
Nucleolar spindle associated protein 1 (NUSAP1) facilitates proliferation of hepatocellular carcinoma cells
Introduction
Liver cancer is evaluated to be the sixth most commonly diagnosed cancer and the fourth leading reason of cancer related death worldwide in 2018, with approximate 841,000 new cases and 782,000 deaths yearly. Among liver cancer, 78–85% of cases are hepatocellular carcinoma (HCC) (1). Therefore, it is essential to investigate the detailed mechanisms of tumorigenicity in HCC.
The most fundamental characteristic of cancer cells is abnormal proliferation (2), which is partly caused by misregulation of cell cycle. The cell cycle of eukaryotic cells includes four phase, namely, G1, S, G2 and M. The phase transition is critically driven by cyclin-dependent kinases (CDKs). As the central regulatory determinant in cell cycle, CDKs are suffered to many levels of regulation, and their activities change dependent on both intracellular and extracellular environment (3,4). The first level of regulation is implemented by cyclins, and another level is by CDKs inhibitors (CKIs) to inhibit phosphorylation of CDKs. Of which, cyclin abundance oscillates during the cell cycle as a result of programmed synthesis and degradation. The G1/S phase transition is driven by CDK2, CDK4, CDK6 and corresponding cyclins. CDK4 and CDK6 are regulated by D-type cyclins: cyclin D1, cyclin D2 and cyclin D3. Cyclin E accumulates very closely to G1/S transition and specifically activates CDK2. Activated CDKs can target and phosphorylate retinoblastoma (p-Rb). p-Rb is identified to be the first suppressor gene. It can repress transcription through binding transactivation domain of E2F family (5). Phosphorylated p-Rb alleviates its inhibition on E2F family and allows the transcription of genes essential for S phase entry and DNA synthesis (6). CDK1 or CDK2 together with cyclin A are indispensable for the continuation of S phase and entry into M phase. CDK1 together with cyclin B also promote entry into M phase, and cyclin A is degraded before cyclin B by the proteasome (7). The intracellular and extracellular signals that affect phase transition of cell cycle play crucial roles in cell cycle progression. Therefore, we aim to investigate the factors that affect the cell cycle transition.
Nucleolar spindle associated protein 1 (NUSAP1) is originally identified as microtubule and chromosome binding protein. It takes an important role in chromosome segregation fidelity (8-10). Recently, it has been documented that NUSAP1 is abnormal expression, which is associated with poor prognosis in astrocytoma (10), prostate cancer (11), oral squamous cell carcinoma (12), prostate cancer (13), and breast cancer (14). However, its expressions and functions are unclear in HCC.
Herein, we found the expression of NUSAP1 significantly elevates and is associated with poor prognosis in HCC. Further assay suggested that upregulation of NUSAP1 can promote cell cycle progression of HCC cells. The above results demonstrate that NUSAP1 might be a prognostic marker for HCC.
Methods
Cell line and tissue specimens
HCC cell HepG2 was purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA) and cultured using Dulbecco’s modified eagle medium (DMEM) (Invitrogen, Carlsbad, CA, USA) that contained 10% fatal bovine serum (FBS) (HyClone, UT, USA). The primary HCC tissues and ANT were collected from Sun Yat-sen Memorial Hospital. For research purpose, we acquired the informed consent of patient involved in the research. This study was approved by institutional ethics committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (No. W2017NJ07). The research has been carried out in accordance with the Declaration of Helsinki [2008] of the World Medical Association.
Plasmids and retroviral infection
For overexpression of NUSAP1, the full-length human NUSAP1 cDNA was amplified and cloned into the pMSCV vector. For downregulation of NUSAP1, 2 human siRNA sequences that target NUSAP1 were sub-cloned into pSuper-retro-puro plasmid. And the siRNA fragments are as follows: Sh#1: GAGCACCAAGAAGCTGAGAAT; Sh#2: GAACCACACAAAGGAAAGCTA. Infection was conducted using retrovirus. To get stable cell lines, we treated the cells using 0.5 µg/mL puromycin. 10 days later, we detected the levels of NUSAP1 in cells.
Quantitative PCR (qPCR)
We used the Trizol reagent (Invitrogen, Carlsbad, CA, USA) to collect total RNA. qPCR was performed using ABI 7500 Fast System (Applied Biosystems, Rockville, MD, USA). We evaluated the relative mount of each gene on the basis of the algorithm: 2-[(Ct of gene)-(Ct of β-Tubulin)]. In the algorithm, the Ct is defined as threshold cycle and we used β-Tubulin as the internal control. The following are the primers of genes: NUSAP1: forward, 5'-CTGACCAAGACTCCAGCCAGAA-3', reverse, 5'-GAGTCTGCGTTGCCTCAGTTGT-3'; Cyclin D1: forward, 5'-TCTACACCGACAACTCCATCCG-3', reverse, 5'-TCTGGCATTTTGGAGAGGAAGTG-3'; Cyclin D3: forward, 5'-AGATCAAGCCGCACATGCGGAA-3', reverse, 5'-ACGCAAGACAGGTAGCGATCCA-3'; Cyclin E1: forward, 5'-TGTGTCCTGGATGTTGACTGCC-3', reverse, 5'-CTCTATGTCGCACCACTGATACC-3'; Cyclin A2: forward, 5'-CTCTACACAGTCACGGGACAAAG-3', reverse, 5'-CTGTGGTGCTTTGAGGTAGGTC-3'; Cyclin B1, forward, 5'-GACCTGTGTCAGGCTTTCTCTG-3', reverse, 5'-GGTATTTTGGTCTGACTGCTTGC-3'.
Western blotting assay
Western blotting assay was carried out on the basis of methods described (15). Whereafter, the membrane was probed by the primary antibodies: anti-NUSAP1, anti-p-Rb, anti-β-Tubulin (Abcam, Cambridge, MA, USA), and subsequently probed using HRP-conjugated secondary antibody (Abcam, Cambridge, MA, USA). And we used β-Tubulin as the loading control.
3-(4,5-Dimethyl-2-thiazolyl)2,5-diphenyl-2H-tetrazolium bromide (MTT) assay
2×103 cells were seeded into 96-well plates, subsequently maintained in 37 °C incubator until indicated time. Whereafter, the cells were treated by 100 µL 0.5 mg/mL sterile MTT reagent (sigma, St Louis, MO, USA) for 4 hours. And then, we added 150 µL dimethyl sulphoxide (DMSO) (sigma, St Louis, MO, USA) into the plates. And the absorbance of cells was detected at 490 nm.
Colony formation assay
Colony formation was used to evaluate the effect of NUSAP1 on proliferation of HCC cells and carried out on the basis of the methods described (16). 5×102 cells were implanted into 6-well plates and maintained in 37 °C incubator for 10 days. Firstly, we used 10% formaldehyde to fix the colonies. And then, 1% crystal violet was applied to stain the colonies for 30 seconds.
Anchorage-independent growth assay
The completed medium that contains 1% agar (sigma, St Louis, MO, USA) was supplemented into the bottom of the cell plates. Then, the mixture that was composed of 500 cells, 0.3% agar and 2 mL complete medium were added into the upper of the plates. The cells were maintained in 37 °C incubator for 10 days and then the colonies with diameter larger than 0.1 mm were counted.
Flow cytometry assay
We collected the cells and fixed with 75% ethanol. Then, we applied 20 µg/mL RNase A and 50 µg/mL propidium iodide to treat the cells for 30 minutes away from light. The cells-treated were detected using FASCS alibur instrument (BD Biosciences, Franklin Lake, New Jersey, USA). Besides, CellQuest 3.3 software was used to evaluate the cells percentages in different phases.
Statistical analysis
Every experiment was repeated at least 3 times. SPSS 20.0 statistical software package was used to perform statistical analysis. The statistical significance between groups was evaluated by Student’s t-test, and the data was demonstrated as mean ± deviation. Survival curves were plotted using the Kaplan-Meier method, and significant analysis was using log-rank test. P<0.05 was considered as statistical significance.
Results
NUSAP1 is significantly upregulated and associated with poor survival in HCC
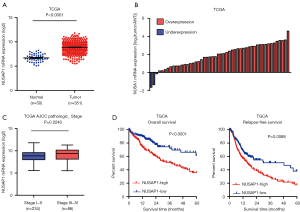
Through analyzing the availably public dataset The Cancer Genome Atlas (TCGA) (https://cancergenome.nih.gov/), the results showed that NUSAP1 expression is significantly upregulated in 351 HCC tissues compared with 50 normal liver tissues (Figure 1A). Subsequently, we further evaluated the NUSAP1 expression in 50 paired tissues. As demonstrated in Figure 1B, NUSAP1 is drastically upregulated in 47 HCC tissues compared with matched adjacent normal liver tissues (ANT). Besides, the correlation between NUSAP1 expression and clinical pathologic characteristics were performed. NUSAP1 expression is significantly upregulated in AJCC stage III-IV compared with that in stage I–II (Figure 1C). Not only that, the patients with high-expression of NUSAP1 showed poorer 5-year overall survival and relapse-free survival than that with low NUSAP1 (Figure 1D).
We also check the NUSAP1 expression in 8 paired fresh HCC tissues and ANT using qPCR and western blotting assay. The results demonstrated that NUSAP1 expression is drastically elevated in HCC tissues than in ANT both on transcription and translation levels (Figure 2).
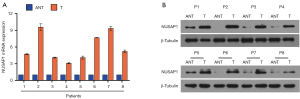
Altogether, NUSAP1 is significantly upregulated in HCC tissues and associated with poor prognosis.
Overexpression of NUSAP1 promotes the proliferation and in vitro tumorigenicity capacity of HCC cells
To investigate the functions of NUSAP1 in HCC progression, the gene set enrichment analysis (GSEA) was carried out using the data from TCGA. The analysis illustrated that NUSAP1 might be involved in cell cycle progression (Figure 3A). Next, the NUSAP1-overexpressed and -silenced stable cell lines were screened using HCC cell HepG2. The mRNA and protein expression of NUSAP1 was examined in the stable cell lines (Figure 3B). Next, MTT and colony formation assay demonstrated that overexpression of NUSAP1 significantly promoted HCC cells proliferation, and knockdown of NUSAP1 drastically inhibited cell proliferation (Figure 3C,D). Besides, anchorage-independent growth assay illustrated that in vitro tumorigenicity capacity of HCC cells promotes in NUSAP1-upregulating cells, while inhibits in NUSAP1-downregulating cells (Figure 3E).
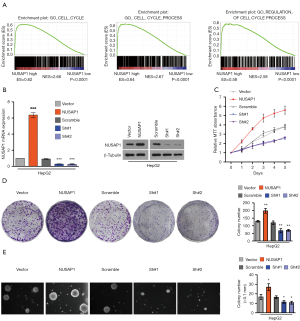
Altogether, overexpression of NUSAP1 promoted proliferation and in vitro tumorigenicity capacity of HCC cells.
NUSAP1 facilitates cell cycle transition in HCC cells
To further explore the mechanisms by which NUSAP1 promotes cell proliferation and in vitro tumorigenicity capacity, GSEA was implemented. The analysis demonstrated that NUSAP1 might be involved in G1/S and G2/M phase transition (Figure 4A). Next, flow cytometric assay was performed. As shown in Figure 4B, overexpression of NUSAP1 increases the percentage of cells in S-phase, but decreases cells in G0/G1 phase. While, downregulation of NUSAP1 showed the opposite results (Figure 4B). Not only that, we evaluated the mRNA expression of cyclins involved in G1/S and G2/M phase transition, cyclin D1, cyclin D3, cyclin E1, cyclin A2, cyclin B1 and the expression of E1F2. The heatmap illustrated that overexpression of NUSAP1 significantly increases their expression and downregulation of NUSAP1 inhibits their expression (Figure 4C). Besides, the phosphorylation level of p-Rb is the critical determinant of G1/S phase transition. Therefore, we evaluated the phosphorylation level of p-Rb by western blotting assay. As shown in Figure 4D, the phosphorylation level of p-Rb enhances in NUSAP1-upregulated cells, while downregulates in NUSAP1-silenced cells, indicating that NUSAP1 can help to G1/S transition.
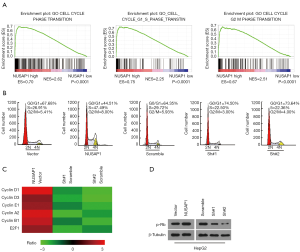
Altogether, overexpression facilitates cell cycle G1/S and G2/M phase transition of HCC cells.
Discussion
Herein, we discovered that the level of NUSAP1 dramatically elevates in HCC tissues and is negatively related with prognosis of patients with HCC. Further analysis demonstrated that upregulation of NUSAP1 promotes the proliferation and in vitro tumorigenicity capacity of HCC cells and facilitates the G1/S and G2/M phase transition.
Aberrant proliferation of cells results in loss of the normal tissue architecture and functions, and the cancer cells obtain the capability for aberrant proliferation. Aberrant proliferation can be caused by multiple intracellular and extracellular signals. It has been reported that IncRNA FAL1 is up-regulated in HCC tissues and accelerates cell proliferation (17). Lin et al. showed that Exportin-T can promote cell proliferation through affecting cell cycle and ubiquitin-mediated proteolysis of HCC cells (18). Liu et al. clarified that PNMA1 significantly elevates in HCC tissues and is associated with poor prognosis (19). Shi and her colleagues suggested that TNNT1 is significant upregulated in breast cancer cells, and upregulation of TNNT1 can promote G1/S transition of breast cancer cells. Especially, TNNT1 can increase the levels of cyclin D1, cyclin E1, cyclin E2 and the phosphorylation of Rb (16). In particular, in consideration of CDKs ultimately driving the cell cycle transition, substantial preclinical test on CKIs are underway. Whereas, the results of clinical trials are disappointing (20). Our study might provide a novel idea on targeting cell cycle. NUSAP1 is upregulated to accelerate cell proliferation in HCC and essential for the integrity of the anaphase spindle and cell division. We speculated that targeting NUSAP1 can lead to abnormal cell division, which might be result in cell apoptosis.
Wu et al. discovered that NUSAP1 also promotes cancer metastasis by activating the Hedgehog signaling pathway in astrocytoma (21). Gordon and his colleagues found that NUSAP1 enhances invasion and metastasis in prostate cancer (11). Whereas, whether NUSAP1 also promotes metastasis in HCC is unclear. We will clarify the above problems in our future study.
In conclusion, NUSAP1 is significantly upregulated in HCC and associated with poor prognosis of patients with HCC. Further assay showed that NUSAP1 can promote cell cycle progression. Our study might provide a novel therapeutic prognostic factor for HCC.
Acknowledgments
Funding: This study was supported by
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tcr.2019.09.28). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. For research purpose, we acquired the informed consent of patient involved in the research. This study was approved by institutional ethics committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (No. W2017NJ07). The research has been carried out in accordance with the Declaration of Helsinki (as revised in 2013) of the World Medical Association.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Morgan DO. Principles of CDK regulation. Nature 1995;374:131-4. [Crossref] [PubMed]
- Nigg EA. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays 1995;17:471-80. [Crossref] [PubMed]
- Harbour JW, Dean DC. Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol 2000;2:E65-7. [Crossref] [PubMed]
- Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 2013;140:3079-93. [Crossref] [PubMed]
- Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat Rev Mol Cell Biol 2008;9:910-6. [Crossref] [PubMed]
- Chou HY, Wang TH, Lee SC, et al. Phosphorylation of NuSAP by Cdk1 regulates its interaction with microtubules in mitosis. Cell Cycle 2011;10:4083-9. [Crossref] [PubMed]
- Mills CA, Suzuki A, Arceci A, et al. Nucleolar and spindle-associated protein 1 (NUSAP1) interacts with a SUMO E3 ligase complex during chromosome segregation. J Biol Chem 2017;292:17178-89. [Crossref] [PubMed]
- Li C, Xue C, Yang Q, et al. NuSAP governs chromosome oscillation by facilitating the Kid-generated polar ejection force. Nat Commun 2016;7:10597. [Crossref] [PubMed]
- Gordon CA, Gong X, Ganesh D, et al. NUSAP1 promotes invasion and metastasis of prostate cancer. Oncotarget 2017;8:29935-50. [Crossref] [PubMed]
- Okamoto A, Higo M, Shiiba M, et al. Down-Regulation of Nucleolar and Spindle-Associated Protein 1 (NUSAP1) Expression Suppresses Tumor and Cell Proliferation and Enhances Anti-Tumor Effect of Paclitaxel in Oral Squamous Cell Carcinoma. PLoS One 2015;10:e0142252. [Crossref] [PubMed]
- Gulzar ZG, McKenney JK, Brooks JD. Increased expression of NuSAP in recurrent prostate cancer is mediated by E2F1. Oncogene 2013;32:70-7. [Crossref] [PubMed]
- Chen L, Yang L, Qiao F, et al. High Levels of Nucleolar Spindle-Associated Protein and Reduced Levels of BRCA1 Expression Predict Poor Prognosis in Triple-Negative Breast Cancer. PLoS One 2015;10:e0140572. [Crossref] [PubMed]
- Ye G, Sun G, Cheng Z, et al. p55PIK regulates alpha-fetoprotein expression through the NF-kappaB signaling pathway. Life Sci 2017;191:104-10. [Crossref] [PubMed]
- Shi Y, Zhao Y, Zhang Y, et al. TNNT1 facilitates proliferation of breast cancer cells by promoting G1/S phase transition. Life Sci 2018;208:161-6. [Crossref] [PubMed]
- Li B, Mao R, Liu C, et al. LncRNA FAL1 promotes cell proliferation and migration by acting as a CeRNA of miR-1236 in hepatocellular carcinoma cells. Life Sci 2018;197:122-9. [Crossref] [PubMed]
- Lin J, Hou Y, Huang S, et al. Exportin-T Promotes Tumor Proliferation and Invasion in Hepatocellular Carcinoma. Mol Carcinog 2019;58:293-304. [Crossref] [PubMed]
- Liu P, Chen B, Gu Y, et al. PNMA1, regulated by miR-33a-5p, promotes proliferation and EMT in hepatocellular carcinoma by activating the Wnt/beta-catenin pathway. Biomed Pharmacother 2018;108:492-9. [Crossref] [PubMed]
- Asghar U, Witkiewicz AK, Turner NC, et al. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 2015;14:130-46. [Crossref] [PubMed]
- Wu X, Xu B, Yang C, et al. Nucleolar and spindle associated protein 1 promotes the aggressiveness of astrocytoma by activating the Hedgehog signaling pathway. J Exp Clin Cancer Res 2017;36:127. [Crossref] [PubMed]


