
Prognostic values of preoperative albumin to globulin ratio for predicting clinical outcome in patients with high-grade gliomas
Introduction
Gliomas were one of the most common primary central nervous system tumors which have an increased morbidity in recent years. And the clinical prognosis of high-grade gliomas (HGG, WHO Grade III to IV) was most with an average survival rate of only dozens of months, even if patients received standardized treatment including surgery, postoperative radiotherapy and chemotherapy (1). Therefore, forecasting for long-term prognosis of patients has a significant guiding for the treatment of patients.
Considerable researches have demonstrated that isocitrate dehydrogenase (IDH), phosphatase and tension homolog (PTEN), epidermal growth factor receptor (EGFR) were significant molecular markers for predicting the prognosis of patients with glioma (2,3). In recent years, many researchers found that the level of preoperative neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR) and albumin-to-globulin ratio (AGR) could predict the clinical outcome of patients with solid malignant tumors such as hepatoma (4), renal cell carcinoma (5,6), lung cancer (7), etc. AGR could reflect the nutritional and systemic inflammation status in patients with tumor (8). For the moment there were limited studies focusing on the clinical prognosis of AGR in glioma patients, and the relationship between AGR level and the survival of patients with HGG was not investigated by far. The present study explored the prognostic significance of preoperative AGR for patients with HGG. And the relationship between AGR level and IDH mutation in HGG was also investigated.
Methods
Two hundred and thirty-two cases of patients who were diagnosed HGG by pathology in The First Affiliated Hospital of Zhengzhou University from May 2011 to July 2015 were enrolled in the study. Within three months before glioma resection, all the patients did not receive additional surgery, radiotherapy and chemotherapy, and have no infection, hypertension, blood system diseases, autoimmune diseases and so on.
The relevant data of the cohort were collected through the medical record system, which included sex, age, preoperative Karnofsky performance score (KPS), extent of resection, hematology data (albumin count and globulin count within one week before surgery), postoperative molecular pathology such as IDH results, etc. The calculation of AGR was that AGR is the ratio of serum albumin and serum globulin. AGR = albumin/globulin.
The patients were followed-up regularly. Time from the operative date to the date of death was considered as overall survival (OS), and the interval from the operative date to the recurrence date was considered as progression-free-survival (PFS). This research was approved by the Ethics Committee of Zhengzhou University. All participants have written the informed consent.
The survival rate was calculated by using the Kaplan-Meier method. The cut-off score of AGR was determined by X-tile. Univariate survival analysis was performed by Log-rank method. Proportional hazards model (Cox model) was performed for multivariate analysis to determine independent prognostic factors. All statistical analyses were implemented to SPSS 25.0 software (IBM, USA) and X-tile software 3.6.1 (http://x-tile.software.informer.com). P<0.05 was considered as statistically significant.
Results
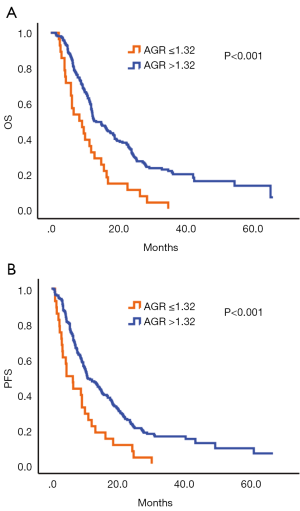
The analysis of central tendency of AGR showed that the minimum was 0.69, the maximum was 2.78, the mean was 1.64 and the Std. Deviation was 0.31. According to the X-tile software, the optimal cut-off score of AGR was 1.32. Then the AGR queue was divided into two groups: AGR ≤1.32 and AGR >1.32. Survival analysis showed that the 3-year survival rate of the two groups was 0.0% vs. 20.5%, respectively. And the difference between the two groups was statistically significant (P<0.001) (Figure 1A). Similarly, the median progression-free survival for the two groups was 0.0% vs. 15.6%, respectively and the difference was statistically significant, too (P<0.001) (Figure 1B).
Univariate analysis of general factors such as sex, age, KPS, extent of resection, radiotherapy, chemotherapy, tumor grade and IDH mutation revealed that degree of tumor resection (OS, P=0.002, PFS, P=0.006), radiotherapy (OS, P<0.001, PFS, P=0.001), chemotherapy (OS, P<0.001, PFS, P<0.001), tumor grade (OS, P<0.001, PFS, P<0.001), IDH mutation (OS, P<0.001, PFS, P<0.001) were the factors which influenced the clinical outcome of patients with HGG. However, sex, age and KPS could not be factors in the clinical prognosis of patients with HGG (P>0.05) (Table 1). The above factors with statistical significance was included in a multivariate analysis. And the results showed that degree of resection (OS, P<0.001, PFS, P=0.002), chemotherapy (OS, P=0.037, PFS, P=0.036), tumor grade (OS, P<0.001, PFS, P<0.001), preoperative AGR (OS, P=0.003, PFS, P=0.007) were independent factors that affected the clinical prognosis of patients with HGG (Table 2). Result of multivariate analysis in IDH wild-type HGG revealed that AGR (OS, P=0.005. PFS, P=0.012) was an independent risk factor that affected the clinical prognosis of patients (Table 3).
Table 1
| Factors | No. of cases | OS | PFS | |||||
|---|---|---|---|---|---|---|---|---|
| 3-year OS (%) | P value | 3-year PFS (%) | P value | |||||
| Sex | ||||||||
| Male | 130 | 19.8 | 0.952 | 13.1 | 0.733 | |||
| Female | 102 | 15.0 | 14.2 | |||||
| Age | ||||||||
| ≤50 | 86 | 29.3 | <0.001* | 19.8 | 0.003* | |||
| >50 | 146 | 10.7 | 9.7 | |||||
| KPS | ||||||||
| ≤80 | 58 | 21.2 | 0.627 | 14.1 | 0.396 | |||
| >80 | 174 | 16.2 | 13.2 | |||||
| Extent of resection | ||||||||
| Gross total | 176 | 17.9 | 0.002* | 13.3 | 0.006* | |||
| Subtotal | 56 | 18.2 | 14.1 | |||||
| RT | ||||||||
| Yes | 177 | 19.6 | <0.001* | 14.2 | 0.001* | |||
| No | 55 | 11.3 | 11.8 | |||||
| CHT | ||||||||
| Yes | 167 | 21.9 | <0.001* | 16.5 | <0.001* | |||
| No | 65 | 5.9 | 4.8 | |||||
| AGR | ||||||||
| ≤1.32 | 28 | 0.0 | <0.001* | 0.0 | <0.001* | |||
| >1.32 | 204 | 20.5 | 15.6 | |||||
| Grade | ||||||||
| III | 59 | 44.6 | <0.001* | 27.9 | <0.001* | |||
| IV | 173 | 7.8 | 8.0 | |||||
| IDH | ||||||||
| IDH wt | 205 | 13.0 | <0.001* | 9.5 | <0.001* | |||
| IDH mut | 27 | 51.3 | 41.3 | |||||
*, P<0.05 showed statistically significant. AGR, albumin-to-globulin ratio; KPS, Karnofsky performance score; RT, postoperative primary radiation therapy; CHT, postoperative primary chemotherapy; IDH, isocitrate dehydrogenase.
Table 2
| Factors | OS | PFS | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Complete resection (yes or no) | 1.965 (1.375–2.807) | <0.001* | 1.741 (1.229–2.466) | 0.002* | |
| Age (≤50 or >50) | 1.184 (0.855–1.641) | 0.310 | 1.167 (0.854–1.596) | 0.333 | |
| RT (yes or no) | 1.127 (0.745–1.705) | 0.571 | 1.095 (0.729–1.644) | 0.663 | |
| CHT (yes or no) | 1.533 (1.026–2.291) | 0.037* | 1.521 (1.027–2.251) | 0.036* | |
| Grade (III or IV) | 2.900 (1.899–4.429) | <0.001* | 2.152 (1.468–3.154) | <0.001* | |
| AGR (≤1.32 or >1.32) | 0.528 (0.347–0.803) | 0.003* | 0.561 (0.369–0.851) | 0.007* | |
*, P<0.05 showed statistically significant. OR, odds ratio; CI, confidence interval; RT, postoperative primary radiation therapy; CHT, postoperative primary chemotherapy; AGR, albumin to globulin ratio; OS, overall survival; PFS, progression free survival; HGG, high-grade glioma.
Table 3
| Factors | OS | PFS | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Complete resection (yes or no) | 2.023 (1.399–2.925) | <0.001* | 1.821 (1.271–2.610) | 0.001* | |
| Age (≤50 or >50) | 1.259 (0.889–1.784) | 0.195 | 1.213 (0.869–1.693) | 0.256 | |
| RT (yes or no) | 1.261 (0.820–1.939) | 0.290 | 1.293 (0.846–1.976) | 0.235 | |
| CHT (yes or no) | 1.436 (0.952–2.167) | 0.084 | 1.385 (0.923–2.077) | 0.116 | |
| Grade (III or IV) | 2.109 (1.313–3.387) | 0.002* | 1.524 (0.992–2.343) | 0.055 | |
| AGR (≤1.32 or >1.32) | 0.548 (0.359–0.838) | 0.005* | 0.582 (0.382–0.886) | 0.012* | |
*, P<0.05 showed statistically significant. OR, odds ratio; CI, confidence interval; RT, postoperative primary radiation therapy; CHT, postoperative primary chemotherapy; AGR, albumin to globulin ratio; IDH, isocitrate dehydrogenase; OS, overall survival; PFS, progression free survival; HGG, high-grade glioma.
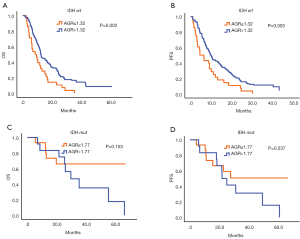
The cutoff values of AGR in IDH wild-type HGG and IDH mutation HGG were 1.32 and 1.77. Statistical analysis of mutation of IDH in sanger sequencing in patients showed that IDH mutation was noted in 27 cases of patients and IDH wild-type was found in 205 cases of patients. In IDH wild-type HGG, the high level of preoperative AGR revealed a considerable better clinical prognosis (3-year OS 0.0% vs. 15.6%, P=0.002) (Figure 2A), (3-year PFS 0.0% vs. 11.3%, P=0.003) (Figure 2B). There was no statistically significant between high AGR group and low AGR group of HGG patients with IDH mutation (OS, P=0.153, PFS, P=0.237) (Figure 2C,D).
Discussion
The immune and nutritional status of tumor patient was closely related to clinical prognosis (8). Numerous studies of solid tumor have shown that AGR could be a prognostic factor in cancer. Our study evaluated the clinical significance of preoperative AGR in 232 patients with HGG. Elevated preoperative AGR (AGR >1.32) indicated a better clinical prognosis and AGR was an independent prognostic factor for OS in patients with HGG. Moreover, in IDH wild-type HGG, higher preoperative AGR had a longer survival time and could further predict the clinical outcome.
Albumin was the most abundant serum protein and normal level of albumin maintained the normal physiological activity. Albumin was produced by the liver, as a biological marker for assessing liver function (9). We are aware that patients with cancer always experience malnutrition which leads to an increased postoperative mortality. And studies have found that malnutrition was involved in immuno-suppression (10,11). The level of albumin was not only related to nutritional status, but also one of the clinical prognostic indices of various malignancies. Study finding showed that low serum albumin level could be risk factors of severe postoperative complications in elderly gastric cancer patients (12). Hypoalbuminemia might ultimately be correlated with biochemical recurrence of prostate cancer (13) and was a predictor of poor surgical outcomes of colon cancer (14). Serum globulin mainly refers to the immune-related proteins which including immunoglobulin, complement, C-reactive protein (CRP), interleukin and so on. Studies have shown that these inflammatory proteins were involved in tumor prognosis. Elevated levels of complement 3 and IgA indicated poor prognosis of patients with lung cancer (15) and elevated levels of CRP was related to survival rate of patients with esophageal carcinoma (16).
Research showed that the level of immunoglobulin in serum was reacted with the inflammatory state of the organism. Almost all the primary tumors existed persistent chronic inflammation, and high levels of immunoglobulin played a major role in the progression and metastasis of cancer (17). A study in nasopharyngeal carcinoma showed that low AGR had a significant correlation to high neutrophil counts (18). Neutrophil could help tumors invade by producing angiogenesis factors (19) and the high NLR indicated a poor prognosis of multiple tumors (20,21). Preoperative AGR reflected the body's nutritional status and inflammatory response. AGR could reduce the influencing factor of body dehydration or water retention which might cause the count change of albumin or globulin and could better reflect the impact of patient’s outcome. A meta-analysis of pretreatment albumin to globulin ratio in cancers revealed that low pretreatment AGR is associated with poor prognosis in human cancers, and AGR should be used as a prognostic marker during cancer therapy (22). For patients with glioblastoma, AGR (>1.75) (23) and AGR (1.87±0.36) (24) have high predictive values for glioblastoma. In hepatoma carcinoma patients, low pretreatment AGR (<1.18) was significantly related to shorter OS (25). In patients with colorectal cancer, a low pretreatment serum AGR (<1.4) was an independent predictor of poor OS (18) and the AGR higher than the cut-off values ranging from 1.15–1.75 was related to better OS (26). Not only in cancer patients, low AGR is a risk factor for cancer incidence and mortality, both short- and long terms, in a generally healthy screened population (27). In our research, the cut-off values of AGR were 1.32. It’s similar to these results above-mentioned.
IDH, TERT and 1p/19q were important molecular pathological index in gliomas. They had important clinical significance to differentiate tumor types and predict prognosis and the prognosis of patients with IDH mutation was better (28-30). In our study, we analyzed the mutation of IDH and showed that the survival time of patients with high AGR was longer in the patients with IDH wild-type. It’s essential to direct the treatment of glioma patients. In the future, we will require more basic medical experimentation to further elucidate the molecular mechanism.
Compared with previous study of AGR in glioblastoma (23), our sample number of this study was larger and covered the HGG (WHO Grade III to IV). So, the results were more representative. In this study, we confirmed the preoperative AGR was correlated with clinical prognosis of patients with HGG, the survival time of patients with high AGR was longer. And the prognosis of patients with high AGR was better in IDH wild type. Therefore, the inexpensive preoperative AGR might predict the clinical prognosis of patients with HGG and then aid the decision-making process in clinical treatment.
Admittedly, some limitations existed in the present study. Our research was a retrospective study. The accuracy of the results might be influenced by systematic bias. Patients of our research only proceed in one medical center.
Conclusions
In conclusion, high preoperative AGR indicated a better clinical prognosis and AGR was an independent prognostic factor for OS in patients with HGG. Moreover, in IDH wild-type HGG, higher preoperative AGR had a longer survival time and could further predict the clinical outcome.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.08.16). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research was approved by the Ethics Committee of Zhengzhou University. All participants have written the informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Labussière M, Boisselier B, Mokhtari K, et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology 2014;83:1200-6. [Crossref] [PubMed]
- Idoate MA, Echeveste J, Diez-Valle R, et al. Biological and clinical significance of the intratumour heterogeneity of PTEN protein expression and the corresponding molecular abnormalities of the PTEN gene in glioblastomas. Neuropathol Appl Neurobiol 2014;40:736-46. [Crossref] [PubMed]
- Peng W, Li C, Wen TF, et al. Neutrophil to lymphocyte ratio changes predict small hepatocellular carcinoma survival. J Surg Res 2014;192:402-8. [Crossref] [PubMed]
- Hutterer GC, Stoeckigt C, Stojakovic T, et al. Low preoperative lymphocyte-monocyte ratio (LMR) represents a potentially poor prognostic factor in nonmetastatic clear cell renal cell carcinoma. Urol Oncol 2014;32:1041-8. [Crossref] [PubMed]
- Hu H, Yao X, Xie X. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol 2017;35:261-70. [Crossref] [PubMed]
- Zhou T, He X, Fang W, et al. Pretreatment albumin/globulin ratio predicts the prognosis for small-cell lung cancer. Medicine (Baltimore) 2016;95:e3097. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [Crossref] [PubMed]
- Cholongitas E, Papatheodoridis GV, Vangeli M, et al. Systematic review: The model for end-stage liver disease-should it replace Child-Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther 2005;22:1079-89. [Crossref] [PubMed]
- Kanda M, Mizuno A, Tanaka C, et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Medicine (Baltimore) 2016;95:e3781. [Crossref] [PubMed]
- Mainous MR, Deitch EA. Nutrition and infection. Surg Clin North Am 1994;74:659-76. [Crossref] [PubMed]
- Kang SC, Kim HI, Kim MG. Low Serum Albumin Level, Male Sex, and Total Gastrectomy Are Risk Factors of Severe Postoperative Complications in Elderly Gastric Cancer Patients. J Gastric Cancer 2016;16:43-50. [Crossref] [PubMed]
- Sejima T, Iwamoto H, Masago T, et al. Low pre-operative levels of serum albumin predict lymph node metastases and ultimately correlate with a biochemical recurrence of prostate cancer in radical prostatectomy patients. Cent European J Urol 2013;66:126-32. [PubMed]
- Lai CC, You JF, Yeh CY, et al. Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int J Colorectal Dis 2011;26:473-81. [Crossref] [PubMed]
- Oner F, Savas I, Numanoglu N. Immunoglobulins and complement components in patients with lung cancer. Tuberk Toraks 2004;52:19-23. [PubMed]
- Shimada H, Nabeya Y, Okazumi S, et al. Elevation of preoperative serum C-reactive protein level is related to poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol 2003;83:248-52. [Crossref] [PubMed]
- Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009;30:1073-81. [Crossref] [PubMed]
- Du XJ, Tang LL, Mao YP, et al. The Pretreatment Albumin to Globulin Ratio Has Predictive Value for Long-Term Mortality in Nasopharyngeal Carcinoma. PLoS One 2014;9:e94473. [Crossref] [PubMed]
- Di Carlo E, Forni G, Lollini P, et al. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood 2001;97:339-45. [Crossref] [PubMed]
- Wang XY, Li K. Neutrophil-to-lymphocyte ratio predicts the survival a patient with post-operative recurrence of non-small cell lung cancer. Chin J Oncol 2014;37:298-302.
- Bambury RM, Teo MY, Power DG, et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol 2013;114:149-54. [Crossref] [PubMed]
- Lv GY, An L, Sun XD, et al. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: a meta-analysis. Clin Chim Acta 2018;476:81-91. [Crossref] [PubMed]
- Xu WZ, Li F, Xu ZK, et al. Preoperative albumin-to-globulin ratio and prognostic nutrition index predict prognosis for glioblastoma. Onco Targets Ther 2017;10:725-33. [Crossref] [PubMed]
- Wang PF, Meng Z, Song HW, et al. Preoperative Changes in Hematological Markers and Predictors of Glioma Grade and Survival. Front Pharmacol 2018;9:886. [Crossref] [PubMed]
- Zhang J, Liu X, Yang Z, et al. The pretreatment albumin to globulin ratio, a validated biomarker, predicts prognosis in hepatocellular carcinoma. J BUON 2016;21:925-34. [PubMed]
- He J, Pan H, Liang W, et al. Prognostic Effect of Albumin-to-Globulin Ratio in Patients with solid tumors: A Systematic Review and Meta-analysis. J Cancer 2017;8:4002-10. [Crossref] [PubMed]
- Suh B, Park S, Shin DW, et al. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann Oncol 2014;25:2260-6. [Crossref] [PubMed]
- Zhang ZY, Chan AK, Ng HK, et al. Surgically treated incidentally discovered low-grade gliomas are mostly IDH mutated and 1p19q co-deleted with favorable prognosis. Int J Clin Exp Pathol 2014;7:8627-36. [PubMed]
- Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med 2015;372:2499-508. [Crossref] [PubMed]
- Zhang ZY, Chan AK, Ding XJ, et al. TERT promoter mutations contribute to IDH mutations in predicting differential responses to adjuvant therapies in WHO grade II and III diffuse gliomas. Oncotarget 2015;6:24871-83. [PubMed]


