Intracystic papillary breast carcinoma with DCIS in a man: a case report
Introduction
Male breast cancer accounts less than 1% of breast cancer diagnosed (1-3). It is mostly associated to BRCA2 mutation, but can be sporadic amongst men affected by gynecomastia or sexual hormones disequilibrium (4). Results from the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program showed histologic grade was not significantly correlated with clinical outcome, unlike what is seen in female patients (5). In that case series male breast cancer patients have had worse survival outcomes compared with those of female patients. An analysis of the Surveillance, Epidemiology and End Results (SEER) data from 2005 to 2010 found 5-year survival rate for male patients was lower than that for female patients (82.8% vs. 88.5%) (6). Specifically intracystic papillary tumours are less than 5% of all men breast cancers (7). Diagnosis is sometimes delayed because ultrasound scan and fine needle cytology may report cystic disease and may not report the papillary component. Surgical excision of the lump with margins in excess of 2 mm is considered satisfactory (7). Sentinel lymph node biopsy (SLNB) is recommended as data have shown the risk of finding an invasive cancer in the final histology. Radiotherapy following intracystic papillary carcinoma alone is of uncertain significance as this form of cancer is usually low grade and rarely recurs (7).
Case presentation
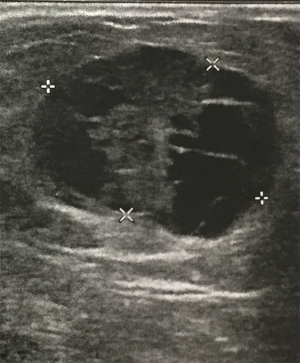
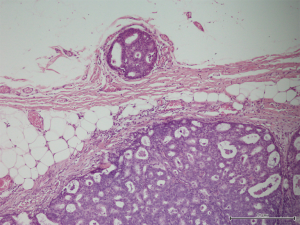
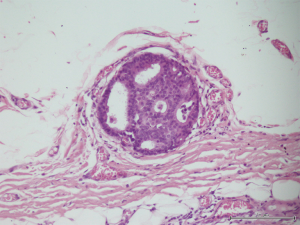
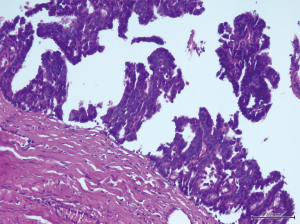
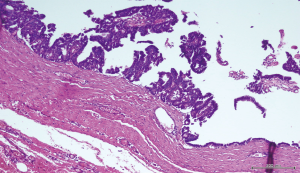
A 56-year-old man with bilateral gynecomastia and without familial history of breast cancer presented with a 50-mm lump in the central quadrant of his left breast. The lump was not painful, and was not associated to nipple discharge. Mammogram showed 50-mm round mass in the left breast behind the nipple. Breast ultrasound scan showed an anechoic mass containing vascularised hyperechoic 2.5 cm lesion (Figure 1). Core biopsy showed papillary neoplasia. The patient underwent left wide local excision and radio-guided sentinel lymph node biopsy. Pathology report showed low-grade intracystic papillary carcinoma (Figures 2,3), surrounded by several foci of ductal carcinoma in situ (DCIS) involving excision margins as shown in Figure 4 and Figure 5. Sentinel nodes were 3 and were negative for metastases. Patient was rescheduled for left total mastectomy. The staging at final pathology report was pT2N0M0. Oestrogen and progesterone receptors were 90%; Ki67% was 13%; HER2/neu oncogene was not amplified. The case was discussed at multidisciplinary meeting and the patient was prescribed hormonal therapy with tamoxifen for 5 years. Radiotherapy was not prescribed.
Discussion
Papillary lesions of the breast are exclusively intraductal neoplasms, although rarely an invasive carcinoma of the breast may have a predominantly papillary architecture. Papillary lesions comprise intraductal papilloma, papilloma with atypical ductal hyperplasia (ADH), papilloma with DCIS, intracystic (encapsulated) papillary carcinoma and intraductal papillary carcinoma (8). Although the diagnosis of papillary lesion is typically not difficult, the distinction among those entities is not always straightforward. Nipple discharge or a palpable mass may be clinically evident, depending on the location and size of the lesion (8). Intracystic papillary carcinoma is morphologically similar to intraductal papillary carcinoma with the exception that the myoepithelial cells are absent at the surrounding thick fibrous capsule. These lesions have been traditionally regarded as a variant of DCIS by most authors given their discrete nodular growth, lack of stromal reaction, and indolent clinical behaviour (8). These lesions often present with an expansile growth pattern and occasional lymph node and distant metastasis have been reported. Intracystic papillary carcinoma has a favourable prognosis with adequate local therapy alone in the absence of DCIS or invasive carcinoma in the surrounding breast tissue (9-12). The presence of concurrent DCIS in the adjacent breast tissue is associated with a higher risk of local recurrence. Based on the overall molecular changes, it is suggested that intracystic papillary carcinoma is closer to DCIS than to invasive carcinoma (8,13). The incidence of nodal metastases is extremely low, thus axillary dissection is not justified. According to Wang et al., the incidence of nodal metastasis was 2.7% amongst a group of 99 women (14). Therefore, an alternative might be sentinel node biopsy (14-16), which is considered prudent among this population (17). Our patient was not scheduled for radiation therapy because the addition of radiation to the treatment of patients has not shown to impact the incidence of local recurrence or likelihood of death compared to those who did not receive radiation (16,17). Grabowski et al. investigated on the long-term prognosis of patients diagnosed with intracystic papillary disease with an analysis of over 900 cases (17). At 10 years, the cumulative survival rate of intracystic papillary disease with in situ component was 96.8% (P=0.75) (17) according to the Californian Cancer Registry (CCR). This database includes information about histology, patient demographics, disease stage at diagnosis and survival, but does not specify adjuvant therapies. Solorzano et al., showed disease-specific survival rate of 100% among forty patients. Only one third of this population had received radiotherapy. Although the indication to endocrine therapy remains unclear and controversial, Fayanju et al. concluded that patients with intracystic papillary carcinoma and DCIS or microinvasion had significantly increased use of endocrine therapy versus patients with pure intracystic papillary carcinoma (P<0.01) (18).
Conclusions
Intracystic papillary breast carcinoma is a rare entity amongst male patients, occurring in less than 5% of patients. Although its rarity, intracystic papillary breast carcinoma is considered a curable disease because of its biology. The optimal treatment remains surgery with clear margins and sentinel node biopsy. If surrounded by DCIS foci, mastectomy is recommended rather than wide local excision. Radiotherapy and chemotherapy are not recommended, whereas hormonal therapy is generally prescribed when hormonal receptors are expressed. Patients with papillary intracystic disease are deemed at excellent prognosis.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office for the focused issue “Rare Tumors of the Breast” published in Translational Cancer Research. This article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.09.40). The focused issue “Rare Tumors of the Breast” was commissioned by the editorial office without any funding or sponsorship. EE served as the unpaid Guest Editor for the focused issue and serves as the unpaid editorial board member of Translational Cancer Research from May 2018 to Apr 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Male Breast Cancer Treatment (PDQ®)–Health Professional Version. Available online: https://www.cancer.gov/types/breast/hp/male-breast-treatment-pdq
- Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary Meeting on Male Breast Cancer: Summary and Research Recommendations. J Clin Oncol 2010;28:2114-22. [Crossref] [PubMed]
- Contractor KB, Kaur K, Rodrigues GS, et al. Male breast cancer: is the scenario changing. World J Surg Oncol 2008;6:58. [Crossref] [PubMed]
- Silvestri V, Barrowdale D, Mulligan AM, et al. Male breast cancer in BRCA1 and BRCA2 mutation carriers: pathology data from the Consortium of Investigators of Modifiers of BRCA1/2. Breast Cancer Res 2016;18:15. [Crossref] [PubMed]
- Vermeulen MA, Slaets L, Cardoso F, et al. Pathological characterisation of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Eur J Cancer 2017;82:219-27. [Crossref] [PubMed]
- Liu N, Johnson KJ, Ma CX. Male Breast Cancer: An Updated Surveillance, Epidemiology, and End Results Data Analysis. Clin Breast Cancer 2018;18:e997-1002. [Crossref] [PubMed]
- Reefy SA, Kameshki R, Sada DA, et al. Intracystic papillary breast cancer: a clinical update. Ecancermedicalscience 2013;7:286. [PubMed]
- Wei S. Papillary Lesions of the Breast: An Update. Arch Pathol Lab Med 2016;140:628-43. [Crossref] [PubMed]
- Lefkowitz M, Lefkowitz W, Wargotz ES. Intraductal (intracystic) papillary carcinoma of the breast and its variants: a clinicopathological study of 77 cases. Hum Pathol 1994;25:802-9. [Crossref] [PubMed]
- Calderaro J, Espie M, Duclos J, et al. Breast intracystic papillary carcinoma: an update. Breast J 2009;15:639-44. [Crossref] [PubMed]
- Harris KP, Faliakou EC, Exon DJ, et al. Treatment and outcome of intracystic papillary carcinoma of the breast. Br J Surg 1999;86:1274. [Crossref] [PubMed]
- Leal C, Costa I, Fonseca D, et al. Intracystic (encysted) papillary carcinoma of the breast: a clinical, pathological, and immunohistochemical study. Hum Pathol 1998;29:1097-104. [Crossref] [PubMed]
- Khoury T, Hu Q, Liu S, et al. Intracystic papillary carcinoma of breast: interrelationship with in situ and invasive carcinoma and a proposal of pathogenesis: array comparative genomic hybridization study of 14 cases. Mod Pathol 2014;27:194-203. [Crossref] [PubMed]
- Wang Y, Lu S, Graves T, et al. Can Sentinel Lymph Node Biopsy Be Spared in Papillary Carcinoma of the Breast? Clin Breast Cancer 2017;17:127-33. [Crossref] [PubMed]
- Hu ZI, Liu C, Fisher PR, et al. Intracystic Papillary Carcinoma of the Breast in a Male Patient. Rare Tumors 2016;8:6050. [Crossref] [PubMed]
- Solorzano CC, Middleton LP, Hunt KK, et al. Treatment and outcome of patients with intracystic papillary carcinoma of the breast. Am J Surg 2002;184:364-8. [Crossref] [PubMed]
- Grabowski J, Salzstein SL, Sadler GR, et al. Intracystic papillary carcinoma: a review of 917 cases. Cancer 2008;113:916-20. [Crossref] [PubMed]
- Fayanju OM, Ritter J, Gillanders WE, et al. Therapeutic management of intracystic papillary carcinoma of the breast: the roles of radiation and endocrine therapy. Am J Surg 2007;194:497-500. [Crossref] [PubMed]