
KISS1 protein expression is associated with worse prognosis in osteosarcoma patients: a long-term follow-up study
Introduction
Osteosarcoma (OS) is a common primary malignant bone tumor occurring in adolescence, and mostly affects males (1). In recent years, several clinical applications, including neo-adjuvant chemotherapy combined with surgery and other comprehensive treatment, have significantly improved its clinical prognosis. However, the 5-year survival rate is still hovering around 50–70% (2) due to early lung metastasis and inflicts a grievous toll on the community and families.
KISS1 gene was found to be cDNA clone fragment (3), located on the long arm of chromosome 1 (1q321q34) (4), which generates a biologically active secretory peptide named kisspeptin-54 (5,6). GPR54 was discovered (7) to be a receptor protein, consisting of seven transmembrane (TM) domains, belonging to the orphan G protein receptor family. In 2001, researchers found human GPR54 homologs, known as AXOR12 (8) or hOT7T175 (5), composed of 398 amino acids. Kisspeptin-54 can specifically bind with receptor GPR54 and perform its biological role (6).
Research on the metastasis mechanism and clinical prognosis in OS have been intensively active of late. However, long-term follow-up reports are rare. This study was conducted for an average of 10.11 years of follow-up and aimed to analyze the relationship between KISS1 and GPR54 protein expression and clinical prognosis in OS patients.
Methods
Samples and data collection
Forty-four paraffin-embedded OS samples and 15 osteochondroma samples were provided by the Pathology Department of the First Affiliated Hospital of Fujian Medical University. The OS samples were conserved during the period from 2005 to 2009 and included 25 males (56.81%) and 19 females (43.19%) with a median age at diagnosis of 18 years (range, 12–74 years; mean age 23.68 years). All patients had been given a period of treatment of chemotherapy (combined treatment of doxorubicin, cyclophosphamide, and ifosfamide) before surgery. Information about gender, age, pathological type, surgical stage, and clinical outcome was collected from the medical record.
The relationship between the expression of KISS1 or GPR54 protein and the clinical prognosis of OS patients were reported in our previous study (9,10) with short-term (average less than 2 years) follow-up results. With a regular annual follow-up, the average follow-up time was 10.11 years (121.36±15.46 months; range, 97 to 162 months) lasting until June 2018. The rates of lung metastasis and mortality have changed, and we here report the most recent follow-up results.
Immunohistochemistry
The immunohistochemical staining of paraffin specimens from patients was used to detect the expression of KISS1 and GPR54 protein. Serial 2.5 µm slices were cut from formalin-fixed and paraffin-embedded OS specimens. First, sections were dewaxed in xylene and rehydrated in a graded series of alcohols before being washed by distilled water. Then, the sections were boiled with high power in the microwave oven to a boiling point, and then continuously boiled at medium-to-low power for 15 minutes with EDTA (0.05 mol/L Tris, 0.001 mol/L EDTA, and pH 9.0) to retrieve antigens. After boiling, sections were washed 3 times with PBS and then treated with 3% H2O2 for blocking unspecific protein-binding sites for 15 minutes. Second, the slides were incubated with a monoclonal mouse anti-KISS1 antibody (1:50, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or rabbit anti-GPR54 antibody (1:700, Phoenix Pharmaceuticals, Burlingame, CA, USA) at 37 °C for 1 hour. Next, slides were incubated with Polymer Helper and Poly Peroxidase-anti-Mouse IgG (GBICO, USA) for 25 minutes. Third, the peroxidase activity was visualized through a color reaction with DAB (3, 3’-diaminobenzidine, Maixin Biotechnology, Fuzhou, China). Brown and yellow colors indicated positive results for KISS1 and GPR54 antigen. Finally, the sections were counterstained with hematoxylin and mounted with coverslips after standard dehydration. Pictures were captured with a BX-51 microscope (Olympus, Japan) by image processing software DP-70 (Olympus, Japan), and have been published with the results of the short-term follow-up studies (9,10).
Statistical analyses
All statistical analyses were conducted by SPSS21 (SPSS Inc., Chicago, IL, USA). The distribution of data was assessed for normality using the Kolmogorov-Smirnov test. Continuous variables were presented as the mean ± standard error of the mean (SEM) or median with range. For normally distributed data, Student’s t-test was used to compare the differences between 2 independent groups. Otherwise, the Mann-Whitney U test was used. Chi-square test was performed to compare differences of rates among different groups. Survival curves were drawn by the Kaplan-Meier method and compared by the log-rank test. Statistical significance was assumed when P<0.05.
Results
Overall clinical prognosis
After more than an average of 10 years of follow-up, the follow-up durations (121.36±15.46 months) ranged from 97 to 162 months, 19 patients were alive, 25 patients died, and the median survival time was 35 months (mean ± SEM 63.05±7.88 months). The overall survival rate was 43.18% (19/44), and the metastatic rate was 65.91% (29/44) in the course of the disease. These data points were 54.55% (24/44) and 61.36% (27/44) respectively, at the early follow-up. Cases of metastasis included 26 lung metastases, 1 bone metastasis, 1 axillary lymph node metastasis, and 1 retroperitoneal metastasis. Furthermore, there was no statistical difference in the expression of KISS1/GPR54 protein in terms of gender, age, pathological subtype, and surgical stage (Table 1).
Table 1
| OS patients | KISS1 (+) (n=20), n (%) | KISS1 (–) (n=24), n (%) | P value | GPR54 (+) (n=36), n (%) | GPR54 (–) (n=8), n (%) | P value |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 12 (60.00) | 13 (54.17) | 0.70 | 21 (58.33) | 4 (50.00) | 0.97 |
| Female | 8 (40.00) | 11 (45.83) | 15 (41.67) | 4 (50.00) | ||
| Age (mean ± SEM) (year) | 24.50±2.68 | 23.00±2.85 | 0.44 | 23.69±2.32 | 23.63±2.83 | 0.39 |
| Locations | ||||||
| Femur | 9 (45.00) | 11 (45.83) | 0.89 | 16 (44.44) | 4 (50.00) | 0.34 |
| Tibia | 7 (35.00) | 7 (29.17) | 13 (36.11) | 1 (12.50) | ||
| Other | 4 (20.00) | 6 (25.00) | 7 (19.44) | 3 (37.50) | ||
| Pathological type | ||||||
| Telangiectatic OS | 1 (5.00) | 2 (8.33) | 0.27 | 2 (5.56) | 1 (12.50) | 0.63 |
| Small cell OS | 2 (10.00) | 0 (0.00) | 2 (5.56) | 0 (0.00) | ||
| Other | 17 (85.00) | 22 (91.67) | 32 (88.89) | 7 (85.50) | ||
| Surgery stages | ||||||
| IIA | 2 (10.00) | 5 (20.83) | 0.57 | 4 (11.11) | 3 (37.50) | 0.19 |
| IIB | 18 (90.00) | 19 (79.17) | 32 (88.89) | 5 (62.50) | ||
| Metastasis | ||||||
| Present | 17 (85.00) | 12 (50.00) | χ2=5.95 | 24 (66.67) | 5 (62.50) | χ2=0.00 |
| Absent | 3 (15.00) | 12 (50.00) | 0.01 | 12 (33.33) | 3 (37.50) | 1.00 |
| Mortality | ||||||
| Death | 15 (75.00) | 10 (41.67) | χ2=4.94 | 22 (61.11) | 3 (37.50) | χ2=0.68 |
| Survival | 5 (25.00) | 14 (58.33) | 0.03 | 14 (38.89) | 5 (62.50) | 0.41 |
| Survival time (months) | 55.35±14.29 | 97.46±13.06 | 0.01 | 73.97±11.81 | 92.75±15.42 | 0.18 |
SEM, standard error of the mean; OS, osteosarcoma.
KISS1 expression and clinical prognosis analysis
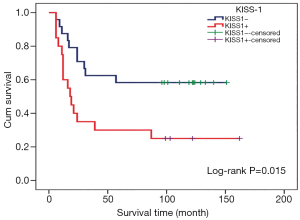
Among patients with KISS1 protein positive expression, the distal metastasis rate was 85% (17/20), the mortality rate was 75% (15/20), the median survival time was 18 months, and the mean survival time was 55.35±14.29 months. As for the KISS1 protein negative patients, the rate of distal metastases and mortality was 50% (12/24) and 41.67% (10/24), and the mean survival time was 97.46±13.06 months. There was significant difference in the mortality rate (Figure 1), metastasis rate (Figure 1), and mean survival time (Figure 2) (P<0.05), suggesting that positive expression of KISS1 protein correlated with more mortality and metastasis, and shorter survival time in OS patients, which is consistent with the results of our early follow-up (9).

GPR54 expression and clinical prognosis analysis
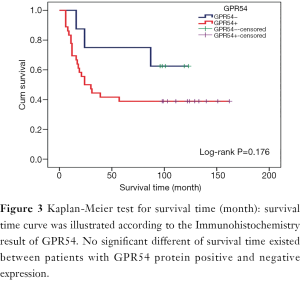
In GPR54 protein positive expression patients, the metastatic rate was 66.67% (24/36), the mortality rate was 61.11% (22/36), the median survival time was 24 months, and the mean survival time was 73.97±11.81 months. In GPR54 protein negative expression patients, the metastasis rate was 62.5% (5/8), the mortality rate was 37.5% (3/8), and the mean survival time was 92.75±15.42 months. There was no significant difference in mortality rate (Figure 1), metastasis rate (Figure 1), or mean survival time (Figure 3) (P>0.05), which is inconsistent with the results of our early follow-up (10).
Furthermore, there were 19 cases with KISS1 and GPR54 proteins, both positive, in which 16 cases were distal metastases, and 14 ended in death. For those 7 cases with both KISS1 and GPR54 proteins negative, 4 cases had distal metastases, and 2 cases ended in death. The analysis showed that there was a significant statistical difference in distal metastases (Figure 1) rather than mortality (Figure 1), indicating that both KISS1 and GPR54 positive expression was negatively correlated with the metastasis rate of patients with OS.
Discussion
The important role of the KISS1/GPR54 system in human tumors has been demonstrated, but the specific effects are still controversial. Some reports have shown that KISS1/GPR54 genes inhibit tumor occurrence or transfer. For instance, it has been reported that B16-BL6 melanoma cell morphology was changed significantly after the transfection of the GPR54 gene, and the ability of invasion and metastasis markedly decreased (11). The lack of KISS1 and GPR54 gene was found to be associated with the occurrence of lymph node metastasis tumors in esophageal cancer (12). Another study (13) reported that KISS-1 expression was decreased in pancreatic cancer tissue, but GPR54 (mRNA) expression increased. Zohrabian et al. (14) discovered that KISS1 gene expression decreased in lung adenocarcinoma tissue. Furthermore, it was reported that KISS1 expression in uveal melanoma, patients with low KISS1 expression were associated with a higher risk of metastatic disease (15). By down-regulating Gαq-p63RhoGEF-RhoA signaling pathways, Cho et al. (16) found that the KISS1 receptor GPR54 gene could delay the development of breast cancer. Recently, Yin et al. (17) reported that KISS1 inhibited the proliferation of OS in vitro by accelerating the processes of apoptosis and autophagy in the cells.
Conversely, the high expression of KISS1/GPR54 showed poor prognosis in several other kinds of malignant tumors. In 2003, it was found that the KISS1/GPR54 gene was highly expressed in all advanced liver cancer tissues (18). Results reported by Gao et al. (19) also showed that the expression level of KISS1 was higher in tumor tissues than benign lesions and normal tissues in the ovarian epithelial tumor. The positive expression rate of KISS1 was also found to be higher in the development of colorectal cancer tumors than normal tissue (20). In addition, Kostakis et al. (21) reported a contradictory phenomenon: the expression of KISS1 was much higher in normal colonic mucosa than in malignant colonic mucosa and higher in more advanced tumors than early tumor tissue. Moreover, among malignant tissues, KISS1 expression was higher in larger tumors (>4 cm) than in smaller ones (≤4 cm), higher in stages III and IV than in stages I and II, and higher in patients with lymph node metastases.
According to the previous studies, the KISS1/GPR54 gene may participate in the occurrence and development process in different tumors through different molecular mechanisms, as an inhibitor in some tumors and a promoter in others. The complex molecular mechanism of KISS1/GPR54 gene in tumors remains to be further studied. Here, we investigated the prognosis of OS patients with KISS1 or GPR54 alone or together.
Among the 36 patients with GPR54 protein positive expression, the KISS1 protein was positively expressed in 19 patients, 16 of whom had distant metastasis, 14 of these patients who occurred distant metastasis resulting in death. For the other 17 patients with KISS1 protein negative expression, distant metastasis occurred in 8 patients, all of whom died. This indicates that the rate of mortality and distal metastasis were both significantly higher in the patients with both KISS1 and GPR54 protein positive expression. On the other hand, among the 20 patients with positive KISS1 protein expression, the only one with negative GPR54 protein expression had lung metastasis and died, which suggests that the KISS1 positive expression was associated with clinical outcomes, but not the expression of GPR54 protein. In contrast, KISS1 protein expression was positively correlated with metastasis in patients with GPR54 protein expression, but not in patients with GPR54 protein absent. What the above indicates is that, as an endogenous receptor of KISS1, GPR54 protein affected the prognosis of patients with OS by integrating with KISS1 protein, and KISS1 protein affected the prognosis perhaps through the GPR54 signaling pathway. The results of the long-term follow-up were distinct from the short-term follow-up results we reported 7 years ago (10).
Some limitations exist in this study. First, this is a retrospective evaluation of a limited number of patients. Second, immunohistochemical staining was the only validated experiment method in this study, but our previous experiments had proven that KISS/GPR54 protein expression in OS cell lines (U-2 OS, Saos-2, MG-63) and KISS protein expression were related with aggressive ability in cell lines. Despite these limitations, this study is noteworthy since we are first to report a long-term clinical prognosis analysis concerning the predictive effect of KISS1/GPR54 protein expression in OS patients.
Conclusions
In summary, after more than an average of 10.11 years of follow-up, we found that KISS1 protein expression significantly increased in OS patients who had distal metastases, with a negative correlation to survival time and survival rate; these findings are consistent with the results of our earlier follow-up. GPR54 protein affects the prognosis of patients with OS due to being integrated with the KISS1 protein but does not affect the prognosis when expressed alone, an observation is inconsistent with the results of early follow-up. The molecular mechanism of the KISS/GPR54 protein system in tumors is very complex, and thus, further study is needed.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.08.21). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All studies were approved by the ethics committee of Fuzhou Second Hospital Affiliated to Xiamen University and the First Affiliated Hospital of Fujian Medical University (FUM-2010-012). Individual informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weber KL. What's new in musculoskeletal oncology. J Bone Joint Surg Am 2005;87:1400-10. [PubMed]
- O'Day K, Gorlick R. Novel therapeutic agents for osteosarcoma. Expert Rev Anticancer Ther 2009;9:511-23. [Crossref] [PubMed]
- Lee JH, Miele ME, Hicks DJ, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst 1996;88:1731-7. [Crossref] [PubMed]
- West A, Vojta PJ, Welch DR, et al. Chromosome localization and genomic structure of the KiSS-1 metastasis suppressor gene (KISS1). Genomics 1998;54:145-8. [Crossref] [PubMed]
- Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001;411:613-7. [Crossref] [PubMed]
- Kaiser UB, Kuohung W. KiSS-1 and GPR54 as new players in gonadotropin regulation and puberty. Endocrine 2005;26:277-84. [Crossref] [PubMed]
- Lee DK, Nguyen T, O'Neill GP, et al. Discovery of a receptor related to the galanin receptors. FEBS Lett 1999;446:103-7. [Crossref] [PubMed]
- Muir AI, Chamberlain L, Elshourbagy NA, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem 2001;276:28969-75. [Crossref] [PubMed]
- Wang FS, Chen H, Wu ZY, et al. KISS1 expression in osteosarcoma: high in chinese clinical cases, but lower in cell lines. Asian Pac J Cancer Prev 2011;12:3229-34. [PubMed]
- Chen H, Wu CY, Lin JH, et al. G protein-coupled receptor 54 gene expression in osteosarcoma and its clinical significance. Chin J Cancer Biother 2012;19:289-93.
- Hori A, Honda S, Asada M, et al. Metastin suppresses the motility and growth of CHO cells transfected with its receptor. Biochem Biophys Res Commun 2001;286:958-63. [Crossref] [PubMed]
- Ikeguchi M, Yamaguchi K, Kaibara N. Clinical significance of the loss of KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in esophageal squamous cell carcinoma. Clin Cancer Res 2004;10:1379-83. [Crossref] [PubMed]
- Masui T, Doi R, Mori T, et al. Metastin and its variant forms suppress migration of pancreatic cancer cells. Biochem Biophys Res Commun 2004;315:85-92. [Crossref] [PubMed]
- Zohrabian VM, Nandu H, Gulati N, et al. Gene expression profiling of metastatic brain cancer. Oncol Rep 2007;18:321-8. [PubMed]
- Martins CM, Fernandes BF, Antecka E, et al. Expression of the metastasis suppressor gene KISS1 in uveal melanoma. Eye (Lond) 2008;22:707-11. [Crossref] [PubMed]
- Cho SG, Wang Y, Rodriguez M, et al. Haploinsufficiency in the prometastasis Kiss1 receptor Gpr54 delays breast tumor initiation, progression, and lung metastasis. Cancer Res 2011;71:6535-46. [Crossref] [PubMed]
- Yin Y, Tang L, Shi L. The metastasis suppressor gene KISS-1 regulates osteosarcoma apoptosis and autophagy processes. Mol Med Rep 2017;15:1286-90. [Crossref] [PubMed]
- Ikeguchi M, Hirooka Y, Kaibara N. Quantitative reverse transcriptase polymerase chain reaction analysis for KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in hepatocellular carcinoma. J Cancer Res Clin Oncol 2003;129:531-5. [Crossref] [PubMed]
- Gao GL, Liu LD, Zou XS, et al. Expression of KiSS-1, matrix metalloproteinase-9, nuclear factor-kappaBp65 in ovarian tumour. Zhonghua Fu Chan Ke Za Zhi 2007;42:34-8. [PubMed]
- Chen SQ, Chen ZH, Lin SY, et al. KISS1 methylation and expression as predictors of disease progression in colorectal cancer patients. World J Gastroenterol 2014;20:10071-81. [Crossref] [PubMed]
- Kostakis ID, Agrogiannis G, Vaiopoulos AG, et al. KISS1 expression in colorectal cancer. APMIS 2013;121:1004-10. [Crossref] [PubMed]


