
PRDX6 promotes proliferation and induces chemo-resistance via peroxidase activity in Toledo diffuse large B-cell lymphoma cells
Introduction
Diffuse large B cell lymphoma (DLBCL) is the most common type of B cell non-Hodgkin’s lymphoma (NHL), and accounts for 30–40% of NHL cases (1). The combined chemotherapy of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) has increased DLBCL survival substantially, with complete remission (CR) in approximately 80% patients (2). Although tremendous progress has been made in the outcomes of DLBCL, 30–40% of patients are refractory to treatment or relapse after initial response to therapy (3). Thus, investigating the molecular mechanisms underlying DLBCL chemo-resistance is critical for improving the treatment response.
Peroxiredoxins (PRDX) family contains six members of peroxidase (PRDX1-PRDX6). PRDX6 is a unique member of PRDXs family which displays both glutathione peroxidase and phospholipase A2 activities (4). The glutathione peroxidase activity of PRDX6 is crucial for reducing cellular H2O2 levels and decreasing oxidative stress-induced lipid peroxidation, membrane damage, and apoptosis (5-7). The phospholipase A2 activity of PRDX6 catalyzes the hydrolysis of the sn-2 fatty acyl ester bond of glycerophospholipids to produce free fatty acids and lysophospholipids, and is critical in phospholipid metabolism and cell signaling (8). Recent evidence suggests that PRDX6 plays important roles in multiple cancers. For example, PRDX6 is upregulated in cancerous hepatoma cell line compared with noncancerous counterpart. The anti-oxidant activity of PRDX6 suppresses peroxide-induced cytotoxicity in hepatoma cell (9). Additionally, the phospholipase A2 activity of PRDX6 is important for PRDX6 promoted proliferation and induction of Src family kinase activation in melanoma cells (10). Moreover, PRDX6 also promotes invasion and metastasis in lung cancer cells via its phospholipase A2 activity (11). However, little is known about the role of PRDX6 in DLBCL, and the underlying molecular mechanism is largely unknown.
In current study, we evaluate the functions of PRDX6 in DLBCL, and demonstrate the potential molecular mechanism behind it. We found that overexpression of PRDX6 significantly promoted proliferation of Toledo DLBCL cells, while downregulation of PRDX6 suppressed the proliferation. Additionally, downregulating the expression of PRDX6 induced apoptosis in Toledo DLBCL cells. Importantly, our data showed that upregulation of PRDX6 alleviated doxorubicin induced apoptosis, while downregulation of PRDX6 produced a synergistic effect on apoptosis when Toledo DLBCL cells treated with doxorubicin. Mechanically, PRDX6 displayed both glutathione peroxidase and phospholipase A2 activities in Toledo DLBCL cells. Interestingly, inhibition of glutathione peroxidase activity of PRDX6 by M-succinate (mercaptosuccinate) treatment reversed PRDX6 promoted proliferation and anti-apoptosis effects, while inhibition of phospholipase A2 activity did not. Our data indicated that PRDX6 was a critical molecule to induce doxorubicin resistance, targeting the glutathione peroxidase activity of PRDX6 could be a promising strategy to overcome doxorubicin resistance.
Methods
Reagents and cells culture
Doxorubicin and Annexin V-FITC Apoptosis Detection Kit were purchased from Sigma-Aldrich. Flag, PRDX6, and GAPDH were purchased from Cell Signaling Technology. Toledo was obtained from the American Type Culture Collection (ATCC) and was cultured in RPMI-1640 (Gibco) supplemented with 10% fetal bovine serum (FBS; Hyclone). Cells were maintained at 37 °C in a humidified 5% CO2 atmosphere.
shPRDX6 stable cell lines established
Knockdown of gene was performed with the specific shRNA delivered by a lentiviral system purchased from Sigma-Aldrich Corp, according to the instruction manual. 293T cells were co-transfected with pMD2.G and psPAX2 compatible packaging plasmids and pLKO.1 plasmid bearing the specific shRNA for 24 h. The cultured medium containing lentivirus was collected. Then Toledo cells were infected with the lentivirus bearing the shRNA in growth medium containing 8 µg/mL polybrene for 24 h. Afterwards, cells were subcultured and selected with 2 µg/mL puromycin. The shRNA constructs targeting the gene and referring to the sequence is: PRDX6 (NM_004905.2): TRCN0000052154 (5'-CCGGCCGAAAGGAGTCTTCACCAAATCGAGTTTGGTGAAGACTCCTTTCGGTTTTTG-3').
PRDX6-flag overexpressed stable cell lines established
The plasmids encoding PRDX6 was generated by PCR amplification and subcloned into the pLVX-DsRed-Monomer-N1 expression vector. The primers for gene PRDX6-flag cloning were as follow: 5'-AACTCGAGATGCCCGGAGGTCTGCTTCTC-3'; 5'-AAGAATTCTTACTTATCGTCGTCATCCTTGTAATCAGGCTGGGGTGTGTAGCGGAG-3'. 5×106 293T cells were transfected with lentiviral vector, psPAX2 and pMD2.G. Supernatants were collected every 24 h between 24 to 72 h after transfection, pulled together and concentrated via ultracentrifugation, and the viral titer was determined by serial dilutions. The multiplicity of infection during transfection was 10. 2×105 cell were treated with polybrene (8 µg/mL) and virus contained supernatants for 24 h. Cells were growth for another 48h. Cells were treated with puromycin (2 µg/mL) for 72 h to establish stable cells.
Small interfering RNA transfection
Toledo cells were seeded in six-well plates. In each well, 100 nM of siRNA and 5 µL of Lipofectamine 2000 were added to Opti-MEM, mixed and then added to the cells. After transfection of siRNAs for 24 or 48 h, RNAi efficiency was determined by western blot. The siRNA was purchased from GenePharma company and the sequence was as follow: si-PRDX6 5'-CCGAAAGGAGTCTTCACCAAA-3'.
Cell counting assay
About 1×105 cells per well were plated in six-well plates. Subsequently, cells were treated. Then, cell count was determined with trypan blue exclusion assay.
Annexin V/PI analysis
Cells treated with indicated concentration of indicated drug for indicated time. Cells were collected and resuspended in the binding buffer (500 µL/sample). Annexin-V-FITC (5 µL/sample) was added to the cells followed by addition of 5 µL/sample PI (propidium iodide). The samples were then incubated for 15 min in the dark at 4 °C and subjected to flow cytometry analysis (12).
Western blot analysis
Cells were harvested and lysed in RIPA buffer. The protein concentration was determined by Bradford method with BSA (Sigma) as the standard. Equal amounts of cell extract were subjected to electrophoresis in SDS-polyacrylamide gel and transferred to nitrocellulose membrane (Millipore). The membranes were blocked and then incubated with flag, PRDX6 and GAPDH (Cell Signaling Technology Corp, Beverly, MA, USA) antibodies at 4 °C overnight, followed by incubation for 1 h room temperature with appropriate secondary antibodies. Antibody binding was detected with an enhanced chemiluminescence kit (Pierce).
Glutathione peroxidase activity and iPLA2 activity assay
Glutathione peroxidase assay (No. 703102) and cPLA2 assay kits (No. 765021) were purchased from Cayman Chemical and iPLA2 and glutathione peroxidase activities were measured according to the manufacturer’s recommendations (13).
Statistics
Statistical analysis was performed using SPSS version 16.0 (SPSS Inc.) and GraphPad Prism 6 (GraphPad Software, Inc.). The Student’s t test and multi-factorial ANOVA were used to make a statistical comparison. The level of significance was set at P<0.05.
Results
PRDX6 is critical for cell proliferation in Toledo DLBCL cells
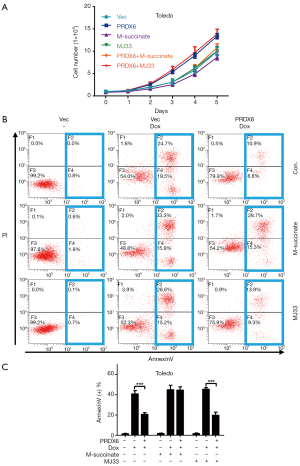
To study the functions of PRDX6 in DLBCL, we evaluated the proliferation of Toledo DLBCL cells at first. We established a stable PRDX6-flag overexpressed cell line (PRDX6 cells) and the control cell line (Vec cells, vector cell) in Toledo DLBCL cells by lentivirus mediated gene transfer. Western blot analysis showed that PRDX6 cells indeed overexpressed PRDX6-flag protein (Figure 1A). We then evaluated proliferation by cell counting assay. As shown in Figure 1B, PRDX6 cells displayed a significant advantage in cell proliferation as compared with Vec cells. To further confirm the effect on proliferation, we established another stable cell line expressed PRDX6 shRNA (shPRDX6 cells) and the control cell line (NC cells, negative control cells) in Toledo DLBCL cells by lentivirus mediated gene transfer. The expression of PRDX6 was down regulated up to approximately 70% in shPRDX6 cells as compared with NC cells (Figure 1B). The proliferation of Toledo DLBCL cells was significantly suppressed by downregulation of PRDX6 (Figure 1B). These data indicated that PRDX6 has an important role in regulating the proliferation of Toledo DLBCL cells.
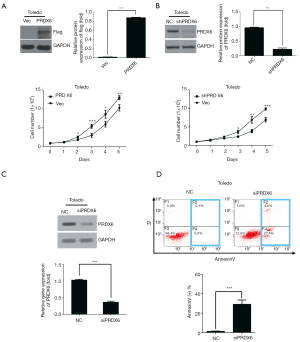
Downregulation of PRDX6 induces apoptosis in Toledo DLBCL cells
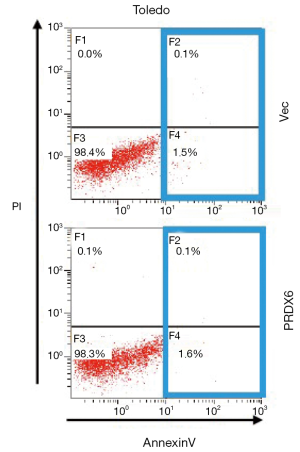
We next evaluated the effect of PRDX6 on apoptosis. As shown in Figure S1, upregulation of PRDX6 did not induce apoptosis in Toledo DLBCL cells. However, downregulating the expression of PRDX6 by siRNA induced a substantial apoptosis in Toledo DLBCL cells (Figure 1C,D). The population of apoptotic cell were 28.97%±3.70% (P<0.001) in siPRDX6 cells and 1.13%±0.33% in NC cells.
PRDX6 induces chemo-resistance in Toledo DLBCL cells
Chemo-resistance is the main obstacle faced in DLBCL treatment. We further detected whether PRDX6 might affect the sensitivity of DLBCL cells to doxorubicin, an important component in R-CHOP regimen. As shown in Figure 2, 0.2 µM doxorubicin treatment (Dox) significantly induced apoptosis in Toledo DLBCL cells (comparing the first and third panel of Figure 2A up, comparing the first and third column of Figure 2B left). The apoptosis populations in control group and Dox group were 2.07%±0.41% and 49.90%±2.74% (P<0.001) respectively. However, overexpression of PRDX6 markedly reduced doxorubicin induced apoptosis (comparing the third and fourth panel of Figure 2A up, comparing the third and fourth column of Figure 2B left). The apoptosis populations in Dox group and Dox+ group were 49.90%±2.74% and 16.07%±1.83% (P<0.001) respectively.
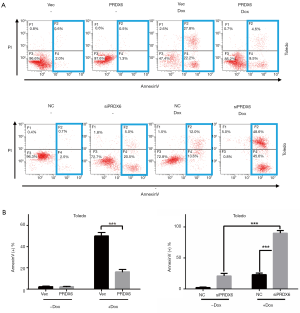
Downregulation of PRDX6 enhances Dox induced apoptosis in Toledo DLBCL cells
We next examined the effect of PRDX6 downregulation on Dox induced apoptosis. As shown in Figure 2A down and 2B right, we treated Toledo with 0.05 µM doxorubicin to induce a moderate level of apoptosis. Low concentration of doxorubicin treatment induced 23.17%±2.00% (P<0.001) level of apoptosis as comparing with 2.36%±0.63% in control group (comparing the first and third panel of Figure 2A down, comparing the first and third column of Figure 2B right). Downregulation of PRDX6 expression by siRNA also induced a moderate level of apoptosis (23.17%±2.00%). Interestingly, downregulation of PRDX6 expression substantially increased doxorubicin induced apoptosis (Figure 2A down and 2B right). The level of apoptosis in the combined group was 90.00%±3.47% which was significantly higher than the apoptosis of Dox group plus siPRDX6 group (Figure 2A down and Figure 2B right). These data suggested that siPRDX6 might induce a synergetic apoptosis effect when combining with doxorubicin treatment.
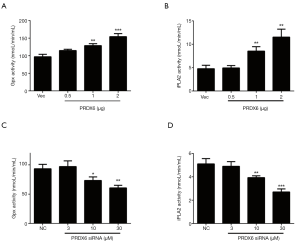
Peroxidase activity is responsible for PRDX6 induced cell growth and anti-apoptotic effects in Toledo DLBCL cells
Since PRDX6 displays both glutathione peroxidase and phospholipase A2 (iPLA2) activities (4). We further examined which activity was responsible for PRDX6 induced proliferation and anti-apoptosis. We upregulated or downregulated PRDX6 expression in a dose-dependent manner by transient transfection. As shown in Figure 3A,B, the glutathione peroxidase and iPLA2 activities were gradually increased as the PRDX6 expression increasing. Conversely, the glutathione peroxidase and iPLA2 activities were gradually suppressed as the PRDX6 expression decreasing (Figure 3C,D). We then inhibited the glutathione peroxidase or iPLA2 activities by M-succinate (mercaptosuccinate) or MJ33 respectively (4,14). As shown in Figure 4A, M-succinate effectively reversed PRDX6 induced proliferation, but MJ33 did not. As shown in Figure 4B,C, PRDX6 alleviated Dox induced apoptosis. However, M-succinate, but not MJ33, overcame PRDX6 induced Dox resistance (Figure 4B,C). These data indicated that the glutathione peroxidase activity was critical for PRDX6 induced proliferation and Dox resistance.
Discussion
In the current study, we explored the function of PRDX6 in DLBCL cells. Our data showed PRDX6 is critical for the proliferation of Toledo DLBCL cells. Additionally, overexpression of PRDX6 conferred Dox-resistance to Toledo DLBCL cells. Moreover, combination of Dox treatment and siPRDX6 induced a synergetic effect on apoptosis. Mechanically, we showed that inhibition of glutathione peroxidase activity of PRDX6 by M-succinate, but not inhibition of phospholipase A2 activity of PRDX6 by MJ33, overcame PDRX6 induced proliferation and anti-apoptosis effects.
DLBCL is a kind of aggressive lymphoma which composes of large, transformed B cells, and displays in diffuse growth pattern (15). The addition of rituximab to CHOP regimen generated a significant improvement in DLBCL treatment in two decades. Although the prognosis of DLBCL has been improved significantly, resistance to the CHOP regimen continues to pose a problem in managing or curing DLBCL (16). Thus, elucidating the mechanism of chemo-resistance and identifying new therapeutic target are urgently needed to improve the quality of patient care and effectiveness of CHOP therapies. In this study, we focused on doxorubicin resistance in DLBCL, which is a main component of CHOP regimen. We showed that PRDX6 expression was critical for inducing doxorubicin resistance. Targeting PRDX6 not only overcame doxorubicin resistance, but also produced a synergetic effect on apoptosis in Toledo DLBCL cells.
PRDX6 is a member of the family of non-selenium thiol peroxidases. Recent studies have shown that PRDX6 are up-regulated in various cancers, including breast cancer (17), lung cancer (18) or tongue squamous cell carcinoma (19). However, there is no study about the function of PRDX6 in DLBCL. We studied the effects of PRDX6 on cell proliferation, apoptosis and drug resistance in DLBCL for the first time. Our data showed that the expression of PRDX6 was important for the cell proliferation and apoptosis in Toledo DLBCL cells. More importantly, PRDX6 was a crucial molecule mediated doxorubicin resistance. Targeting PRDX6 by siRNA produced synergetic effect on apoptosis when combined with doxorubicin treatment. These data suggested a promising therapeutic potential of PRDX6 in clinical treatment of DLBCL.
PRDX6 is a unique bifunctional enzyme in PRDX family that contains, in addition to its peroxidase activity, iPLA2 activity (4). Previous studies indicated that the peroxidase activity and iPLA2 activity might have different function. For example, the peroxidase activity of PRDX6 was important for cell growth. This effect could be blocked by M-succinate, which was a peroxidase activity inhibitor, but not MJ33, which was a iPLA2 activity inhibitor (11). Conversely, the invasion ability was mainly supported by iPLA2 activity (11). Consistently, our study supported that PRDX6 promoted Toledo DLBCL cells proliferation via peroxidase activity. Importantly, we showed that the anti-apoptosis function of PRDX6 was also supported by peroxidase activity, but not iPLA2 activity. These data indicated that inhibition of PRDX6 peroxidase activity might also an effective strategy to overcome PRDX6 induced drug resistance in DLBCL.
Together, our study explored the tumor promoting function of PRDX6 in DLBCL. Our data implied that PRDX6 was a crucial factor to induce drug resistance. Targeting PRDX6 expression or peroxidase activity could be an effective strategy to overcome drug resistance in clinical DLBCL treatment.
Acknowledgments
Funding: This work was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.08.36). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The institutional ethical approval and individual informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Nakajima Y, Tomita N, Itabashi M, et al. Analysis of outcomes in patients with supra-diaphragmatic vs infra-diaphragmatic diffuse large B cell lymphoma treated with R-CHOP therapy. Leuk Res 2015;39:198-203. [Crossref] [PubMed]
- Ziepert M, Hasenclever D, Kuhnt E, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol 2010;28:2373-80. [Crossref] [PubMed]
- Chen JW, Dodia C, Feinstein SI, et al. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J Biol Chem 2000;275:28421-7. [Crossref] [PubMed]
- Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med 2005;38:1422-32. [Crossref] [PubMed]
- Manevich Y, Sweitzer T, Pak JH, et al. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci U S A 2002;99:11599-604. [Crossref] [PubMed]
- Wang Y, Phelan SA, Manevich Y, et al. Transgenic mice overexpressing peroxiredoxin 6 show increased resistance to lung injury in hyperoxia. Am J Respir Cell Mol Biol 2006;34:481-6. [Crossref] [PubMed]
- Hooks SB, Cummings BS. Role of Ca2+-independent phospholipase A2 in cell growth and signaling. Biochem Pharmacol 2008;76:1059-67. [Crossref] [PubMed]
- Walsh B, Pearl A, Suchy S, et al. Overexpression of Prdx6 and resistance to peroxide-induced death in Hepa1-6 cells: Prdx suppression increases apoptosis. Redox Rep 2009;14:275-84. [Crossref] [PubMed]
- Schmitt A, Schmitz W, Hufnagel A, et al. Peroxiredoxin 6 triggers melanoma cell growth by increasing arachidonic acid-dependent lipid signalling. Biochem J 2015;471:267-79. [Crossref] [PubMed]
- Ho JN, Lee SB, Lee SS, et al. Phospholipase A2 activity of peroxiredoxin 6 promotes invasion and metastasis of lung cancer cells. Mol Cancer Ther. 2010;9:825-32. [Crossref] [PubMed]
- Machado-Neto JA, Coelho-Silva JL, Santos FPS, et al. Autophagy inhibition potentiates ruxolitinib-induced apoptosis in JAK2V617F cells. Invest New Drugs 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Yun HM, Park KR, Lee HP, et al. PRDX6 promotes lung tumor progression via its GPx and iPLA2 activities. Free Radic Biol Med 2014;69:367-76. [Crossref] [PubMed]
- Kim TS, Sundaresh CS, Feinstein SI, et al. Identification of a human cDNA clone for lysosomal type Ca2+-independent phospholipase A2 and properties of the expressed protein. J Biol Chem 1997;272:2542-50. [Crossref] [PubMed]
- Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 2018;24:679-90. [Crossref] [PubMed]
- Lazzarino M, Orlandi E, Paulli M, et al. Treatment outcome and prognostic factors for primary mediastinal (thymic) B-cell lymphoma: a multicenter study of 106 patients. J Clin Oncol 1997;15:1646-53. [Crossref] [PubMed]
- Liu FJ, Wang XB, Cao AG. Screening and functional analysis of a differential protein profile of human breast cancer. Oncol Lett 2014;7:1851-6. [Crossref] [PubMed]
- Rostila A, Puustinen A, Toljamo T, et al. Peroxiredoxins and tropomyosins as plasma biomarkers for lung cancer and asbestos exposure. Lung Cancer 2012;77:450-9. [Crossref] [PubMed]
- Yanagawa T, Omura K, Harada H, et al. Peroxiredoxin I expression in tongue squamous cell carcinomas as involved in tumor recurrence. Int J Oral Maxillofac Surg 2005;34:915-20. [Crossref] [PubMed]


