immunosensor Construction. ACS Appl Mater Interfaces 2018;10:15913-9. [Crossref] [PubMed]
- Valster A, Tran NL, Nakada M, et al. Cell migration and invasion assays. Methods 2005;37:208-15. [Crossref] [PubMed]
- Wu J, Liu T, Rios Z, et al. Heat Shock Proteins and Cancer. Trends Pharmacol Sci 2017;38:226-56. [Crossref] [PubMed]
- Tokunaga M, Baron B, Kitagawa T, et al. Active Hexose-correlated Compound Down-regulates Heat Shock Factor 1, a Transcription Factor for HSP27, in Gemcitabine-resistant Human Pancreatic Cancer Cells. Anticancer Res 2015;35:6063-7. [PubMed]
- Sevin M, Kubovcakova L, Pernet N, et al. HSP27 is a partner of JAK2-STAT5 and a potential therapeutic target in myelofibrosis. Nat Commun 2018;9:1431. [Crossref] [PubMed]
- Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol 2017;14:611-29. [Crossref] [PubMed]
- Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015;527:525-30. [Crossref] [PubMed]
- Cordonnier T, Bishop JL, Shiota M, et al. Hsp27 regulates EGF/β-catenin mediated epithelial to mesenchymal transition in prostate cancer. Int J Cancer 2015;136:E496-507. [Crossref] [PubMed]
- Konda JD, Olivero M, Musiani D, et al. Heat-shock protein 27 (HSP27, HSPB1) is synthetic lethal to cells with oncogenic activation of MET, EGFR and BRAF. Mol Oncol 2017;11:599-611. [Crossref] [PubMed]
- Kim K, Punj V, Kim JM, et al. MMP-9 facilitates selective proteolysis of the histone H3 tail at genes necessary for proficient osteoclastogenesis. Genes Dev 2016;30:208-19. [PubMed]


